Abstract
This paper focusses on the morphological and viscoelastic properties of the cicada tymbal from the species Dundubia rufivena. Morphological details were determined by scanning electron and fluorescence microscopy, while the viscoelastic properties were determined by dynamic mechanical thermal analysis, and further supported by differential scanning calorimetry. We find that water evaporation from the tymbal begins at 71.1 C and the glass transition for the tymbal, which is a chitin–resilin composite, is on average 150 C, though there is considerable heterogeneity in the material of the tymbal, as indicated by the half height peak width of the tymbal (35.3 C) and the shoulder peak indicative of a second phase and hence glass transition at on average, 168 C. This second phase is assumed to reflect the effects of large-scale molecular pinning and restructuring at resilin–chitin interfaces (possibly via specific binding domains). In addition, we elucidate that the predominantly resilin regions of the tymbal of Dundubia rufivena is reinforced by a polygonal mesh of chitin, a morphological feature that has not been described in any previous research on the cicada tymbal. We provide evidence for nonlinear elasticity in the tymbal by comparing the storage modulus of the tymbal at different frequencies and loading amplitudes.
1. Introduction
Cicadas are amongst the loudest insects in the animal kingdom [1], with sounds reaching up to 115 dB [2]. It produces this sound from a specialised structure called a tymbal, which is located, one on each side, of the cicada’s abdomen. Sound is generated in this structure on contraction of a tymbal muscle, attached to a tymbal membrane, which causes it to buckle inwards to create the audible click. In species such as Cyclochila australasiae, the tymbal has also been found to act as a Helmholtz resonator, which excites and sustains sound at a certain frequency (4 kHz), storing and releasing mechanical energy throughout the process of tymbal movement [3]. The resonator is made up of two structures, the tympana (membranous structures) and the air sac [2,4]. After sound is produced by the tymbal, it is transferred to the air sac, which then creates resonant vibrations, which are in turn radiated to the environment through the tympana [2,3], analogous in many ways to how loudspeakers are built up [5,6]. Bennet-Clark [7] reported that, as a primary resonator [3], the tymbal has the components of a resonant system (mass and compliance). The major mass element is the tymbal plate, whereas the major elastic elements are the dorsal resilin pad and tymbal ribs. When buckling inward, tymbal compliance was found to be 0.02 m/N, while the outward buckling was reported as being lower, at 0.012 m/N. Asymmetrical inward and outward buckling compliance indicates that the buckling of each rib releases stored energy into the resilin, and that this might involve a nonlinear deformation. This is expected, since resilin is essentially a nonlinear rubbery material with hyperelastic characteristics [8]. In addition, increases in the tymbal compliance are related to its geometrical makeup, in particular, to the slenderness ratios of the long ribs, which buckle, causing an inward movement of the tymbal plate. Josephson and Young [9] furthermore report that tymbal bucking brings about an increase in body temperature. This is due to the presence of high contraction frequencies from the large tymbal muscles. They observed a rise of ca. 12 C from the ambient temperature during a song. Lower temperature increases were also detected from several other body parts, comprising the thorax and the large air sac, which were deemed likely to be warmed by the activity of the tymbal muscles during a song. Although the measurement of temperature excess in these body parts of the cicada has been performed, data reporting a temperature increase in the tymbal itself is still unavailable, as is data related to the thermomechanical performance of the tymbal. High frequency vibrations in tymbals (of at least 4 kHz) are highly likely to result in increased tymbal temperatures and this in turn may affect the viscoelastic response of the tymbal.
Most cicadas share similar tymbal features, and the tymbal function is the same in all cases, such that the tymbal determines the song frequency and the abdominal cavity acts as a resonator. Different species of cicada do nevertheless exhibit different mechanisms for sound radiation [10]. Importantly, any damage to tymbal structures, such as the tymbal ribs, will likely destroy a tymbal’s ability to buckle and disable its ability to sing [7]. This is one possible reason for why the tymbal is constructed as a viscoelastic composite of both harder (chitin) and softer (resilin) materials, as combinations of these two materials in insects reduce damage under the conditions of dynamic mechanical loading [11,12,13]. In this paper, we aim to develop further insights into the structures and viscoelastic properties of the cicada tymbal from the species Dundubia rufivena.
2. Materials and Methods
2.1. Species Collection and Tymbal Preparation
Tymbals were dissected from males of the Dundubia rufivena species, which were captured in Tamantirto, Kasihan, Bantul Regency—7.8256389 110.3263697 (051612.2 S latitude and 1103263697 E longitude) Yogyakarta, Indonesia between October and November 2019. The cicadas were captured at night using a light trap and euthanised using chloroform in an insect bottle, after which they were dry preserved in an oven at 40 C. Tymbals can only be found on cicada males (two per male) and four males were captured for these tests. As such, a total of eight tymbals were available for testing. Tymbals were separated from the surrounding cicada cuticle using scalpel and tweezers. Samples were further moisturised in a humidity chamber for 24 h prior to testing, in order to ensure that the tymbals were hydrated.
2.2. Scanning Electron Microscopy (SEM)
Scanning electron microscopy (Hitachi TM4000, Tokyo, Japan) was performed to more closely examine the tymbal structure (n = 1). A tymbal was sputter coated with gold under vacuum for 5 s, resulting in a coating layer thickness of approximately 30 nm. Tymbals were scanned in SEI mode at an accelerating voltage of 5 kV to avoid burning of the biological tissue of the tymbal.
2.3. Fluorescence Microscopy
Fluorescence microscopy was conducted to differentiate chitin structures from resilin structures within the tymbal (n = 3). Tymbals removed from the body were glued to a piece of flat board, exposing the external surface of the tymbal for microscopic analysis. A Dinolite AM4115FUT was used with Ex/Em 375 nm/410 nm, a Dinolite AM4115T-GRFBY with Ex/Em 480 nm/610 nm and a Dinolite AD4113T-12 V with Ex/Em 395 nm/430 nm.
2.4. Dynamic Mechanical Thermal Analysis (DMTA)
The viscoelastic properties of the tymbals were measured using a DMTA machine (Tritec 2000 DMA, Burladingen, Germany). Tymbals (n = 2) were subjected to a multi-frequency temperature sweep from 25 C to 180 C under dynamic tensile deformation at 1 Hz and 10 Hz at a displacement of 0.05 mm. The Tritec 2000 DMA was additionally used to conduct tensile tests on the tymbal at a constant (room) temperature of 25 C, which is the tymbal muscle temperature before and after singing according to Josephson and Young [9] using a single frequency mode at 1 Hz and 10 Hz and displacements of 0.01 mm, 0.02 mm and 0.05 mm. The tymbals were glued at either end to a piece of thick card using a fast setting epoxy. The card was then cut across its centre to a position corresponding with the centre of the tymbal. This was to ensure that the tymbal could be loaded in tension through the card and was necessary since the tymbal was of an atypical geometry that could not be held by the specimen grips of the machine. An example image of the set up is shown in Figure 1.
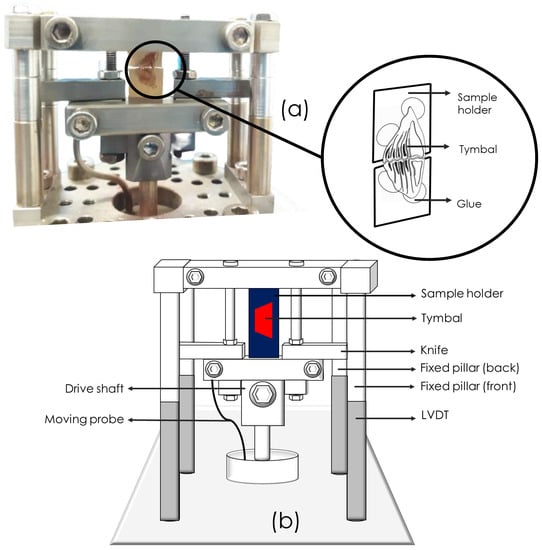
Figure 1.
Clamping set up for the cicada tymbal within the DMTA showing (a) a photo of the cicada tymbal as set up in the DMTA and a schematic drawing to highlight the tymbal the glue and the sample holding card, and (b) a schematic of the complete DMTA test set up.
2.5. Differential Scanning Calorimetry (DSC)
A differential scanning calorimeter (PerkinElmer DSC 8000, Waltham, MA, USA) was used to determine the thermal transitions that tymbals undergo under a progressively increasing temperature. The weight of the tymbal was measured before DSC measurements were taken (n = 2), using an analytical scale (Ohaus, Parsippany, NJ, USA). The DSC was set to run from 40 C to 250 C at a heating rate of 20 C/minute.
3. Results
Cicada body length was measured as being on average 29.2 mm (SD = ±2.03 mm, CoV = 6.95%), while the maximum cicada body width was measured as being on average 9.5 mm (SD = ±0.47, CoV = 4.94%). The average tymbal length was measured as 4.04 mm (SD = ±0.35, CoV = 8.68%) and the average maximum width measured was 2.90 mm (SD = ±0.2, CoV = 6.9%). Figure 2 shows in (a) an SEM image of an entire tymbal from the species Dundubia rufivena. In this image, the chitin ribs and other chitin structures can be seen as protuberant bands, with the rest being resilin rich regions [3,14]. Closer observation by Ex/Em 480 nm/610 nm autofluorescent imaging (b–c) shows that there are chitin rich support structures, many of which form a polygonal mesh, within the resilin rich regions. This is reaffirmed in (d) by Ex/Em 395 nm/430 nm autofluorescent imaging, where the polygonal chitin structures embedded within the resilin rich regions are once again noticeable as the parts that do not autofluoresce. Interestingly, we note that there is heavy resilin loading at interfaces of larger chitin supports in the tymbal as seen in (e) under Ex/Em 395 nm/430 nm and (f) under Ex/Em 375 nm/410 nm, and that there are also large patches of resilin rich regions between chitin supports as shown in (g) under Ex/Em 395 nm/430 nm. While there are several reports that distinguish the chitin ribs from the resilin connecting them, this is the first report of the existence of a polygonal chitin mesh within the resilin rich regions of the tymbal. We presume this polygonal mesh serves to support the soft rubbery resilin, and may even enable outward buckling (a return to structural form) after inward buckling.
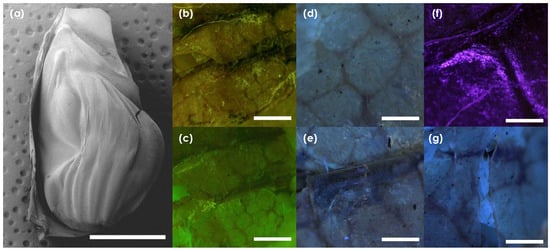
Figure 2.
(a) SEM image of a tymbal from the species Dundubia rufivena (scale = 1.5 mm); (b,c) Ex/Em 480 nm/610 nm autofluorescent image showing chitin support structures and polygonal chitin structures: (b,c scale bars = 0.3 mm); (d) polygonal chitin structures shown under Ex/Em 395 nm/430 nm; (e) resilin autofluorescence around chitin ribs shown under Ex/Em 395 nm/430 nm; (f) resilin autofluorescence around chitin joints under Ex/Em 375 nm/410 nm; and (g) large patches of resilin autofluorescence between chitin supports shown under Ex/Em 395 nm/430 nm: (d–g scale bars = 0.15 mm).
Table 1 shows the effect of loading frequency and loading amplitude on the tensile properties of the tymbal. Generally, we note that, at a higher loading frequency (strain rate), the modulus of the tymbal is higher, which is characteristic of a viscoelastic composite. In addition, we note that the storage modulus decreases as a function of increasing loading amplitude under the same strain rate, which indicates nonlinear elasticity of the tymbal. We assume that nonlinear elasticity is an effect introduced by the presence of rubber-like resilin, which is known to have hyperelastic properties [8]. Figure 3 shows curves for the storage modulus and tan- under a temperature sweep for two tymbals from different cicadas. The difference between the two is noted by the shaded regions on the graph. Taking an average of the two curves, the tymbal can be seen to have a glass transition in the region of 150 C, and its storage modulus decreases as a function of increasing temperature reaching approximately 0.07GPa at 37 C, which, according to Josephson and Young, (1979) is the maximum temperature the tymbal muscle reaches during singing. Additionally, the tymbal shows considerable heterogeneity as indicated by the half height peak width of the tymbal, which is 35.3 C. The presence of two different phases within the tymbal is further indicated by the shoulder peaks on the tan- curves, which have an average glass transition of 168 C. The tan- peak is on average 0.57, indicating that the tymbal is a high damping structure for a solid material, which we presume is borne primarily from the resilin component of the composite. As such, the tymbal of Dundubia rufivena is a structure that is very effective at absorbing and dispersing dynamic mechanical energy. The amount of energy that can be absorbed by the tymbal is measured as the area under the tan- curve, and is on average 37.6 C.

Table 1.
Storage modulus as a function of frequency and displacement for the tymbals.
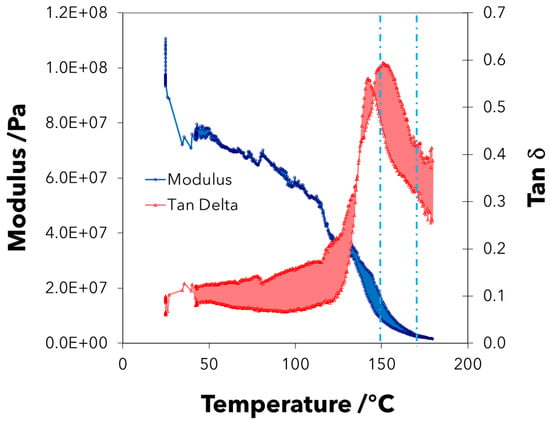
Figure 3.
Storage modulus and tan- curves for two tymbal samples with shaded differences as a function of temperature. Broken turquoise lines indicate the two averaged Tg transitions.
The DSC curve in Figure 4 shows that the tymbal has a broad endothermic peak at 71.1 C. As the temperature is increased, the tymbal undergoes a glass transition as shown by a small endothermic peak at around 150 C, confirming glass transition temperature measurements by DMTA as described previously.
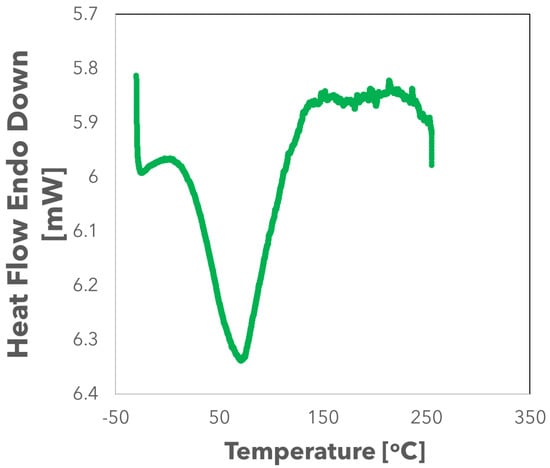
Figure 4.
Thermal transitions of a cicada tymbal analysed by DSC with temperature ranges of −35 C to 250 C.
4. Discussion
As reported by Lombardi and Kaplan [15], rubber-like regions of insect exoskeleton with the characteristics of high and reversible elasticity, embedded by chitin lamellae, exhibit elastomeric properties. Such structures are essentially composite structures, and the cicada tymbal is shown here to be a composite with viscoelastic properties and, as such, the tymbal is a strain rate dependent structure. While resilin provides elasticity within the frequently extending and retracting tymbal [8,16], chitin provides structural rigidity and support. While chitin has been known to form the harder structures in cicada tymbals, such as the ribs, it is revealed herein that chitin additionally forms a polygonal mesh within the resilin rich regions of the tymbal. The Young’s modulus of resilin is about 0.6–2 MPa [17,18,19,20,21] while the Young’s modulus of chitin nanofibres can be over 150 GPa [17]. Thus, we postulate herein that, while resilin enables flexibility, chitin enables speedy spring-back mechanisms, and that the polygonal chitin mesh is therefore of high importance to elastic return. This position can be further strengthened through the strain rate dependent nature of resilin as compared against resilin–chitin composites. Pure resilin from a dragonfly wing, for example, undergoes its glass transition at a frequency of 106.3 Hz [22], while a resilin–chitin composite, such as is found in a cockroach, will transition at a higher strain rate of 200 kHz [23]. Resilin is understood to form a composite interface with chitin via a chitin binding domain where it undergoes polymerisation, which in turn generates dityrosine bridges [24]. Mushi [25] suggests that resilin with chitin binding domain could integrate the nanoscale ductility of resilin to insect cuticle. Here, we note that there are resilin rich regions at interfaces to chitin rich supports in the tymbal and suggest that these rich regions are necessary to delocalise high stresses at the resilin–chitin interfaces, thereby decreasing the chances of interfacial failure.
Previous observation of resilin–chitin thermal transitions [8] note that the resilin with a chitin binding domain displays a broad endothermic peak at 65 C, while Lombardi and Kaplan [15] find this peak is at 83 C. In the present work, for the tymbal of Dundubia rufivena, we find this peak is at 71.1 C (cf. Figure 4). This peak is understood to indicate the beginning stages of bound water molecule evaporation [8]. It should be noted that water evaporation from pure chitin begins at 66.05 C [26] and thus deviations from this are a likely result of water evaporation from resilin and resilin–chitin binding regions, at temperatures higher than 150 C, which is the glass transition temperature identified herein for the cicada tymbal. Above this temperature, there -turn transitions are possible (at temperatures from 201 C and above), leading to more ordered structures in the resilin; however, this transition is understood to continue to 240 C, before the resilin decomposes at 250 C [8]. This temperature of 201 C, at which -turn transitions occur, is much higher than the second glass transition noted in our DMTA study, which occurs at 168 C. Choudhury [23] suggests that certain regions of resilin tend to be unordered if chitin is not present. Conversely, resilin tends to develop -structures when it interacts with chitin, leading to the formation of a more ordered resilin structure with decreased compliance as compared to the compliance of pure resilin. In addition, Lombardi and Kaplan [15] note from circular dichroism (CD) studies that the resilin sequence of the -structures in resilin, 39% can be attributed to -sheets 27.8% can be attributed to -turns. As such, we suggest that the second glass transition temperature, measured from our DMTA work, relates to resilin–chitin interactions, and the pinning and restructuring of resilin molecules as a result of their interactions with chitin (and possibly also directly from resilin–chitin binding at specific domains).
Previous research focussed on DMA analyses of pure resilin has shown that the glassy state storage modulus of pure resilin is 30 MPa, while the rubbery state storage modulus is 1.6 MPa [22]. When comparing against our DMTA results, the storage modulus of the tymbal, which is a resilin–chitin composite, is notably higher than this, and this reflects the stiffer chitin component of a composite tymbal as well as possible restructuring of resilin through resilin–chitin interactions. Previous observations of chitin–resilin composites [25] show that the chitin network provides tensile strength and stiffness to the resilin. Wigglesworth [27] also notes that chitin is responsible for a cuticle’s tough elastic properties. Likewise, Appel and co-workers [28] propose that chitin microfibrils combined with proteins will contribute significantly to cuticle stiffness. Chitin fibre reinforcements in insect cuticles have other mechanical benefits such as improved energy storage [29] and fatigue endurance [30], and this in turn is important since the tymbal will endure high cycle loads during song. The discovery of the polygonal chitin mesh in this paper is hypothesised, therefore, to be of additional benefit in resisting fatigue failure as the tymbal vibrates during songs.
5. Conclusions
In this paper, we elucidate the morphological, viscoelastic and thermal transition properties of cicada tymbal from the species Dundubia rufivena. We find that this species of cicada has a polygonal chitin mesh embedded within the resilin of the tymbal and suggest that the mesh improves fatigue endurance and elastic return during a song. The tymbal is a resilin–chitin composite, and its thermal transition properties are therefore different to those of pure resilin or pure chitin. Here, we report that water evaporation begins at 71.1 C and that there are two glass transition temperatures, one at 150 C and a second at 168 C. There is heterogeneity in the resilin component of the chitin–resilin composite tymbal, and we suggest that there is a pure resilin phase providing one glass transition temperature, and a secondary resilin phase that results from binding with the chitin to form -structures that alter the glass transition properties of the resilin as well as from molecular pinning at interfaces. We show herein that the tymbal of Dundubia rufivena is a viscoelastic structure, with nonlinear elastic properties.
Author Contributions
Conceptualization, P.A.; methodology, C.R., P.R.S., F. and P.A.; validation, C.R. and P.A.; formal analysis, P.R.S., F., C.R. and P.A.; investigation, F., P.R.S., C.R. and P.A.; resources, B.R. and P.A.; data curation, F., P.R.S., C.R. and P.A.; writing—original draft preparation, F. and P.R.S.; writing—review and editing, P.A.; visualization, P.A.; supervision, P.A.; project administration, P.A.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as Universitas Gadjah Mada already has legally authorised permission to conduct field survey and sample collection accordingly. The sample are not on the list of protected animals. According to Law of the Republic of Indonesia No. 18 Year 2009 Article 66 about Animal Welfare (Undang Undang Republik Indonesia No. 18 Tahun 2009 Pasal 66 Tentang Kesejahteraan Hewan), the action of collecting, handling, placement, killing and transporting animals must be conducted in the best possible manner such that the animals are free from pain, fear, mistreatment, and abuse. However, that Law does not require an Ethical Approval arrangement for performing the actions mentioned above, during research involving the animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author on request.
Acknowledgments
We acknowledge Sukirno for his assistance in teaching Fahrunnida and Puspa Restu Sayekti on cicada collection using a light trap. We also acknowledge Gestono, Sukisti, Gilang Pangestu and Bagus Kodrat Istianto for their assistance in cicada collection and preservation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bennet-Clark, H.C. How Cicadas Make Their Noise. Sci. Am. 1998, 278, 58–61. [Google Scholar] [CrossRef]
- Young, D. Do cicadas radiate sound through their ear-drums? J. Exp. Biol. 1990, 151, 41–56. [Google Scholar] [CrossRef]
- Young, D.; Bennet-Clark, H.C. The role of the tymbal in cicada sound production. J. Exp. Biol. 1995, 198, 1001–1019. [Google Scholar] [CrossRef] [PubMed]
- Bennet-Clark, H.C.; Young, D. A model of the mechanism of sound production in cicadas. J. Exp. Biol. 1992, 173, 123–153. [Google Scholar] [CrossRef]
- Schroeder, T.B.; Houghtaling, J.; Wilts, B.D.; Mayer, M. It’s not a bug, it’s a feature: Functional materials in insects. Adv. Mater. 2018, 30, 1705322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelsen, A.; Nocke, H. Biophysical aspects of sound communication in insects. In Advances in Insect Physiology; Treherne, J.E., Berridge, M.J., Wigglesworth, V.B., Eds.; Academic Press: London, UK, 1974; Volume 10, pp. 247–296. [Google Scholar]
- Bennet-Clark, H.C. Tymbal mechanics and the control of song frequency in the cicada Cyclochila australasiae. J. Exp. Biol. 1997, 200, 1681–1694. [Google Scholar] [CrossRef]
- Qin, G.; Hu, X.; Cebe, P.; Kaplan, D.L. Mechanism of resilin elasticity. Nat. Commun. 2012, 3, 1003. [Google Scholar] [CrossRef] [Green Version]
- Josephson, R.K.; Young, D. Body temperature and singing in the bladder cicada, Cystosoma saundersii. J. Exp. Biol. 1979, 80, 69–81. [Google Scholar] [CrossRef]
- Fonseca, P.J.; Popov, A.V. Sound radiation in a cicada: The role of different structures. J. Comp. Physiol. A 1994, 175, 349–361. [Google Scholar] [CrossRef]
- Fauziyah, S.; Alam, C.; Soesilohadi, R.C.H.; Retnoaji, B.; Alam, P. Morphological and mechanical characterisation of the hindwing nodus from the Libellulidae family of dragonfly (Indonesia). Arthropod Struct. Dev. 2014, 43, 415–422. [Google Scholar] [CrossRef]
- Fauziyah, S.; Soesilohadi, R.C.H.; Retnoaji, B.; Alam, P. Dragonfly wing venous cross-joints inspire the design of higher-performance bolted timber truss joints. Compos. Part B Eng. 2016, 87, 274–280. [Google Scholar] [CrossRef]
- Gorb, S.N. The jumping mechanism of cicada Cercopis vulnerata (Auchenorrhyncha, Cercopidae): Skeleton–muscle organisation, frictional surfaces, and inverse-kinematic model of leg movements. Arthropod Struct. Dev. 2004, 33, 201–220. [Google Scholar] [CrossRef]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; LaJeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, E.C.; Kaplan, D.L. Preliminary characterization of resilin isolated from the cockroach, Periplaneta americana. In Materials Research Society Symposium Proceedings; Viney, C., Case, S.T., Waite, J.H., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1993; pp. 3–7. [Google Scholar]
- Andersen, S.O. Characterization of a new type of cross-linkage in resilin, a rubber-like protein. Biochim. Biophys. Acta 1963, 69, 249–262. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Weis-Fogh, T. Molecular Interpretation of the Elasticity of Resilin, a Rubber-like Protein. J. Mol. Biol. 1961, 3, 648–667. [Google Scholar] [CrossRef]
- Weis-Fogh, T. Thermodynamic Properties of Resilin, a Rubber-like Protein. J. Mol. Biol. 1961, 3, 520–531. [Google Scholar] [CrossRef]
- Su, R.S.; Kim, Y.; Liu, J.C. Resilin: Protein-based elastomeric biomaterials. Acta Biomater. 2014, 10, 1601–1611. [Google Scholar] [CrossRef]
- Gosline, M. Elastic properties of rubber-like proteins and highly extensive tissues. In Mechanical Properties of Biological Materials; Vincent, J.F.V., Currey, J.D., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 331–357. [Google Scholar]
- King, R.J. Dynamic Mechanical Properties of Resilin. Ph.D. Dissertation, Virginia Tech, Blacksburg, VA, USA, 2010. [Google Scholar]
- Choudhury, U. Dynamic Mechanical Properties of Cockroach (Periplaneta Americana) Resilin. Ph.D. Dissertation, Virginia Tech, Blacksburg, VA, USA, 2012. [Google Scholar]
- Qin, G.; Lapidot, S.; Numata, K.; Hu, X.; Meirovitch, S.; Dekel, M.; Podoler, I.; Shoseyov, O.; Kaplan, D.L. Expression, Cross-Linking, and Characterization of Recombinant Chitin Binding Resilin. Biomacromolecules 2009, 10, 3227–3234. [Google Scholar] [CrossRef]
- Mushi, N.E. Chitin nanofibers, networks and composites—Preparation, structure and mechanical properties. Ph.D. Thesis, KTH, Royal Institute of Technology, Stockholm, Sweden, 2014. [Google Scholar]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The insect cuticle. Biol. Rev. Camb. Philos. Soc. 1948, 23, 408–451. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.; Heepe, L.; Lin, C.P.; Gorb, S.N. Ultrastructure of dragonfly wing veins: Composite structure of fibrous material supplemented by resilin. J. Anat. 2015, 227, 561–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrow, M.; Shaw, S.R.; Sutton, G.P. Resilin and chitinous cuticle form a composite structure for energy storage in jumping by froghopper insects. BMC Biol. 2008, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirks, J.H.; Parle, E.; Taylor, D. Fatigue of insect cuticle. J. Exp. Biol. 2013, 216, 1924–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).