Poly(L-lactide) Epimerization and Chain Scission in the Presence of Organic Bases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Typical Epimerization Reactions
2.3. Synthesis of Acetate End-Capped Poly(L-lactide) PLLA
2.4. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiesewetter, M.K.; Shin, E.J.; Hedrick, J.L.; Waymouth, R.M. Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar] [CrossRef]
- Dove, A.P. Organic Catalysis for Ring-Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Ottou, W.N.; Sardon, H.; Mecerreyes, D.; Vignolle, J.; Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 2016, 56, 64–115. [Google Scholar] [CrossRef] [Green Version]
- Nederberg, F.; Connor, E.F.; Möller, M.; Glauser, T.; Hedrick, J.L. New paradigms for organic catalysts: The first organocatalytic living polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M.; et al. Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Brown, H.A.; de Crisci, A.G.; Hedrick, J.L.; Waymouth, R.M. Amidine-Mediated Zwitterionic Polymerization of Lactide. ACS Macro Lett. 2012, 1, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Sherck, N.J.; Kim, H.C.; Won, Y.-Y. Elucidating a Unified Mechanistic Scheme for the DBU-Catalyzed Ring-Opening Polymerization of Lactide to Poly(lactic acid). Macromolecules 2016, 49, 4699–4713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, R.C.; Lohmeijer, B.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef]
- Simón, L.; Goodman, J.M. The Mechanism of TBD-Catalyzed Ring-Opening Polymerization of Cyclic Esters. J. Org. Chem. 2007, 72, 9656–9662. [Google Scholar] [CrossRef]
- Zhang, L.; Nederberg, F.; Pratt, R.C.; Waymouth, R.M.; Hedrick, J.L.; Wade, C.G. Phosphazene Bases: A New Category of Organocatalysts for the Living Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2007, 40, 4154–4158. [Google Scholar] [CrossRef]
- Yang, H.; Xu, J.; Pispas, S.; Zhang, G. Hybrid Copolymerization of ε-Caprolactone and Methyl Methacrylate. Macromolecules 2012, 45, 3312–3317. [Google Scholar] [CrossRef]
- Zhao, J.; Pahovnik, D.; Gnanou, Y.; Hadjichristidis, N. A “Catalyst Switch” Strategy for the Sequential Metal-Free Polymerization of Epoxides and Cyclic Esters/Carbonate. Macromolecules 2014, 47, 3814–3822. [Google Scholar] [CrossRef]
- Liu, S.; Ren, C.; Zhao, N.; Shen, Y.; Li, Z. Phosphazene Bases as Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromol. Rapid Commun. 2018, 39, 1800485. [Google Scholar] [CrossRef]
- Bonduelle, C.; Martín-Vaca, B.; Cossío, F.P.; Bourissou, D. Monomer versus Alcohol Activation in the 4-Dimethylaminopyridine-Catalyzed Ring-Opening Polymerization of Lactide and LacticO-Carboxylic Anhydride. Chem.—A Eur. J. 2008, 14, 5304–5312. [Google Scholar] [CrossRef]
- Coulembier, O.; Dubois, P. 4-dimethylaminopyridine-based organoactivation: From simple esterification to lactide ring-opening “Living” polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1672–1680. [Google Scholar] [CrossRef]
- Nogueira, G.; Favrelle, A.; Bria, M.; Ramalho, J.P.P.; Mendes, P.J.; Valente, A.; Zinck, P. Adenine as an organocatalyst for the ring-opening polymerization of lactide: Scope, mechanism and access to adenine-functionalized polylactide. React. Chem. Eng. 2016, 1, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Stanley, N.; Chenal, T.; Jacquel, N.; Saint-Loup, R.; Ramalho, J.P.P.; Zinck, P. Organocatalysts for the Synthesis of Poly(ethylene terephthalate-co-isosorbide terephthalate): A Combined Experimental and DFT Study. Macromol. Mater. Eng. 2019, 304, 1900298. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Glausser, T.; Hedrick, J.L. Organocatalytic chain scission of poly (lactides): A general route to controlled molecular weight, functionality and macromolecular architecture. Chem. Commun. 2001, 20, 2066–2067. [Google Scholar] [CrossRef]
- Leibfarth, F.A.; Moreno, N.; Hawker, A.P.; Shand, J.D. Transforming polylactide into value-added materials. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4814–4822. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Tsukegi, T.; Motoyama, T.; Shirai, Y.; Nishida, H.; Endo, T. Racemization behavior of l,l-lactide during heating. Polym. Degrad. Stab. 2007, 92, 552–559. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Jiao, H.; Schulze, J.; Tietz, W.; Kühlein, K.; Börner, A. Studies on the epimerization of diastereomeric lactides. Tetrahedron Lett. 2011, 52, 1027–1030. [Google Scholar] [CrossRef]
- Zhu, J.-B.; Chen, E.Y.-X. From meso-Lactide to Isotactic Polylactide: Epimerization by B/N Lewis Pairs and Kinetic Resolution by Organic Catalysts. J. Am. Chem. Soc. 2015, 137, 12506–12509. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Serra, A. Polylactones: 6. Influence of various metal salts on the optical purity of poly(L-lactide). Polym. Bull. 1985, 14, 487–502. [Google Scholar] [CrossRef]
- Meimoun, J.; Favrelle-Huret, A.; Bria, M.; Merle, N.; Stoclet, G.; De Winter, J.; Mincheva, R.; Raquez, J.M.; Zinck, P. Epimerization and chain scission of polylactides in the presence of an organic base, TBD. Polym. Degrad. Stab. 2020, 181, 109188. [Google Scholar] [CrossRef]
- Coulembier, O.; Moins, S.; Raquez, J.-M.; Meyer, F.; Mespouille, L.; Duquesne, E.; Dubois, P. Thermal degradation of poly(l-lactide): Accelerating effect of residual DBU-based organic catalysts. Polym. Degrad. Stab. 2011, 96, 739–744. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Polymerization of l,l-Lactide Initiated by Aluminum Isopropoxide Trimer or Tetramer. Macromolecules 1998, 31, 2114–2122. [Google Scholar] [CrossRef]
- Feng, L.-D.; Sun, B.; Bian, X.-C.; Chen, Z.-M.; Chen, X.-S. Determination of d-lactate content in poly(lactic acid) using polarimetry. Polym. Test. 2010, 29, 771–776. [Google Scholar] [CrossRef]
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I.A. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales. J. Org. Chem. 2005, 70, 1019–1028. [Google Scholar] [CrossRef]
- Kubota, Y.; Hanaoka, T.; Takeushi, K.; Sugi, Y. An efficient synthesis of aryl esters by palladium catalyzed carbonylation of 4-bromobiphenyl. Synlett 1994, 1994, 515–517. [Google Scholar] [CrossRef]
- Warhurst, D.C.; Craig, J.C.; Adagu, I.S.; Meyer, D.J.; Lee, S.Y. The relationship of physico-chemical properties and structure to the differential antiplasmodial activity of the cinchona alkaloids. Malar. J. 2003, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Schwesinger, R.; Schlemper, H. Peralkylated Polyaminophosphazenes—Extremely Strong, Neutral Nitrogen Bases. Angew. Chem. Int. Ed. 1987, 26, 1167–1169. [Google Scholar] [CrossRef]
- Ishikawa, T. Superbases for Organic Synthesis: Guanidines, Amidines and Phosphazenes and Related Organocatalysts; Wiley: Chichester, UK, 2009. [Google Scholar]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; van Nostrum, C.F.; Kettenes-Van den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly(lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Van Nostrum, C.F.; Veldhuis, T.F.J.; Bos, G.W.; Hennink, W.E. Hydrolytic degradation of oligo(lactic acid): A kinetic and mechanistic study. Polymer 2004, 45, 6779–6787. [Google Scholar] [CrossRef]
- Stukenbroeker, T.S.; Bandar, J.S.; Zhang, X.; Lambert, T.H.; Waymouth, R.M. Cyclopropenimine Superbases: Competitive Initiation Processes in Lactide Polymerization. ACS Macro Lett. 2015, 4, 853–856. [Google Scholar] [CrossRef] [Green Version]
- Iwahashi, H.; Oka, T.; Abiko, A. An onium salt-catalyzed direct polycondensation of lactic acid. Chem. Lett. 2008, 37, 708–709. [Google Scholar] [CrossRef]
- Kadota, J.; Pavlović, D.; Desvergne, J.-P.; Bibal, B.; Peruch, F.; Deffieux, A. Ring-Opening Polymerization of l-Lactide Catalyzed by an Organocatalytic System Combining Acidic and Basic Sites. Macromolecules 2010, 43, 8874–8879. [Google Scholar] [CrossRef]
- Coady, D.J.; Fukushima, K.; Horn, H.W.; Rice, J.E.; Hedrick, J.L. Catalytic insights into acid/base conjugates: Highly selective bifunctional catalysts for the ring-opening polymerization of lactide. Chem. Commun. 2011, 47, 3105. [Google Scholar] [CrossRef]
- Coulembier, O.; Josse, T.; Guillerm, B.; Gerbaux, P.; Dubois, P. An imidazole-based organocatalyst designed for bulk polymerization of lactide isomers: Inspiration from Nature. Chem. Commun. 2012, 48, 11695. [Google Scholar] [CrossRef]
- Makiguchi, K.; Kikuchi, S.; Yanai, K.; Ogasawara, Y.; Sato, S.; Satoh, T.; Kakuchi, T. Diphenyl phosphate/4-dimethylaminopyridine as an efficient binary organocatalyst system for controlled/living ring-opening polymerization of L-lactide leading to diblock and end-functionalized poly(L-lactide)s. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1047–1054. [Google Scholar] [CrossRef]
- Miao, Y.; Stanley, N.; Favrelle, A.; Bousquet, T.; Bria, M.; Mortreux, A.; Zinck, P. New acid/base salts as co-catalysts for the organocatalyzed ring opening polymerization of lactide. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 659–664. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Z.; Li, Z.; Zhu, Y.; Zhang, C.; Luo, Z.; Guo, T.; Gao, Y.; Zhang, L.; Guo, K. Biocompatible and low-cost pyridinium halides catalysts promoted ring-opening polymerizations of cyclic esters in bulk. Eur. Polym. J. 2020, 127, 109570. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Jehanno, C.; Zhu, H.; Coulembier, O.; Mecerreyes, D.; Sardon, H. Stereoretention in the Bulk ROP of l-Lactide Guided by a Thermally Stable Organocatalyst. Macromolecules 2021, 54, 6214–6225. [Google Scholar] [CrossRef]



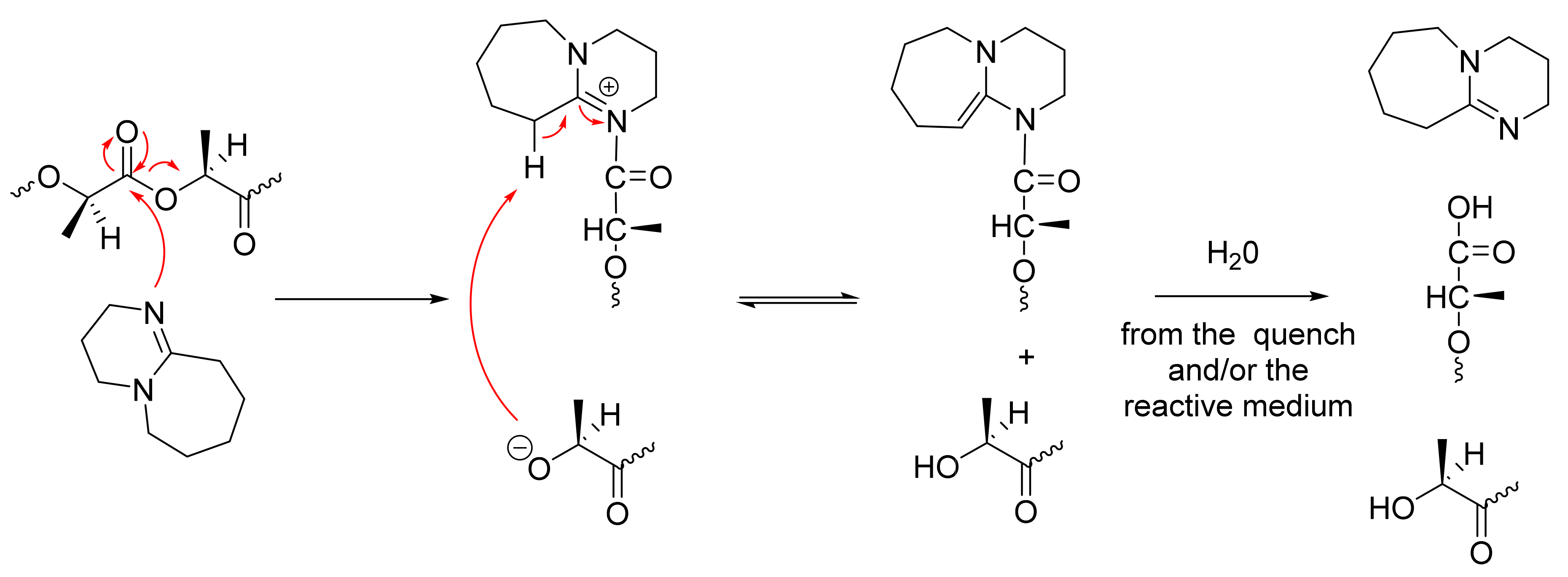
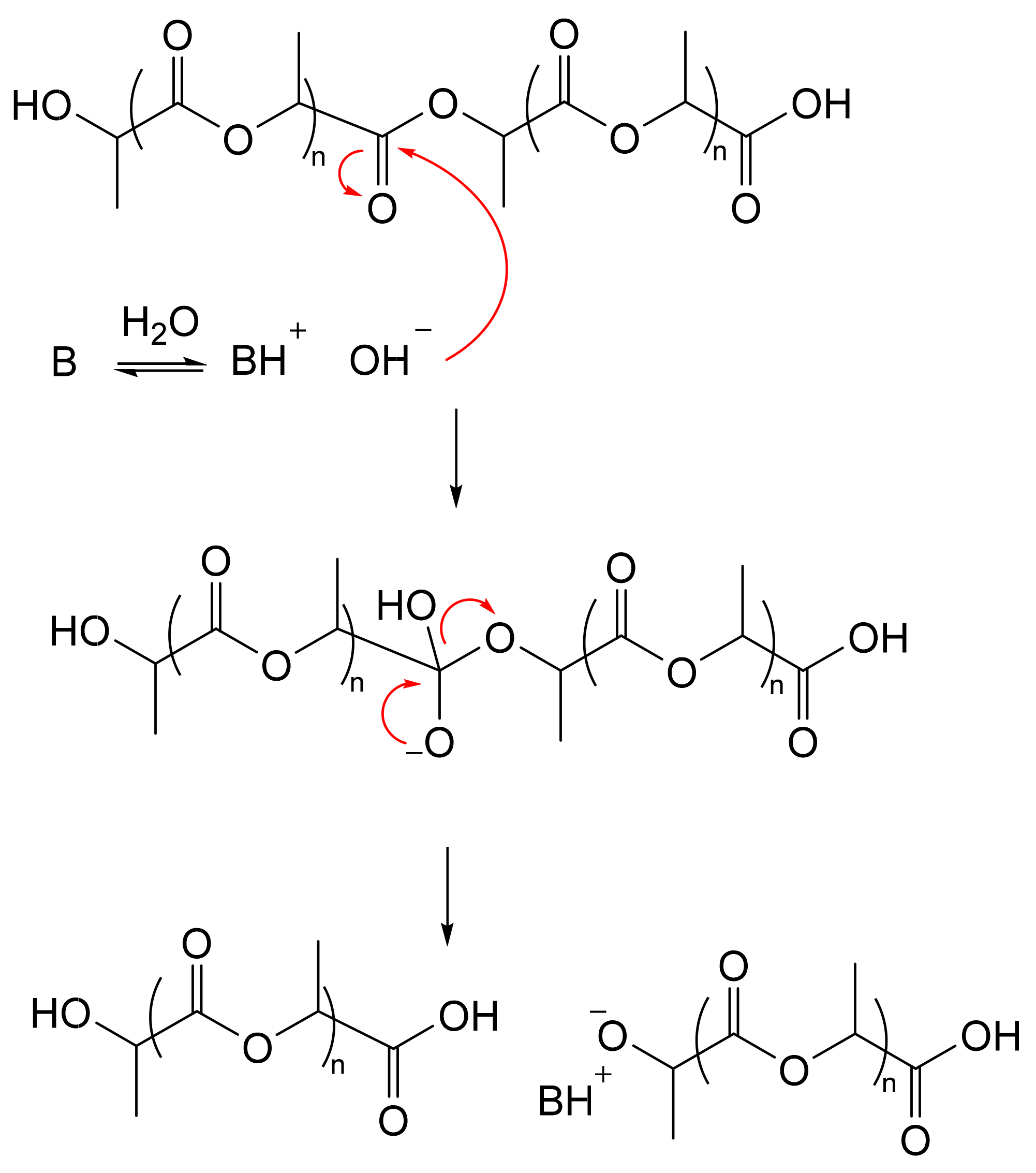
| Entry | Catalyst | pKaH b | Epimerization D (%) d | Mn corrected (g/mol) e | ÐMe | |
|---|---|---|---|---|---|---|
| Starting PLLA | - | −162.3 | 0 | 35,400 | 1.7 | |
| 1 | No catalyst | - | −159.4 | 0.9 | 33,500 | 1.8 |
| 2 | NEt3 | 18.8 CH3CN [30] | −152.0 | 3.2 | 31,400 | 1.8 |
| 3 | DMAP | 17.9 CH3CN [30] 9.7 H2O [31] | −154.5 | 2.4 | 19,200 | 1.8 |
| 4 | DBU | 24.3 CH3CN [30] 11.9 H2O [31] | −43.5 | 36.6 | 5800 | 1.5 |
| 5 | TBD | 26.0 CH3CN [30] | −64.9 | 30 | 6000 | 1.6 |
| 6 | Quinine | 8.6 H2O [32] | −158.2 | 1.3 | 13,200 | 1.8 |
| 7 | Quinidine | 8.6 H2O [32] | −151.0 | 3.4 | 12,100 | 1.9 |
| 8 | Cinchonidine | 8.4 H2O [32] | −155.9 | 2.0 | 11,500 | 2.0 |
| 9 | Cinchonine | 8.4 H2O [32] | −151.2 | 3.4 | 12,000 | 1.9 |
| 10 | BEMP | 27.6 CH3CN [33] | −149.9 | 3.8 | 10,600 | 1.8 |
| 11 | t-BuP4 | 42.7 CH3CN [34] | −142.8 | 6.0 | 8300 | 2.1 |
| Entry | Amount of Catalyst (mol%) | Time (h) | Epimerization D (%) c | Mn NMR d (g/mol) | Mn corrected SEC (g/mol) e | ÐMe | |
|---|---|---|---|---|---|---|---|
| Starting PLLA | - | - | −153.3 | 0 | nd | 61,000 | 1.6 |
| 12 | 0 | 48 | −152.0 | 0.3 | nd | 61,000 | 1.6 |
| 13 | 0.25 | 48 | −148.1 | 1.6 | 16,800 | 16,000 | 1.7 |
| 14 | 0.65 | 6 | −138.6 | 4.7 | 15,600 | 17,000 | 1.9 |
| 15 | 0.65 | 48 | −128.5 | 8.0 | 10,000 | 10,100 | 1.8 |
| 16 | 1 | 6 | −137.7 | 5.0 | 13,000 | 12,000 | 1.8 |
| 17 | 1 | 48 | −127.8 | 8.3 | 5000 | 7200 | 1.6 |
| 18 | 3 | 1 | −142.0 | 3.7 | 13,800 | 12,000 | 2.1 |
| 19 | 3 f | 6 | −59.4 | 30.6 | 2400 | 3200 | 1.6 |
| 20 | 3 f | 48 | −27.2 | 41.1 | 900 | 1000 | 1.8 |
| Entry | Catalyst | mol % vs. PLA | Epimerization D (%) c | Mn corrected (g/mol) d | ÐMd | |
|---|---|---|---|---|---|---|
| 21 | TBD | 0.5 | −136.3 | 8.0 | 11,000 | 1.8 |
| 22 | DPP | 0.5 | −149.3 | 4.0 | 12,100 | 1.9 |
| 23 | TBD/DPP | 0.5/0.5 | −147.0 | 4.7 | 25,600 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meimoun, J.; Favrelle-Huret, A.; Winter, J.D.; Zinck, P. Poly(L-lactide) Epimerization and Chain Scission in the Presence of Organic Bases. Macromol 2022, 2, 236-246. https://doi.org/10.3390/macromol2020016
Meimoun J, Favrelle-Huret A, Winter JD, Zinck P. Poly(L-lactide) Epimerization and Chain Scission in the Presence of Organic Bases. Macromol. 2022; 2(2):236-246. https://doi.org/10.3390/macromol2020016
Chicago/Turabian StyleMeimoun, Julie, Audrey Favrelle-Huret, Julien De Winter, and Philippe Zinck. 2022. "Poly(L-lactide) Epimerization and Chain Scission in the Presence of Organic Bases" Macromol 2, no. 2: 236-246. https://doi.org/10.3390/macromol2020016
APA StyleMeimoun, J., Favrelle-Huret, A., Winter, J. D., & Zinck, P. (2022). Poly(L-lactide) Epimerization and Chain Scission in the Presence of Organic Bases. Macromol, 2(2), 236-246. https://doi.org/10.3390/macromol2020016







