Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate)
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
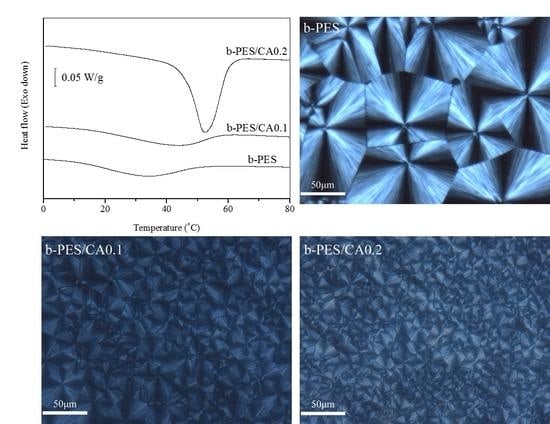
3.1. Influence of CA on the Nonisothermal and Isothermal Melt Crystallization Behaviors of b-PES
3.2. Spherulitic Morphology and Crystal Structure Studies of Neat and Nucleated b-PES
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujimaki, T. Processability and Properties of Aliphatic Polyesters, ‘BIONOLLE’, Synthesized by Polycondensation Reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Ueda, A.; Chatani, Y.; Tadokoro, H. Structure Studies of Polyesters. IV. Molecular and Crystal Structure of Poly(ethylene succinate) and Poly(ethylene oxalate). Polym. J. 1971, 2, 387–397. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable Poly(ethylene succinate) (PES). 1. Crystal Growth Kinetics and Morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable Poly(ethylene succinate) (PES). 2. Crystal Morphology of Melt-crystallized Ultrathin film and Its Change after Enzymatic Degradation. Biomacromolecules 2000, 1, 713–720. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Crystallization Behavior of Biodegradable Poly(ethylene succinate) from the Amorphous State. Polymer 2003, 44, 5429–5437. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinnami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Nonisothermal Crystallization Kinetics of Poly(butylene succinate) and Poly(ethylene succinate). Polym. J. 2004, 36, 642–646. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D.; Achilias, D. Effect of Molecular Weight on the Cold-crystallization of Biodegradable Poly(ethylene succinate). Thermochim. Acta 2007, 457, 41–54. [Google Scholar] [CrossRef]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. DSC and TMDSC study of Melting Behavior of Poly(butylene succinate) and Poly(ethylene succinate). Polymer 2003, 44, 7781–7785. [Google Scholar] [CrossRef]
- Iwata, T.; Doi, Y.; Isono, K.; Yoshida, Y. Morphology and Enzymatic Degradation of Solution-grown Single Crystals of Poly(ethylene succinate). Macromolecules 2001, 34, 7343–7348. [Google Scholar] [CrossRef]
- Tezuka, Y.; Ishii, N.; Kasuya, K.; Mitomo, H. Degradation of Poly(ethylene succinate) by Mesophilic Bacteria. Polym. Degrad. Stab. 2004, 84, 115–121. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D. Synthesis and Properties of Novel Biodegradable/Biocompatible Poly[propyleneco-(ethylene succinate)] Random Copolyesters. Macromol. Chem. Phys. 2009, 210, 1408–1421. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z. Crystallization and Melting Behavior of Biodegradable Poly(ethylene succinate-co-6mol% butylene succinate). J. Appl. Polym. Sci. 2011, 122, 105–111. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Crystallization Kinetics, Morphology, and Mechanical Properties of Novel Poly(ethylene succinate-co-octamethylene succinate). Polym. Test. 2015, 48, 125–132. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Synthesis and Properties of Novel Poly(ethylene succinate-co-decamethylene succinate) Copolymers. RSC Adv. 2015, 5, 103713–103721. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, Z. Synthesis, Crystallization Kinetics and Morphology of Novel Poly(ethylene succinate-co-ethylene adipate) Copolymers. CrystEngComm 2012, 14, 3586–3595. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Crystallization Kinetics, Morphology, and Mechanical Properties of Novel Biodegradable Poly(ethylene succinate-co-ethylene suberate) Copolyesters. Ind. Eng. Chem. Res. 2016, 55, 10286–10293. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Isothermal and Nonisothermal Crystallization Kinetics of Novel Biobased Poly(ethylene succinate-co-ethylene sebacate) Copolymers from the Amorphous State. J. Therm. Ana. Calorim. 2017, 129, 801–808. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, K.; Su, Z.; Qiu, Z. Thermal Behavior, Mechanical and Rheological Properties, and Hydrolytic Degradation of Novel Branched Biodegradable Poly(ethylene succinate) Copolymers. Polym. Test. 2018, 66, 64–69. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.; Jin, H.; Park, J.; Yoon, J. Biodegradability of Ethyl and n-octyl Branched Poly(ethylene adipate) and Poly(butylene succinate). Eur. Polym. J. 2001, 37, 1843–1847. [Google Scholar] [CrossRef]
- Kim, E.; Bae, J.; Im, S.; Kim, B.; Han, Y. Preparation and Properties of Branched Polybutylenesuccinate. J. Appl. Polym. Sci. 2001, 80, 1388–1394. [Google Scholar] [CrossRef]
- Jin, H.; Kim, D.; Kim, M.; Lee, I.; Lee, H.; Yoon, J. Synthesis and Properties of Poly(butylene succinate) with n-hexenyl Side Branches. J. Appl. Polym. Sci. 2001, 81, 2219–2226. [Google Scholar] [CrossRef]
- Chae, H.; Park, S.; Kim, B.; Kim, D. Effect of Methyl Substitution of the Ethylene Unit on the Physical Properties of Poly(butylene succinate). J. Polym. Sci. Polym. Phys. 2004, 42, 1759–1766. [Google Scholar] [CrossRef]
- Wang, G.; Gao, B.; Ye, H.; Xu, J.; Guo, B. Synthesis and Characterizations of Branched Poly(butylene succinate) Copolymers with 1,2-octanediol Segments. J. Appl. Polym. Sci. 2010, 117, 2538–2544. [Google Scholar] [CrossRef]
- Pan, P.; Shan, G.; Bao, Y.; Weng, Z. Crystallization Kinetics of Bacterial Poly(3-hydroxylbutyrate) Copolyesters with Cyanuric Acid as A Nucleating Agent. J. Appl. Polym. Sci. 2013, 129, 1374–1382. [Google Scholar] [CrossRef]
- Weng, M.; Qiu, Z. Effect of Cyanuric Acid on The Crystallization Kinetics and Morphology of Biodegradable Poly(L-lactide) as an Efficient Nucleating Agent. Thermochimi. Acta 2014, 577, 41–45. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Qin, S.; Liu, J.; Bi, C.; Liang, R.; Dong, T.; Feng, X. Effects of Cyanuric Acid on Crystallization Behavior, Polymorphism, and Phase Transition of Poly(butylene adipate). Ind. Eng. Chem. Res. 2015, 54, 8048. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, Z. Enhanced Crystallization Rate of Biodegradable Poly(ε-caprolactone) by Cyanuric Acid as an Efficient Nucleating Agent. Chin. J. Polym. Sci. 2017, 35, 1517–1523. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D. Crystallization and Melting Behavior of Three Biodegradable Poly(alkylene succinates). A Comparative Study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Verschoor, G.; Keulen, E. Electron Density Distribution in Cyanuric Acid. I. An X-ray Diffraction Study at Low Temperature. Acta Cryst. 1971, B27, 134–145. [Google Scholar] [CrossRef]







| Samples | b-PES | b-PES/CA0.1 | b-PES/CA0.2 |
|---|---|---|---|
| Tp (°C) | 33.4 | 43.1 | 52.3 |
| ΔHc (J/g) | 10.7 | 11.5 | 30.4 |
| Xc (%) | 6.0 | 6.4 | 16.9 |
| Tc (°C) | n | k (min−n) | t0.5 (min) | |
|---|---|---|---|---|
| Neat b-PES | 55 | 2.5 | 8.07 × 10−3 | 5.94 |
| 60 | 2.5 | 4.97 × 10−3 | 7.21 | |
| 65 | 2.6 | 1.33 × 10−3 | 11.08 | |
| 70 | 2.5 | 2.57 × 10−4 | 23.58 | |
| b-PES/CA0.1 | 55 | 1.9 | 2.92 × 10−1 | 1.58 |
| 60 | 2.0 | 1.61 × 10−1 | 2.08 | |
| 65 | 2.1 | 4.72 × 10−2 | 3.59 | |
| 70 | 1.9 | 2.15 × 10−2 | 6.22 | |
| b-PES/CA0.2 | 55 | 2.2 | 2.88 × 10−1 | 1.49 |
| 60 | 2.5 | 1.26 × 10−1 | 1.98 | |
| 65 | 2.1 | 5.62 × 10−2 | 3.31 | |
| 70 | 2.3 | 1.17 × 10−2 | 5.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Qiu, Z. Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate). Macromol 2021, 1, 112-120. https://doi.org/10.3390/macromol1020009
Zhang K, Qiu Z. Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate). Macromol. 2021; 1(2):112-120. https://doi.org/10.3390/macromol1020009
Chicago/Turabian StyleZhang, Kangjing, and Zhaobin Qiu. 2021. "Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate)" Macromol 1, no. 2: 112-120. https://doi.org/10.3390/macromol1020009
APA StyleZhang, K., & Qiu, Z. (2021). Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate). Macromol, 1(2), 112-120. https://doi.org/10.3390/macromol1020009