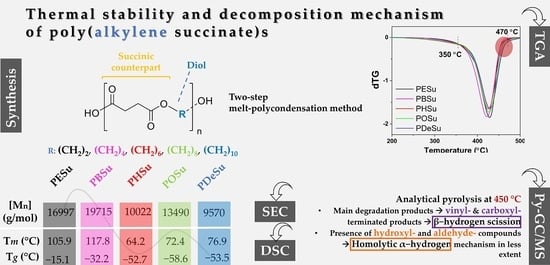
Thermal Stability and Decomposition Mechanism of Poly(alkylene succinate)s
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
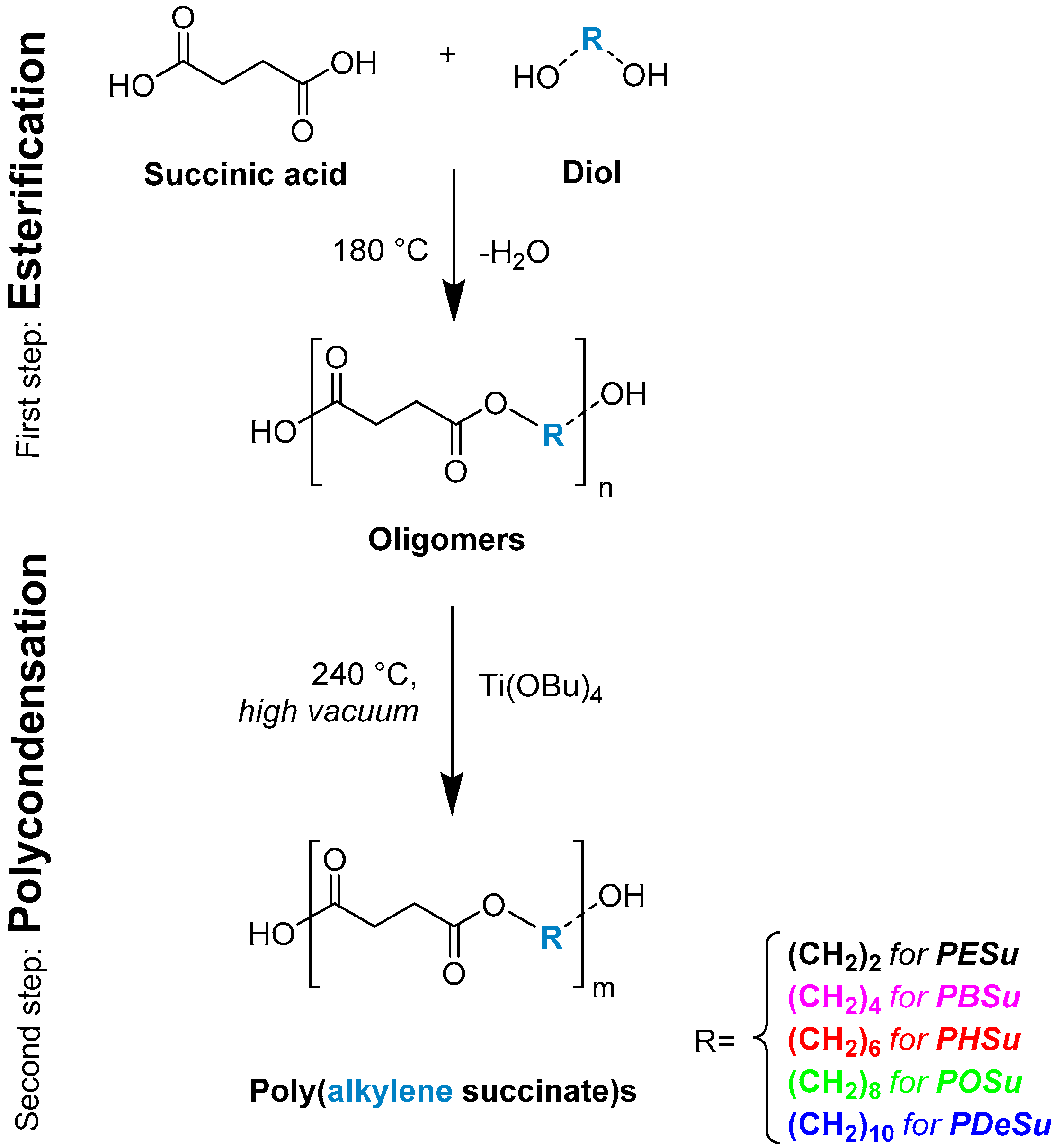
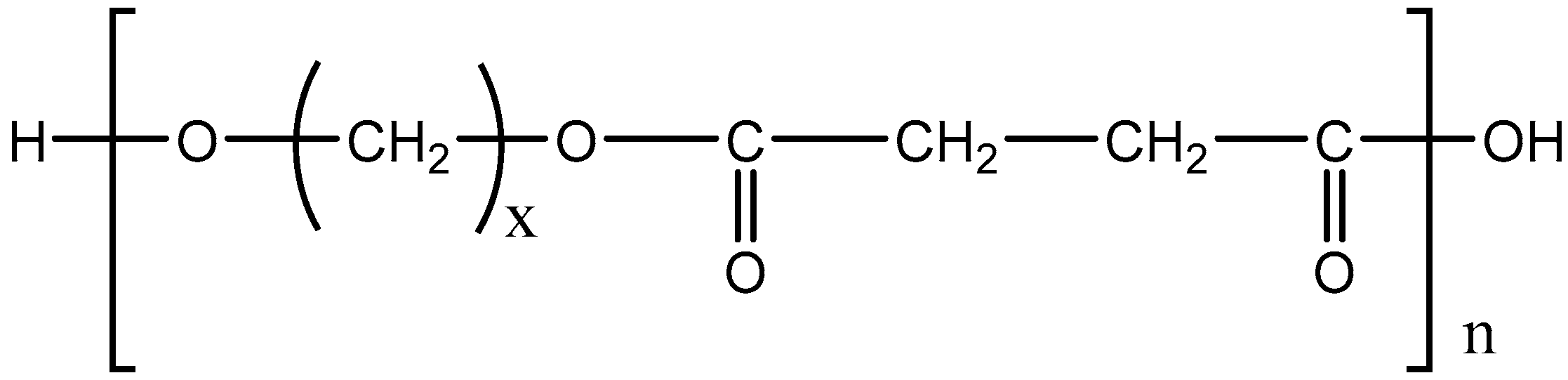
2.2. Synthesis of Poly(alkylene succinate) Polyesters
2.3. Polymer Characterization
2.3.1. Size Exclusion Chromatography (SEC)
2.3.2. Differential Scanning Calorimetry (DSC)
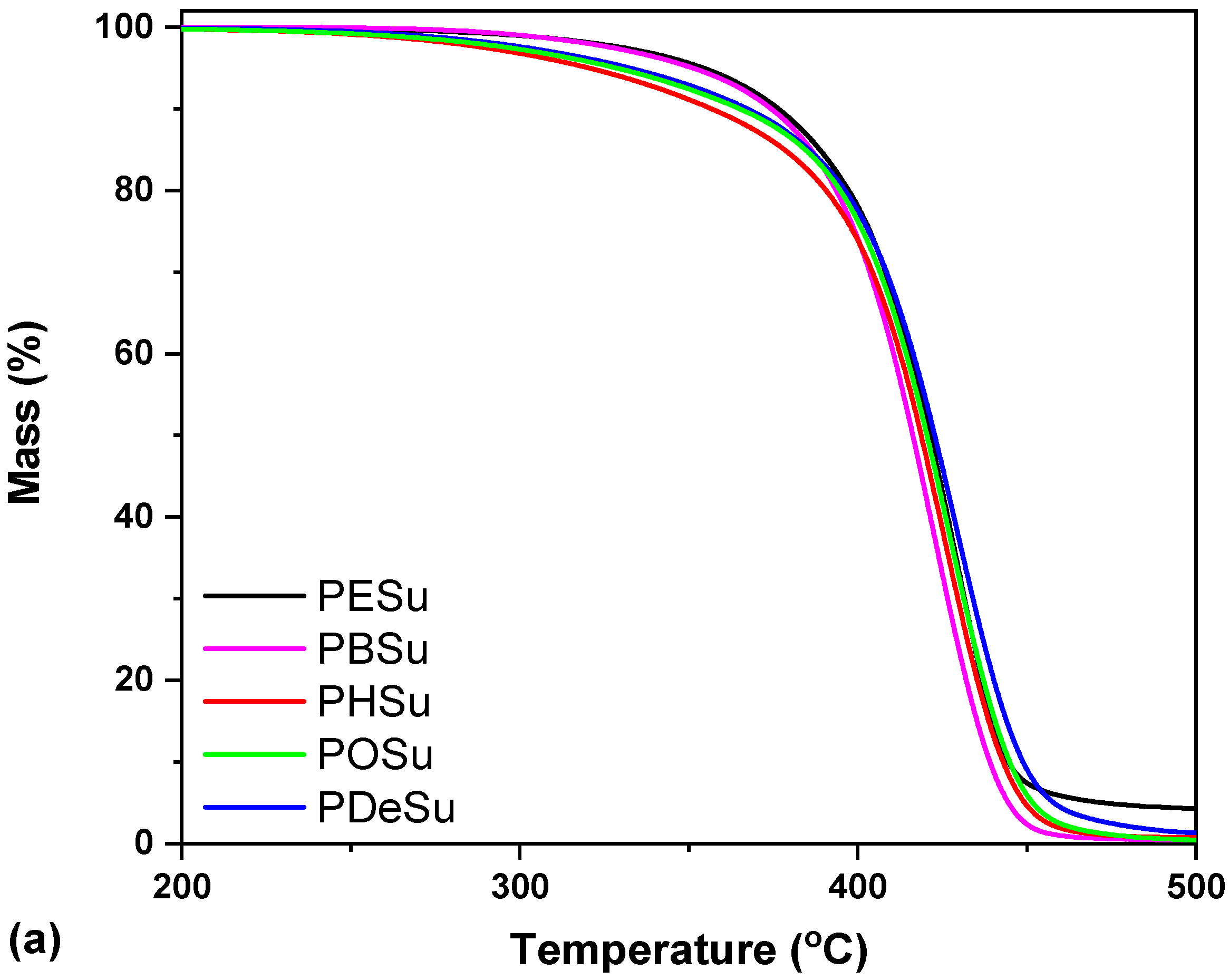
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py–GC/MS)
3. Results
3.1. Characterization of the Prepared Polyesters
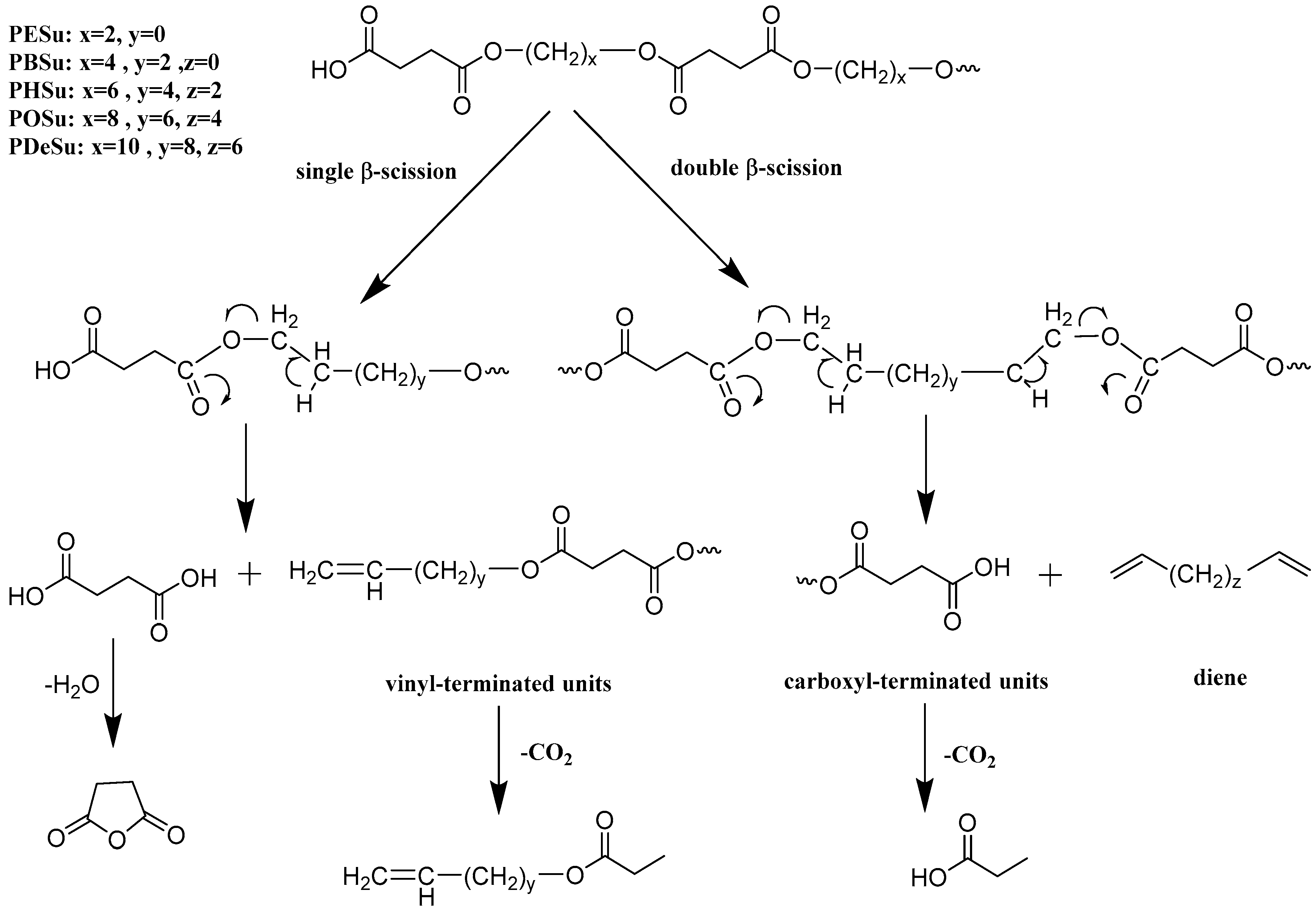
3.2. Evaluation of the Decomposition Mechanism Using Py–GC/MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales-Huerta, J.C.; de Ilarduya, A.M.; Muñoz-Guerra, S. Modulating the Tg of Poly(alkylene succinate)s by inserting bio-based aromatic units via ring-opening copolymerization. Polymers 2017, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic acid: A new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Ranucci, E.; Liu, Y.; Lindblad, M.S.; Albertsson, A.C. New biodegradable polymers from renewable sources. High molecular weight poly(ester carbonate)s from succinic acid and 1,3-propanediol. Macromol. Rapid Commun. 2000, 21, 680–684. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Zhang, S.; Zhang, W.; Yang, Q.; Dong, W.; Jiang, M.; Ma, J.; Xin, F. Bio-based succinic acid: An overview of strain development, substrate utilization, and downstream purification. Biofuels Bioprod. Biorefining 2020, 14, 965–985. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic acid: Technology development and commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Papadimitriou, S.A.; Karavas, E.; Avgoustakis, K. Novel biodegradable polyester poly(propylene succinate): Synthesis and application in the preparation of solid dispersions and nanoparticles of a water-soluble drug. AAPS Pharm. Sci. Tech. 2009, 10, 138–146. [Google Scholar] [CrossRef]
- Arandia, I.; Mugica, A.; Zubitur, M.; Arbe, A.; Liu, G.; Wang, D.; Mincheva, R.; Dubois, P.; Müller, A.J. How composition determines the properties of isodimorphic poly(butylene succinate- ran -butylene azelate) random biobased copolymers: From single to double crystalline random copolymers. Macromolecules 2015, 48, 43–57. [Google Scholar] [CrossRef]
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy Benjamin. Biofuels Bioprod. Biorefining 2013, 6, 246–256. [Google Scholar] [CrossRef]
- Sousa, A.F.; Silvestre, A.J.D. Plastics from renewable sources as green and sustainable alternatives. Curr. Opin. Green Sustain. Chem. 2022, 33, 100557. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Influence of chemical structure on physicochemical properties and thermal decomposition of the fully bio-based poly (propylene succinate-co-butylene succinate)s. Polym. Test. 2020, 83, 106337. [Google Scholar] [CrossRef]
- Abdelghafour, M.M.; Orbán, Á.; Deák, Á.; Lamch, Ł.; Frank, É.; Nagy, R.; Ádám, A.; Sipos, P.; Farkas, E.; Bari, F.; et al. The effect of molecular weight on the solubility properties of biocompatible poly(Ethylene succinate) polyester. Polymers 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, M.S.; Jeon, H.; Koo, J.M.; Eom, Y.; Choi, S.; Shin, G.; Oh, D.X.; Hwang, S.Y.; Park, J. Highly reinforced poly(butylene succinate) nanocomposites prepared from chitosan nanowhiskers by in-situ polymerization. Int. J. Biol. Macromol. 2021, 173, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Puiggalí, J.; Díaz, A.; Katsarava, R. Bio-Based Aliphatic Polyesters from Dicarboxylic Acids and Related Sugar and Amino Acid Derivatives; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081009703. [Google Scholar]
- Hwang, S.Y.; Yoo, E.S.; Im, S.S. The synthesis of copolymers, blends and composites based on poly(butylene succinate). Polym. J. 2012, 44, 1179–1190. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers II. Biodegradation of different polymer groups. TrAC Trends Anal. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-based polyesters for biomedical applications: A review in memory of our beloved colleague and friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Kurokawa, H.; Doi, Y. Solid-state microstructures, thermal properties, and crystallization of biodegradable poly(butylene succinate) (PBS) and its copolyesters. Biomacromolecules 2001, 2, 605–613. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of molecular weight on thermal degradation mechanism of the biodegradable polyester poly(ethylene succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable poly(ethylene succinate) (PES). 1. Crystal growth kinetics and morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation mechanism of poly (ethylene succinate) and poly (butylene succinate): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Y.; Ma, H.; Jia, C.; Sun, X.; Yang, Y.; Liu, J.; Yang, J. Linear Diamides Derivative-Nucleated Biodegradable Poly(ethylene succinate) Polyester: Crystallization Kinetics and Aggregated Structure Manipulated by Hydrogen Bond Interaction. J. Polym. Environ. 2021, 29, 3605–3617. [Google Scholar] [CrossRef]
- Lu, S.F.; Chen, M.; Chen, C.H. Mechanisms and kinetics of thermal degradation of poly(butylene succinate-co-propylene succinate)s. J. Appl. Polym. Sci. 2012, 123, 3610–3619. [Google Scholar] [CrossRef]
- Parcheta, P.; Koltsov, I.; Datta, J. Fully bio-based poly (propylene succinate) synthesis and investigation of thermal degradation kinetics with released gases analysis. Polym. Degrad. Stab. 2018, 151, 90–99. [Google Scholar] [CrossRef]
- Liu, Y.; Ranucci, E.; Lindblad, M.S.; Albertsson, A.C. New biodegradable polymers from renewable sources: Polyester-carbonates based on 1,3-propylene-co-1,4-cyclohexanedimethylene succinate. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2508–2519. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Thermo-oxidative processes in biodegradable poly(butylene succinate). Polym. Degrad. Stab. 2009, 94, 1825–1838. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-based poly (butylene succinate): Recent progress, challenges and future opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Righetti, M.C.; Di Lorenzo, M.L.; Cinelli, P.; Gazzano, M. Temperature dependence of the rigid amorphous fraction of poly(butylene succinate). RSC Adv. 2021, 11, 25731–25737. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Z.; Sun, Y.; Zhu, Z.; Xie, Z.; Ma, H.; Yin, J.; Wang, J.; Yang, J. Biocompatible linear diamides derivative-nucleated biodegradable poly(ethylene succinate): Tailored crystallization kinetics, aggregated structure and thermal degradation. Polym. Degrad. Stab. 2021, 183, 109428. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Isothermal melt crystallization kinetics study of cellulose nanocrystals nucleated biodegradable Poly(ethylene succinate). Polymer 2021, 227, 123869. [Google Scholar] [CrossRef]
- Jia, C.; Zhou, S.; Xie, Z.; Wang, L.; Yang, Y.; Sun, X.; Xie, Y.; Yang, J. Crystallization kinetics, aggregated structure and thermal stability of biodegradable poly(ethylene succinate) manipulated by a biocompatible layered metal phosphonate as an efficient nucleator. Polym. Int. 2021, 70, 1264–1272. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Chrissafis, K.; Paraskevopoulos, K.M.; Triantafyllidis, K.S.; Antonakou, E.V. Investigation of thermal degradation mechanism of an aliphatic polyester using pyrolysis-gas chromatography-mass spectrometry and a kinetic study of the effect of the amount of polymerisation catalyst. Polym. Degrad. Stab. 2007, 92, 525–536. [Google Scholar] [CrossRef]
- Bikiaris, R.; Christodoulou, E.; Kostoglou, M.; Kasimatis, M.; Iatrou, H.; Nikolaidis, N. Paliperidone palmitate depot microspheres based on biocompatible poly (alkylene succinate) polyesters as long-acting injectable formulations. J. Drug Deliv. Sci. Technol. 2022, 68, 103056. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Kasimatis, M.; Iatrou, H.; Kyritsis, A.; Bikiaris, D.N. Synthesis, Crystallization, Structure Memory Effects, and Molecular Dynamics of Biobased and Renewable Poly(n-alkylene succinate)s with n from 2 to 10. Macromolecules 2021, 54, 1106–1119. [Google Scholar] [CrossRef]
- Chrysafi, I.; Ainali, N.M.; Bikiaris, D.N. Thermal degradation mechanism and decomposition kinetic studies of poly(Lactic acid) and its copolymers with poly(hexylene succinate). Polymers 2021, 13, 1365. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Roumeli, E.; Chrissafis, K.; Lioutas, C.; Triantafyllidis, K.; Bikiaris, D.; Boccaccini, A.R. Thermal degradation kinetics and decomposition mechanism of PBSu nanocomposites with silica-nanotubes and strontium hydroxyapatite nanorods. Phys. Chem. Chem. Phys. 2014, 16, 4830–4842. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Li, B.G. High Molecular Weight Polyesters Derived from Biobased 1,5-Pentanediol and a Variety of Aliphatic Diacids: Synthesis, Characterization, and Thermo-Mechanical Properties. ACS Sustain. Chem. Eng. 2017, 5, 6159–6166. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review on thermal decomposition and combustion of thermoplastic polyesters. Polym. Adv. Technol. 2004, 15, 691–700. [Google Scholar] [CrossRef]
- Vijayakumar, C.T.; Ponnusamy, E.; Balakrishnan, T.; Kothandaraman, H. Thermal and Pyrolysis Studies of Copolyesters. J. Polym. Sci. A1 1982, 20, 2715–2725. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.G. Alternative optimization routes for improving the performance of poly(3-hydroxybutyrate) (PHB) based plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Zhang, Y.; Enomoto, Y.; Iwata, T. Synthesis of homo- and copolyesters containing divanillic acid, 1,4-cyclohexanedimethanol, and alkanediols and their thermal and mechanical properties. Polym. Degrad. Stab. 2021, 192, 109706. [Google Scholar] [CrossRef]
- Vuorinen, E.; Hakkarainen, M. Method development for the analysis of biodegradable polymers. Int. J. Metrol. Qual. Eng. 2010, 1, 29–32. [Google Scholar] [CrossRef][Green Version]
- Sin, M.C.; Gan, S.N.; Annuar, M.S.M.; Tan, I.K.P. Thermodegradation of medium-chain-length poly(3-hydroxyalkanoates) produced by Pseudomonas putida from oleic acid. Polym. Degrad. Stab. 2010, 95, 2334–2342. [Google Scholar] [CrossRef]
- Terakado, O.; Ueda, M.; Hirasawa, M. Thermal degradation of polyester-metal oxide mixtures: Fibrous morphology of carbonaceous compounds and pyrolysis products distribution. J. Anal. Appl. Pyrolysis 2010, 89, 183–190. [Google Scholar] [CrossRef]
- Qiu, T.; Ge, F.; Li, C.; Lu, S. Study of the thermal degradation of flame-retardant polyester GFRP using TGA and TG-FTIR-GC/MS. J. Therm. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Liu, W.; Wang, N.; Han, J.; Xu, J.; Li, Z.; Qin, W. Thermal degradation behaviors and evolved products analysis of polyester paint and waste enameled wires during pyrolysis. Waste Manag. 2020, 107, 82–90. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Rapisarda, M.; Perna, S.; Mirabella, E.F.; La Carta, S.; Puglisi, C.; Valenti, G. Determination of polyethylene in biodegradable polymer blends and in compostable carrier bags by Py-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2016, 117, 72–81. [Google Scholar] [CrossRef]
- Kandare, E.; Kandola, B.K.; Price, D.; Nazaré, S.; Horrocks, R.A. Study of the thermal decomposition of flame-retarded unsaturated polyester resins by thermogravimetric analysis and Py-GC/MS. Polym. Degrad. Stab. 2008, 93, 1996–2006. [Google Scholar] [CrossRef]
- Guo, W.; Leu, W.T.; Hsiao, S.H.; Liou, G.S. Thermal degradation behaviour of aromatic poly(ester-amide) with pendant phosphorus groups investigated by pyrolysis-GC/MS. Polym. Degrad. Stab. 2006, 91, 21–30. [Google Scholar] [CrossRef]




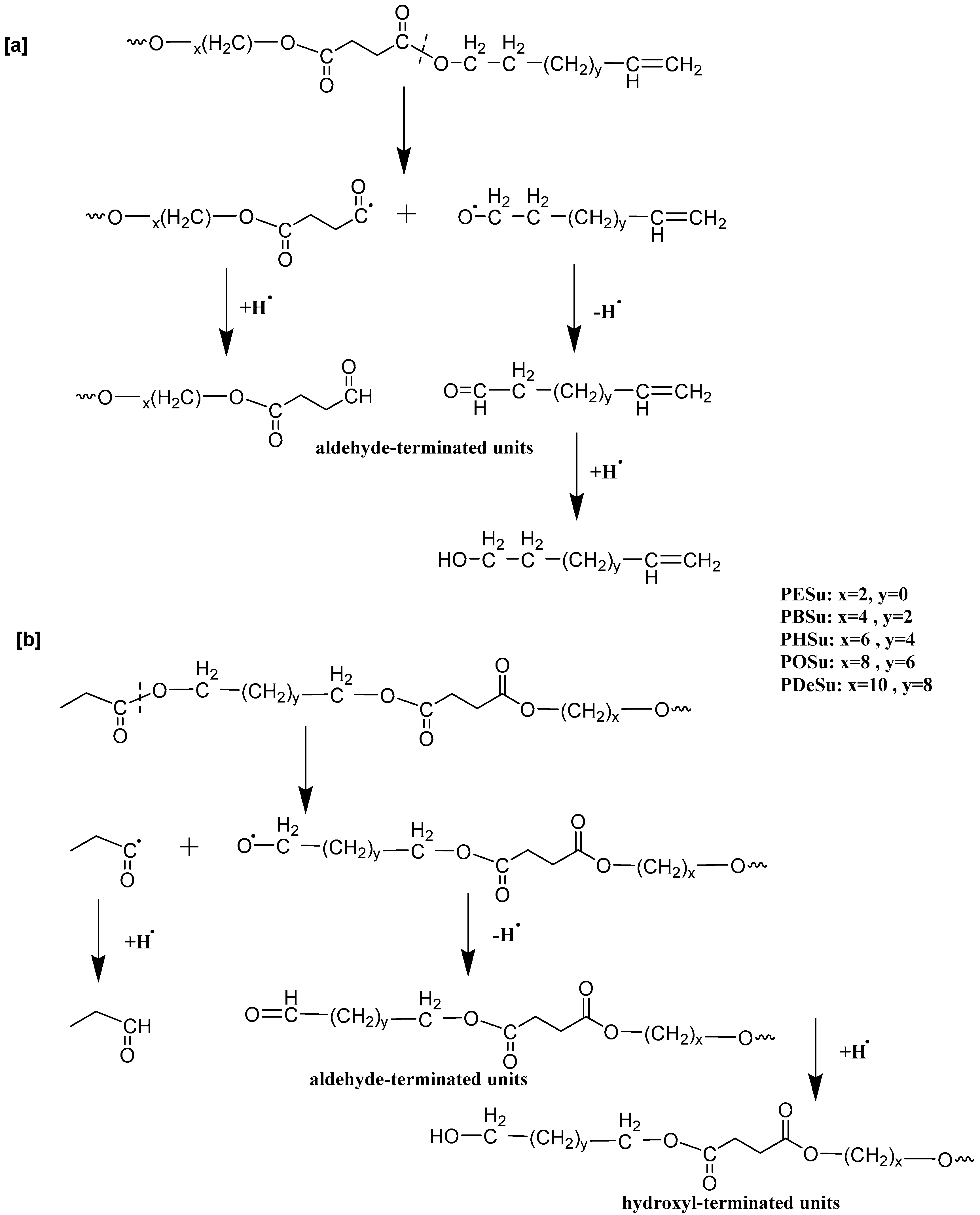
| Weight (g) | ||||
|---|---|---|---|---|
| Succinic Acid | Used Diol | Ti(OBu)4 | ||
| Sample name | PESu | 24.6 | 13.6 (1,2-ethanediol) | 0.02 |
| PBSu | 20.5 | 17.2 (1,4-Butanediol) | 0.017 | |
| PHSu | 16.5 | 18.2 (1,6-hexanediol) | 0.015 | |
| POSu | 15.4 | 21.05 (1,8-octanediol) | 0.013 | |
| PDeSu | 13.81 | 22.46 (1,10-decanediol) | 0.012 | |
| Samples | [Mn] (g/mol) | PDI | Tm (°C) | Tg (°C) |
|---|---|---|---|---|
| PESu | 16,997 | 1.95 | 105.9 | −15.1 |
| PBSu | 19,715 | 1.54 | 117.8 | −32.2 |
| PHSu | 10,022 | 1.92 | 64.2 | −52.7 |
| POSu | 13,490 | 1.73 | 72.4 | −58.6 |
| PDeSu | 9570 | 1.94 | 76.9 | −53.5 |
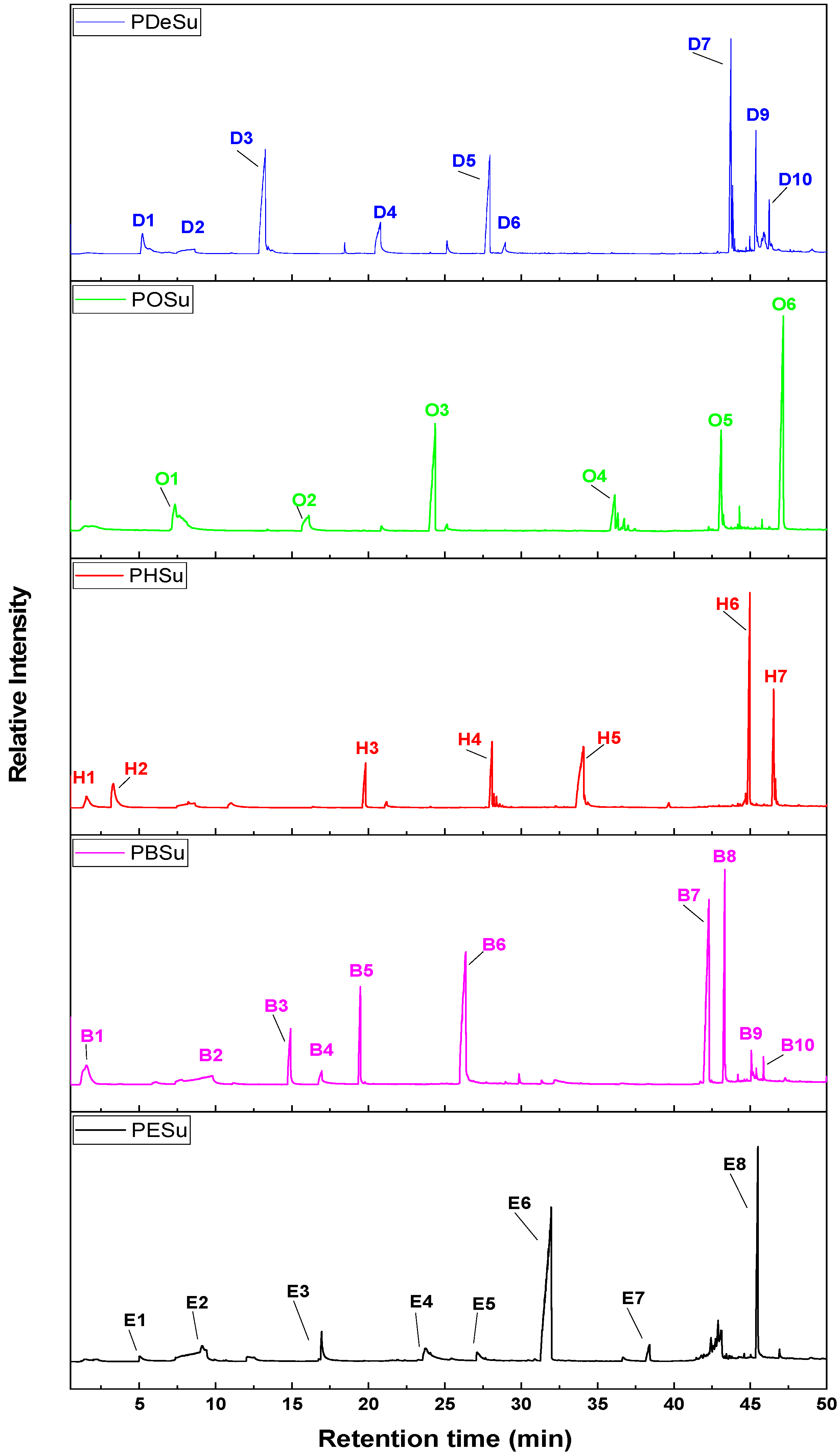
| Peak Name | Retention Time (min) | Peak Area (%) | m/z (amu) | Assigned Compound |
|---|---|---|---|---|
| E1 | 5.09 | 6 | 45, 57, 75, 88, 100 | Propanoic acid, 2-hydroxyethyl ester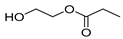 |
| E2 | 9.05 | 8 | 45, 56, 84, 100, 118 | Succinic anhydride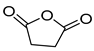 |
| E3 | 16.95 | 14 | 45, 55, 73, 101, 114, 129, 145, 172 | Butanedioic acid, diethyl ester |
| E4 | 23.65 | 11 | 45, 55, 73, 101, 114, 127, 145, 158, 176, 189 | 2-hydroxyethyl vinyl succinate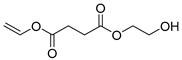 |
| E5 | 27.24 | 6 | 45, 57, 73, 101, 114, 127, 145, 158, 171, 201 | Allyl (2-hydroxyethyl) succinate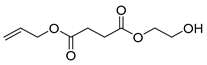 |
| E6 | 31.86 | 72 | 45, 55, 73, 84, 99, 114, 126, 145, 158, 189, 258 | 2-(acryloyloxy)ethyl (2-hydroxyethyl) succinate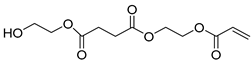 |
| E7 | 38.33 | 10 | 45, 55, 70, 84, 99, 114, 126, 145, 158, 189, 270, 289 | 2-hydroxyethyl (2-((4 oxobutanoyl)oxy)ethyl) succinate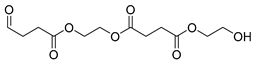 |
| E8 | 45.45 | 100 | 45, 55, 73, 84, 99, 114, 126, 145, 158, 189, 243, 270, 289, 303, 333 | 2-((4-(2-hydroxyethoxy)-4-oxobutanoyl)oxy)ethyl vinyl succinate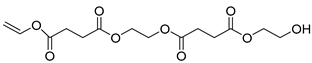 |
| Peak Name | Retention Time (min) | Peak Area (%) | m/z (amu) | Assigned Compound |
|---|---|---|---|---|
| Β1 | 1.50 | 9 | 45, 54, 72 | 2-Propenoic acid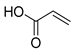 |
| B2 | 9.46 | 5 | 45, 55, 74, 100 | Succinic anhydride |
| B3 | 14.92 | 26 | 45, 54, 73, 87, 101, 114, 131, 144 | 4-(but-3-en-1-yloxy)-4-oxobutanoic acid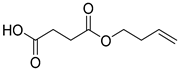 |
| B4 | 16.91 | 8 | 45, 54, 73, 83, 101, 131, 164 | 1,6-dioxecane-2,5-dione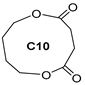 |
| B5 | 19.48 | 46 | 45, 55, 73, 80, 101, 108, 125, 155 | Di(but-3-en-1-yl) succinate |
| B6 | 26.36 | 62 | 45, 55, 73, 89, 101, 119, 155, 213, 244 | But-3-en-1-yl (4-hydroxybutyl) succinate |
| B7 | 42.27 | 81 | 45, 55, 71, 87, 101, 114, 127, 154, 173, 226, 254, 273, 288 | 4,4′-(butane-1,4-diylbis(oxy))bis(4-oxobutanoic acid)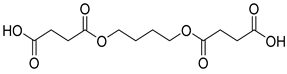 |
| B8 | 43.31 | 100 | 45, 55, 73, 80, 101, 108, 119, 155, 173, 228 | 4-(acryloyloxy)butyl 4-oxobutanoate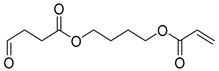 |
| B9 | 45.08 | 15 | 45, 55, 73, 80, 101, 108, 119, 155, 173, 227, 273, 327 | But-3-en-1-yl (4-((4-oxobutanoyl)oxy)butyl) succinate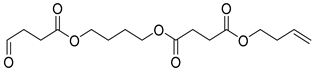 |
| B10 | 45.86 | 13 | 45, 55, 73, 80, 101, 108, 119, 155, 173, 227, 273, 345 | 4-(4-((4-(but-3-en-1-yloxy)-4-oxobutanoyl)oxy)butoxy)-4-oxobutanoic acid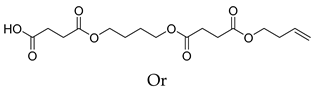 Or 1,6,11,16-Tetraoxacycloicosane-2,5,12,15-tetraone 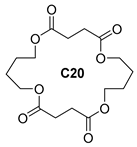 |
| Peak Name | Retention Time (min) | Peak Area (%) | m/z (amu) | Assigned Compound |
|---|---|---|---|---|
| H1 | 1.54 | 7 | 54, 67, 79 | 3-Methylcyclopentene |
| H2 | 3.29 | 12 | 54, 67, 82 | 1,5-hexadiene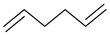 Cyclohexene Or 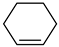 |
| H3 | 19.81 | 21 | 45, 54, 67, 82, 101, 131, 144, 154, 170 | 6-hydroxyhexyl acrylate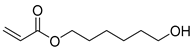 |
| H4 | 28.07 | 31 | 45, 55, 67, 82, 101, 119, 154, 183, 201, 242 | 4-(hex-5-en-1-yloxy)-4-oxobutanoic acid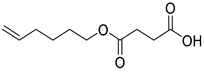 |
| H5 | 34.06 | 28 | 45, 55, 67, 83, 101, 119, 154, 183, 201, 219 | 4-((6-hydroxyhexyl)oxy)-4-oxobutanoic acid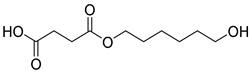 |
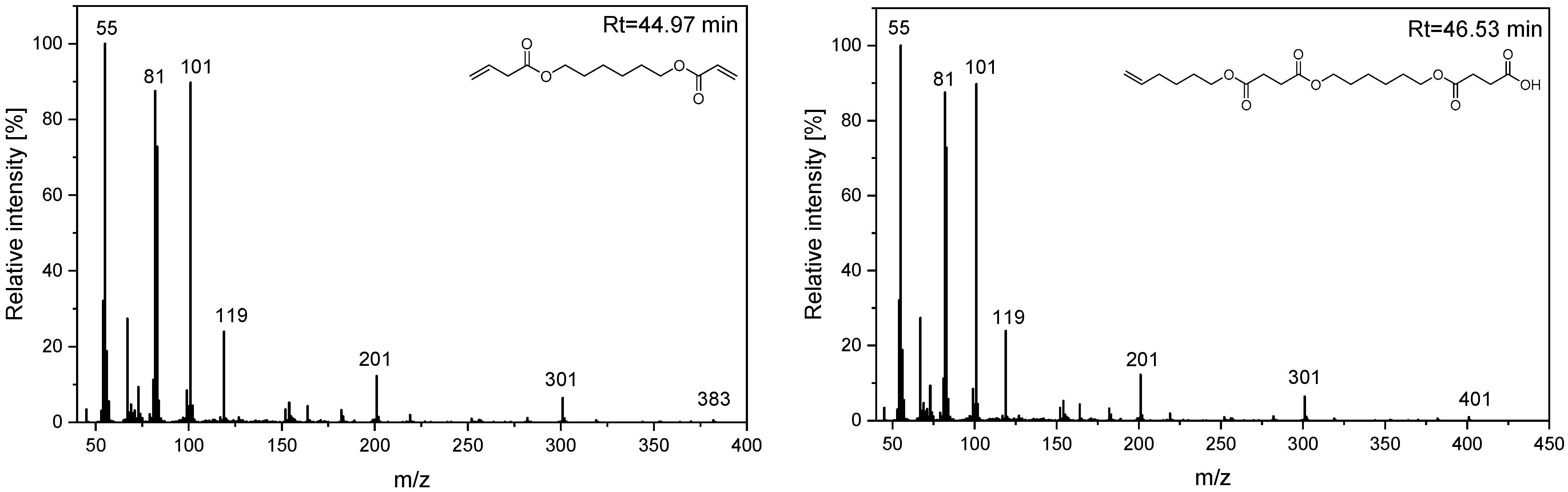
| H6 | 44.97 | 100 | 45, 55, 67, 82, 101, 119, 154, 182, 201, 301, 382 | Hex-5-en-1-yl (6-((4-oxobutanoyl)oxy)hexyl) succinate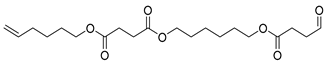 |
| H7 | 46.53 | 55 | 45, 55, 67, 82, 101, 119, 154, 182, 201, 301, 383, 401 | 4-((6-((4-(hex-5-en-1-yloxy)-4-oxobutanoyl)oxy)hexyl)oxy)-4-oxobutanoic acid |
| Peak Name | Retention Time (min) | Peak Area (%) | m/z (amu) | Assigned Compound |
|---|---|---|---|---|
| O1 | 7.34 | 13 | 55, 67, 81, 95, 110 | Oct-7-en-1-ol 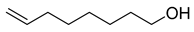 |
| O2 | 16.02 | 8 | 55, 67, 81, 95, 110, 116, 129 | Octane-1,8-diol |
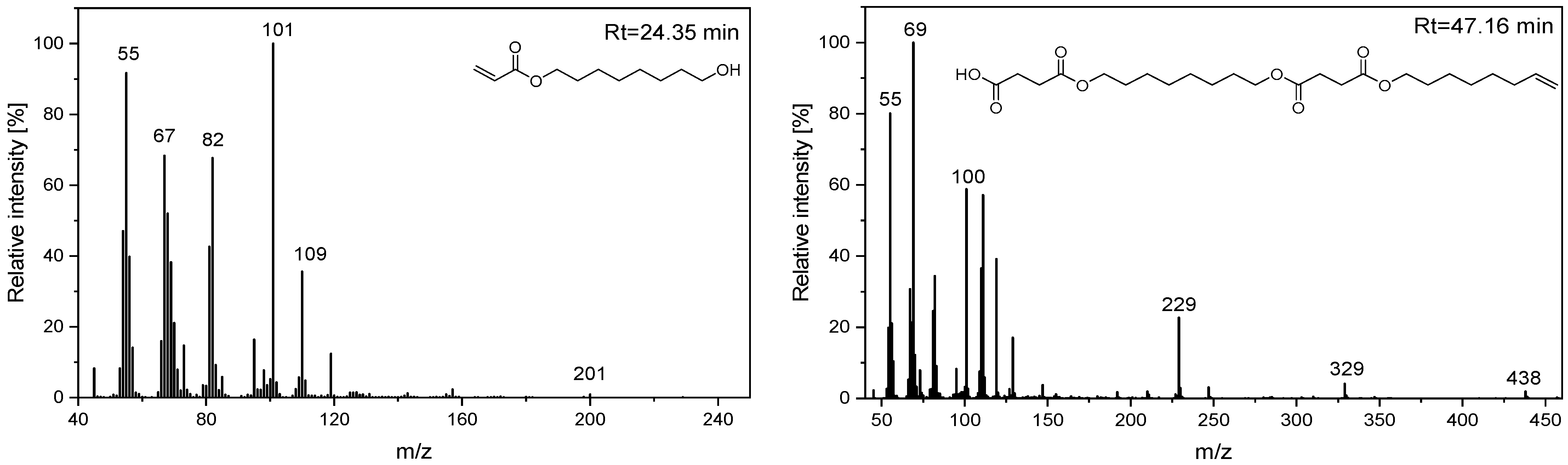
| O3 | 24.35 | 50 | 55, 67, 81, 95, 101, 110, 119, 143, 157, 172, 180, 200 | 8-hydroxyoctyl acrylate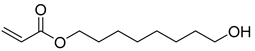 |
| O4 | 36.13 | 17 | 55, 69, 81, 101, 111, 119, 135, 149, 169, 211, 229 | 8-hydroxyoctyl 4-oxobutanoate |
| O5 | 43.09 | 47 | 55, 69, 81, 101, 111, 119, 135, 149, 169, 211, 247 | 4-((8-hydroxyoctyl)oxy)-4-oxobutanoic acid |
| O6 | 47.16 | 100 | 55, 69, 82, 101, 111, 119, 147, 192, 229, 247, 329, 438, 457 | 4-((8-((4-(oct-7-en-1-yloxy)-4-oxobutanoyl)oxy)octyl)oxy)-4-oxobutanoic acid |
| Peak Name | Retention Time (min) | Peak Area (%) | m/z (amu) | Assigned Compound |
|---|---|---|---|---|
| D1 | 5.22 | 10 | 55, 67, 81, 95, 110, 123, 138 | 1,9-decadiene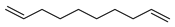 |
| D2 | 8.63 | 4 | 45, 56, 67, 100 | Succinic anhydride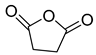 |
| D3 | 13.24 | 49 | 55, 67, 81, 95, 109, 123, 138, 156 | 9-decen-1-ol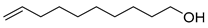 |
| D4 | 20.79 | 15 | 55, 67, 82, 95, 109, 126, 174 | 1,10-decanediol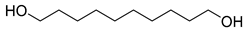 |
| D5 | 27.93 | 46 | 55, 68, 82, 101, 110, 119, 138, 171, 183, 228 | 10-hydroxydecyl acrylate |
| D6 | 28.93 | 5 | 55, 67, 82, 101, 109, 119, 138, 256 | 4-(dec-9-en-1-yloxy)-4-oxobutanoic acid |
| D7 | 43.73 | 100 | 55, 69, 83, 101, 110, 119, 138, 157, 165, 239, 257, 374 | 4,4′-(decane-1,10-diylbis(oxy))bis(4-oxobutanoic acid)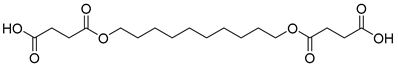 |
| D8 | 43.85 | 32 | 55, 68, 83, 101, 119, 138, 157, 239, 257, 394 | Di(dec-9-en-1-yl) succinate |
| D9 | 45.37 | 57 | 55, 69, 83, 101, 119, 137, 155, 173, 207, 239, 257, 276 | 4-((10-hydroxydecyl)oxy)-4-oxobutanoic acid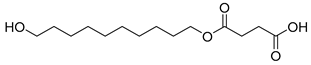 |
| D10 | 46.23 | 25 | 55, 69, 83, 101, 119, 137, 157, 213, 239, 257, 313, 331, 357 | 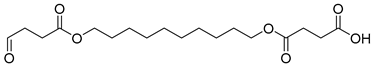 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikiaris, R.D.; Ainali, N.M.; Christodoulou, E.; Nikolaidis, N.; Lambropoulou, D.A.; Papageorgiou, G.Z. Thermal Stability and Decomposition Mechanism of Poly(alkylene succinate)s. Macromol 2022, 2, 58-77. https://doi.org/10.3390/macromol2010004
Bikiaris RD, Ainali NM, Christodoulou E, Nikolaidis N, Lambropoulou DA, Papageorgiou GZ. Thermal Stability and Decomposition Mechanism of Poly(alkylene succinate)s. Macromol. 2022; 2(1):58-77. https://doi.org/10.3390/macromol2010004
Chicago/Turabian StyleBikiaris, Rizos D., Nina Maria Ainali, Evi Christodoulou, Nikolaos Nikolaidis, Dimitra A. Lambropoulou, and George Z. Papageorgiou. 2022. "Thermal Stability and Decomposition Mechanism of Poly(alkylene succinate)s" Macromol 2, no. 1: 58-77. https://doi.org/10.3390/macromol2010004
APA StyleBikiaris, R. D., Ainali, N. M., Christodoulou, E., Nikolaidis, N., Lambropoulou, D. A., & Papageorgiou, G. Z. (2022). Thermal Stability and Decomposition Mechanism of Poly(alkylene succinate)s. Macromol, 2(1), 58-77. https://doi.org/10.3390/macromol2010004