Abstract
Nickel-based ethylene polymerization catalysts have unique features, being able to produce macromolecules with a variable content of branches, resulting in polymers ranging from semicrystalline plastics to elastomers to hyperbranched amorphous waxes and oils. In addition to Brookhart’s α-diimine catalysts, iminopyridine Ni(II) complexes are among the most investigated systems. We report that Ni(II) complexes bearing aryliminopyridine ligands with bulky substituents both at the imino moiety and in the 6-position of pyridine afford either hyperbranched low molecular weight polyethylene oils or prevailingly linear crystalline polyethylenes or both, depending on the ligand structure and the reaction conditions. The formation of multiple active species in situ is suggested by analysis of the post-polymerization catalyst residues, showing the partial reduction of the imino function. Some related arylaminopyridine Ni(II) complexes were also synthesized and tested, showing a peculiar behavior, i.e., the number of branches of the produced polyethylenes increases while ethylene pressure increases.
1. Introduction
Following the breakthrough discoveries of Ni(II) α-diimine catalysts by Brookhart et al. [1,2], a huge number of studies from both academia and industry focused on the development of new late-transition metal olefin polymerization catalysts over the last two decades. Among the large variety of ligand structural variations, the arylimino-pyridyl moiety was one of the most investigated, after the independent successful studies of penta-coordinate 2,6-bis(arylimino)pyridyl Fe(II) and Co(II) complexes by the groups of Brookhart [3] and Gibson [4]. In addition to catalysts supported by such tridentate [N,N,N] ligands, a large number of complexes of bidentate [N,N] iminopyridine ligands with divalent metals like Fe(II), Co(II), and especially Ni(II) and Pd(II) have been synthesized and tested in the polymerization of ethylene [5,6] and in the copolymerization of ethylene with polar vinyl monomers, such as acrylates [7,8]. Simple modification of the steric and electronic features of the (imino)pyridine ligands and their usually easy preparation allowed a fine tuning of the polymerization/oligomerization performance of their metal complexes. E.g., limiting the discussion to Ni(II) complexes, early studies by Laine et al. [9] reported complexes bearing 2,6-alkyl-phenyl substituents on the imino moiety, producing nearly linear or moderately methyl branched low molecular weight polyethylenes (see Scheme 1A). Introduction of a methyl substituent in the 6-position of pyridine resulted in the production of a more linear polyethylene with slightly higher molecular weight and lower activity [10]. A related ligand modification introducing a 2,6-dialkylphenyl substituent in the 6-position of pyridine resulted in prevailing ethylene dimerization to 1-butene with minor amounts of C6, C8, and higher oligomers [11]. A large number of complexes with increasing steric bulk on the arylimino moiety of the ligand, e.g., o-benzhydryl, dibenzhydryl or dibenzocycloheptyl substituents but no substituents on the pyridine moiety, have been synthesized and tested by the group of Sun [12,13,14,15,16,17,18], resulting in thermally stable and highly active catalysts yielding low molecular weight moderately branched polyethylenes (Scheme 1B). Incorporation of very bulky 8-arylnaphtyl substituents on the imino moiety [19], blocking only one of the two coordination sites at the metal center, resulted in catalysts producing moderately branched polyethylenes with increased molecular weight (∼104 g mol−1) (Scheme 1C).
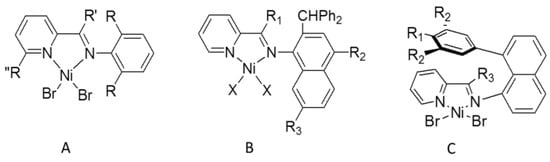
Scheme 1.
Some relevant previously reported iminopyridine Ni(II) catalysts.: (A) arylimino-pyridine complexes [9,10,11]; (B) benzhydryl-substituted arylimino-pyridine complexes [14,15,16], and (C) “half-sandwich” pyridine-imine complexes [19].
In a previous paper [20], we reported the production of hyperbranched low molecular weight polyethylene oils by the iminopyridine complex 1 (see Scheme 2) activated by AlEt2Cl. Both the typical degree of branching (≈100 branches per 1000 carbons) and the molecular weight of the produced samples (≈1000 Da) were poorly sensitive to the reaction conditions. However, during that study, we noticed that when the polymerization was performed at high monomer pressure and low temperature (e.g., 45 atm and 20 °C), traces of solid polymer were obtained together with the low molecular weight polyethylene oils. We have now investigated complex 1 and some related Ni(II) complexes in ethylene polymerization also at sub-ambient temperature and high pressures, resulting in the production of solid polymers, in some cases consisting of fractions with different crystallinities, either as the only product or together with a methanol-soluble oily fraction. The possible role of the in situ formation of multiple active species owing to ligand structure modifications has been evaluated by analysis of the catalyst residues after the reaction and by the synthesis and testing of the corresponding aminopyridine Ni(II) complexes. For the latter catalysts, we have found a dependence of the branching degree on the ethylene pressure, which is opposite to that usually found, i.e., the number of branches increases at increasing pressure.
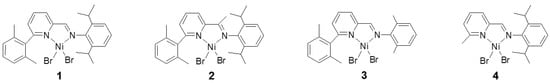
Scheme 2.
Iminopyridine Ni complexes (1)–(4).
2. Experimental Section
2.1. General Conditions
All procedures sensitive to air or moisture were performed under a nitrogen atmosphere using standard Schlenk techniques. Glassware used were dried in an oven at 120 °C overnight and exposed three times to vacuum–nitrogen cycles. Solvents were dried by refluxing over either CaH2 for dichloromethane or metallic sodium for toluene and o-dichlorobenzene and then distilled under nitrogen. Deuterated solvents were purchased from Aldrich and stored in the glovebox over 3 Å molecular sieves before use. All other reagents were purchased from Aldrich and used as received. Ethylene was purchased from SON and used without further purification.
2.2. Characterization Methods
2.2.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
The NMR spectra were recorded on Bruker Advance 400 and Bruker 600 MHz Ascend 3 HD spectrometers.
1H NMR spectra are referenced using the residual solvent peak at δ 7.26 for CDCl3 and δ: 6.93, 7.19 for C6D4Cl2. 13C NMR spectra are referenced using the residual solvent peak at δ 77. 16 for CDCl3 and δ: 127.19, 130.04, 132.39 for C6D4Cl2.
2.2.2. Size-Exclusion Chromatography (SEC)
The molecular weights (Mn and Mw) and the molecular mass distribution (Mw/Mn) of the oily hyperbranched oligoethylene samples were measured by Size-exclusion chromatography (SEC) at 30 °C, using THF as solvent, an eluent flow rate of 1 mL/min, and narrow polystyrene standards as reference. The measurements were performed on a Waters 1525 binary system equipped with a Waters 2414 RI detector using four Styragel columns (range 1000–1,000,000 Å). Molecular weights (Mn and Mw) and polydispersities (Mw/Mn) of crystalline polyethylene samples were determined by high-temperature size exclusion chromatography (HT-SEC) using Waters GPC-V200 RI detector at 135 °C using 1,2-dichlorobenzene as solvent and Styragel columns (range 1 × 107–1 × 103).
2.2.3. Differential Scanning Calorimetry (DSC)
Melting points (Tm) and crystallization points (Tc) of the polymer samples were measured by DSC using aluminum pans and a DSC 2920 TA Instruments apparatus, calibrated with indium. Measurements were performed under nitrogen flow with a heating rate of 10 °C min−1 in the range of −100 to +200 °C. DSC data were processed with TA Universal Analysis v2.3 software and are reported for the second heating cycle.
2.3. Ligands and Complexes Synthesis
Ligands L1–L6 and complexes 1–4 were synthesized as described in our previous papers [20,21,22]. Complexes 5–6 were synthesized following a procedure previously reported for similar complexes [23].
2.3.1. Synthesis of Complex 5
To a solution of L5 (0.48 mmol, 0.180 g) in dry dichloromethane, [(DME)NiBr2] (0.43 mmol, 0.133 g) was added. The reaction mixture was stirred at room temperature for 16 h. The solvent was removed under vacuum and the residues were washed with dry hexane and dried to obtain a green solid (0.241 g, 95% yield).
2.3.2. Synthesis of Complex 6
To a solution of L6 (0.39 mmol, 0.150 g) in dry dichloromethane, [(DME)NiBr2] (0.35 mmol, 0.108 g) was added. The reaction mixture was stirred at room temperature for 24 h. The solvent was removed under vacuum and the residues were washed with dry hexane and dried to obtain a green solid (0.196 g, 93% yield).
2.4. General Procedure for Ethylene Polymerization
Ethylene polymerizations were carried out in a stainless-steel autoclave equipped with a magnetic stirrer. The reactor was first dried overnight at 120 °C in an oven, cooled under vacuum, then pressurized with ethylene and vented three times. The reactor was thermostated at defined temperatures, charged with toluene, the catalyst and AlEt2Cl as cocatalyst and then pressurized at the prescribed ethylene pressure. The mixture was stirred for a defined time under constant ethylene pressure. Then the mixture was vented and poured into acidified methanol. The resulting solid (when present) was filtered. The solution was treated with hexane and water, then the organic layer was dried over MgSO4, filtered and the volatiles were distilled off in a rotavapor. The resulting waxy or oily residues were dried in vacuo overnight at 80 °C.
3. Results
As mentioned in the Introduction, we have previously reported that complex 1 (see Scheme 2) promote ethylene polymerization after activation with AlEt2Cl affording low molecular weight (Mn~103 g/mol) polyethylene oils under a variety of reaction conditions (T = 20 ÷ 50 °C; ethylene pressure 1 ÷ 50 atm) [20].
We have now tested complex 1 after activation with 200 equiv of AlEt2Cl under 50 atm of monomer pressure at 0 °C (see run 1, Table 1): under these conditions, a solid polymer was obtained as the prevailing product, in addition to the usual oily fraction recovered from the reaction solution (see the Experimental section for details). 1H and 13C NMR analysis of the solid and the liquid fractions (see Figures S1–S4) showed that the latter is a hyperbranched polyethylene similar to those previously reported [20], while the former is a moderately branched polyethylene containing prevalently methyl branches. DSC analysis of the solid polymer showed two melting endotherms, a broad one around 90 °C and a sharp one at 125 °C; similarly, two crystallization exotherms were observed (at 113 and 79 °C) when cooling the sample (see Figure S5). Changing the conditions of the DSC analysis (e.g., using different heating or cooling rates) did not affect the results. SEC analysis of the solid polymer indicated that the Mw was one order of magnitude higher than that of the oily product and a rather broad polydispersity (Ð = 3.1).

Table 1.
Ethylene polymerizations by complexes 1–4.
The solid polymer sample was, thus, fractioned using boiling heptane in a Kumagawa extractor, resulting in a soluble (75%) and an insoluble (25%) fraction: the latter was analyzed by 1H and 13C NMR, indicating a substantially linear polyethylene (methyl branches < 1%) with no detectable unsaturated end groups (see Figures S6 and S7); accordingly, DSC analysis showed only a melting endotherm at 130 °C (Figure S8). The heptane-soluble fraction was analyzed similarly, resulting a more branched, lower melting polyethylene (Figure S9).
Subsequently, we have tested several related complexes bearing different substituents at the pyridino and at the imino moieties (complexes 2–4, see Scheme 2).
Complexes (2)–(4) were initially tested in the homopolymerization of ethylene after activation with AlEt2Cl (200 equiv) at 40 °C and 6 atm monomer pressure (see Table 1, runs 2–4): under these reaction conditions, all the complexes afforded hyperbranched low molecular weight polyethylene oils, soluble in methanol, similar to those produced by complex 1 [20].
Characterization of the polymer samples by 1H and 13C NMR analyses is reported in the Supplementary Material. As observed for 1, multigram-quantities of low molecular weight polyethylene oils were obtained under higher catalyst and monomer concentrations and for longer reaction times, suggesting that the catalysts are stable under these conditions (see runs 5–8). Complexes 2, bearing a ketimino ligand, afforded polyethylenes having higher molecular weights (see runs 2, 5, 6, Table 1) with respect to that produced by the previously reported corresponding aldimino complex 1 [20].
Complex 2 was then tested under 50 atm of monomer pressure at 0 °C (see run 9, Table 1): in this case, only a solid polymer was produced, while no oily product was recovered from the reaction mixture. NMR analysis indicated a moderately branched polyethylene (5% branches, see Figures S12 and S13), while DSC showed very broad melting endotherms centered around 32 and 71 °C and very broad crystallization exotherms centered around 57 and 24 °C (see Figure S14).
The production of fractions of macromolecules with different structures and molecular weights suggest that different active species can be generated in situ by activation with AlEt2Cl. With the aim to shed some light on the latter hypothesis, two polymerization runs were carried out using complexes 2 and 3 activated by AlEt2Cl at 0 °C under 1 atm of ethylene for 1 h (see Scheme 3). The reactions were quenched with acidified methanol, and water was added; the mixtures were extracted with dichloromethane and, after solvent removal, the crude product was filtered through a silica column and eluted with hexane and then dichloromethane. The product isolated from the filtrate was analyzed by 1H NMR, showing the presence of both the imine ligands and the corresponding amines deriving by imine reduction. Interestingly, ligand modification after the polymerization run was more relevant for the aldimine complex 3 than for the ketimine complex 2, since the fraction of produced amine was 11% for the former and only 5% for the latter. Moreover, performing the same experiment at higher temperature (40 °C) using the ketimine complex 2 resulted in the formation of 22% of amine (see Figures S15 and S16). Following the suggestion of the reviewer, we have performed a control experiment in the absence of ethylene, resulting in the production of the original imino and the reduced amino ligands, as observed in the presence of ethylene, and an additional amine species bearing an ethyl group on the carbon in alpha position with respect to N atom (see Figure S17) [25].
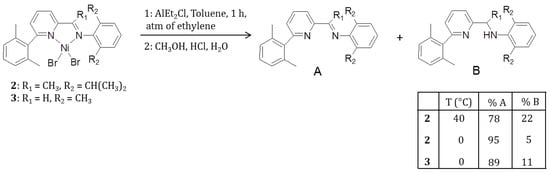
Scheme 3.
Investigation of the post-polymerization catalyst fate.
Following the above findings, we decided to synthesize the authentic amine Ni complexes 5 and 6 (see Scheme 4) having ligand structures corresponding to the imine complexes 1 and 2, respectively. The complexes were obtained in good yields by allowing us to react the amine ligands and dimethoxyethane nickel dibromide in CH2Cl2 at room temperature for 16 h (see the Experimental section).
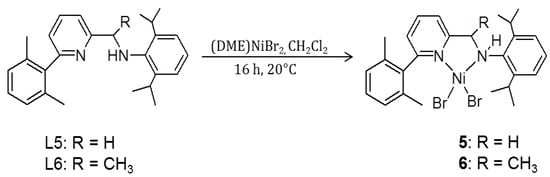
Scheme 4.
Synthesis of pyridylamino complexes 5 and 6.
Pyridilamino complex 5, after activation with AlEt2Cl, was tested in the polymerization of ethylene at 0 °C under 50 atm of monomer, resulting in the exclusive production of low molecular weight highly branched polyethylene oils (see run 10, Table 2). In contrast, the corresponding pyridylimino complex 1 under similar conditions (although using higher dilution, see the Experimental Section) produced a mixture of solid polyethylene and an oily fraction, with significantly higher yield (cf. run 1, Table 1). Pyridylamino complex 6 under the same conditions also afforded low molecular weight highly branched polyethylene oils, although in very low amount (see run 11, Table 2). Complexes 5 and 6 were also tested at T = 40 °C under either 10 or 50 atm of ethylene pressure: in all cases low yields of hyperbranched polyethylene oils were obtained. Interestingly, for both catalysts the branching degree was higher at 50 atm than at 10 atm (cf. run 12 vs. run 13 and run 14 vs. run 15), at variance with all the literature results reported for Ni(II) catalysts affording branched polyethylenes via a ”chain walking” mechanism [2].

Table 2.
Ethylene polymerization by aminopyridine complexes 5 and 6.
4. Discussion
It is well known that Ni(II) catalysts operate via a “chain-walking” mechanism of polymerization, involving a number of β-hydride eliminations and reinsertions with opposite regiochemistry: in this way, a variable content of branches of different lengths are introduced in the polymer chain [2]. As established in the literature, the degree of branching depends on temperature, monomer pressure, and catalyst structure: higher branching is favored by higher polymerization temperature, lower monomer pressure and larger steric bulk in the axial positions of the square-planar coordination sphere [1,2,26,27,28,29,30]. So, very different products can be obtained from polymerization of ethylene only, ranging from substantially linear and crystalline materials to elastomers to hyperbranched polyethylene waxes and oils [1,2,26,27,28,29,30,31].
However, the above framework cannot explain the concomitant production of the different types of macromolecules that we have observed in the above reported polymerization runs by our Ni(II) catalysts bearing aryliminopyridine ligands with bulky substituents both at the imino moiety and in the 6-position of pyridine. On the other hand, there are many literature examples pointing to the easy modification in situ of olefin polymerization catalysts based on imino complexes activated by aluminum alkyls [32,33,34,35], resulting in the reduction of the imino functionality. Moreover, Gibson reported examples of reactions of α-diimine and pyridylimine ligands with, e.g., AlMe3, resulting in Al imino-amide and pyridyl-amide complexes arising from methyl group transfer from the aluminium centre to the backbone carbon of the imine ligand [34]. So, it is reasonable to hypothesize that multiple active species are generated under the examined conditions.
As a matter of fact, our experiments analyzing the fate of the iminopyridine Ni(II) catalysts after the polymerization run showed the partial transformation of the original ligands in the corresponding aminopyridines. This finding supports the multi-site nature of our catalyst systems, at least under certain conditions.
Looking for some further evidence on the matter, we have synthesized and tested two aminopyridine Ni(II) complexes (5 and 6) having structures similar to the iminopyridine complexes 2 and 3. While aminopyridine Ni(II) complexes have been previously reported as efficient ethylene polymerization catalysts [36,37], only traces of uncharacterized oligomers were obtained using aminopyridine complexes having aryl groups in the 6-position of the pyridine moiety, similar to 5 and 6 [37].
Our polymerization results have shown that, under similar conditions (T = 0 °C, P = 50 atm), the aminopyridine complexes produce only low amounts of hyperbranched polyethylene oils, while the iminopyridine analogs afford mixtures of different types of macromolecules (see above), again suggesting the possible modification of the imino functionality. Interestingly, for the aminopyridine complexes more branched polyethylenes are produced at higher monomer pressure, at variance with the usual behavior of Ni(II) catalysts. The latter could be an interesting feature, since one of the main limitations of Brookhart’s Ni(II) catalysts for practical applications in the field of elastomeric materials obtained by ethylene feed only (without the need of comonomers such as propene or 1-hexene) is the fact that the polymers obtained under the high pressures required by the industrial processes are substantially linear. Further investigation in this direction is in progress in our laboratories.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/macromol1020010/s1, Figure S1: 1H-NMR spectrum of an oily hyperbranched oligoethylene sample, Figure S2: 13C-NMR spectrum of an oily hyperbranched oligoethylene sample, Figure S3: 1H-NMR spectrum of a crystalline polyethylene sample, Figure S4: 13C-NMR spectrum of a crystalline polyethylene sample, Figure S5: DSC thermogram of a crystalline polyethylene sample, Figure S6: 1H-NMR spectrum of the heptane-insoluble part of a crystalline polymer sample, Figure S7: 13C-NMR spectrum of the heptane-insoluble part of a crystalline polymer sample, Figure S8: DSC thermogram of the heptane-insoluble fraction of a crystalline polymer sample, Figure S9: DSC thermogram of the heptane-soluble fraction of a crystalline polymer sample, Figure S10: 1H-NMR spectrum of a typical low MW oily polyethylene sample, Figure S11: 13C-NMR spectrum of a typical low MW oily polyethylene sample, Figure S12: 1H-NMR spectrum of a crystalline polyethylene sample, Figure S13: 13C-NMR spectrum of a crystalline polyethylene sample, Figure S14: DSC thermogram of a crystalline polyethylene sample, Figure S15: 1H-NMR spectra of the products of reaction between complex 3 and AlEt2Cl, Figure S16: 1H-NMR spectra of the products of reaction between complex 2 and AlEt2Cl.
Author Contributions
Conceptualization, C.P.; methodology, C.P. and I.D.; investigation, Z.S. and I.D.; data curation, Z.S. and I.D.; writing—original draft preparation, C.P. and I.D.; writing—review and editing, C.P.; supervision, C.P.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Salerno, grant FARB 2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the technical assistance of Patrizia Oliva for NMR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Hoarau, O.D.; Spitzmesser, S.K.; White, A.J.P.; David, J.; Williams, D.J. Iron and Cobalt Ethylene Polymerization Catalysts: Variations on the Central Donor. Inorg. Chem. 2003, 42, 3454–3465. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino) pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: N-Imine-Donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Guo, L.; Liu, W.; Chen, C. Late transition metal catalyzed α-olefin polymerization and copolymerization with polar monomers. Mater. Chem. Front. 2017, 1, 2487–2494. [Google Scholar] [CrossRef]
- Laine, T.V.; Klinga, M.; Leskelä, M. Synthesis and X-ray Structures of New Mononuclear and Dinuclear Diimine Complexes of Late Transition Metals. Eur. J. Inorg. Chem. 1999, 1999, 959–964. [Google Scholar] [CrossRef]
- Laine, T.V.; Piironen, U.; Lappalainen, K.; Klinga, M.; Aitola, E.; Leskelä, M. Pyridinylimine-Based nickel(II) and palladium(II) complexes: Preparation, structural characterization and use as alkene polymerization catalysts. J. Organomet. Chem. 2000, 606, 112–124. [Google Scholar] [CrossRef]
- Irrgang, T.; Keller, S.; Maisel, H.; Kretschmer, W.; Kempe, R. Sterically Demanding Iminopyridine Ligands. Eur. J. Inorg. Chem. 2007, 2007, 4221–4228. [Google Scholar] [CrossRef]
- Mahmood, Q.; Sun, W.-H. N, N-Chelated nickel catalysts for highly branched polyolefin elastomers: A survey. R. Soc. Open Sci. 2018, 5, 180367. [Google Scholar] [CrossRef]
- Vignesh, A.; Zhang, Q.; Ma, Y.; Liang, T.; Sun, W.-H. Attaining highly branched polyethylene elastomers by employing modified α-diiminonickel(II) catalysts: Probing the effects of enhancing fluorine atom on the ligand framework towards mechanical properties of polyethylene. Polymer 2020, 187, 122089. [Google Scholar] [CrossRef]
- Sun, W.-H.; Song, S.; Li, B.; Redshaw, C.; Hao, X.; Li, Y.-S.; Wang, F. Ethylene polymerization by 2-iminopyridylnickel halide complexes: Synthesis, characterization and catalytic influence of the benzhydryl group. Dalton Trans. 2012, 41, 11999–12010. [Google Scholar] [CrossRef]
- Yue, E.; Xing, Q.; Zhang, L.; Shi, Q.; Cao, X.-P.; Wang, L.; Redshaw, C.; Sun, W.-H. Synthesis and characterization of 2-(2-benzhydrylnaphthyliminomethyl) pyridylnickel halides: Formation of branched polyethylene. Dalton Trans. 2014, 43, 3339–3346. [Google Scholar] [CrossRef]
- Yue, E.; Zhang, L.; Xing, Q.; Cao, X.-P.; Hao, X.; Redshaw, C.; Sun, W.-H.; Sun, W.-H. 2-(1-(2-Benzhydrylnaphthylimino) ethyl)-pyridylnickel halides: Synthesis, characterization and ethylene polymerization behavior. Dalton Trans. 2014, 43, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Mahmood, Q.; Hao, X.; Sun, W.-H. Synthesis and Ethylene Polymerization of 8-(fluorenylarylimino)-5, 6, 7-Trihydroquinolylnickel Chlorides: Tailoring Polyethylenes by Adjusting Fluorenyl Position and Adduct Et2Zn. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1910–1919. [Google Scholar] [CrossRef]
- Zada, M.; Vignesh, A.; Guo, L.; Zhang, R.; Zhang, W.; Ma, Y.; Sun, Y.; Sun, W.-H. Sterically and Electronically Modified Aryliminopyridyl-Nickel Bromide Precatalysts for an Access to Branched Polyethylene with Vinyl/Vinylene End Groups. ACS Omega 2020, 5, 10610–10625. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Allen, K.E.; White, P.S.; Daugulis, O.; Brookhart, M. Synthesis of Branched Polyethylene with “Half-Sandwich” Pyridine-Imine Nickel Complexes. Organometallics 2016, 35, 1756–1760. [Google Scholar] [CrossRef]
- D’Auria, I.; Milione, S.; Caruso, T.; Balducci, G.; Pellecchia, C. Synthesis of hyperbranched low molecular weight polyethylene oils by an iminopyridine nickel(II) catalyst. Polym. Chem. 2017, 8, 6443–6454. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Alterio, M.C.; Talarico, G.; Pellecchia, C. Alternating Copolymerization of CO2 and Cyclohexene Oxide by New Pyridylamidozinc(II) Catalysts. Macromolecules 2018, 51, 9871–9877. [Google Scholar] [CrossRef]
- Saki, Z.; D’Auria, I.; Dall’Anese, A.; Milani, B.; Pellecchia, C. Copolymerization of Ethylene and Methyl Acrylate by Pyridylimino Ni(II) Catalysts Affording Hyperbranched Poly(ethylene-co-methylacrylate)s with Tunable Structures of the Ester Groups. Macromolecules 2020, 53, 9294–9305. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Gao, H.; Zhu, F.; Wu, Q. Design of Thermally Stable Amine–Imine Nickel Catalyst Precursors for Living Polymerization of Ethylene: Effect of Ligand Substituents on Catalytic Behavior and Polymer Properties. Chem. Eur. J. 2014, 20, 3225–3233. [Google Scholar] [CrossRef]
- Wiedemann, T.; Voit, G.; Tchernook, A.; Roesle, P.; Götter-Schnetmann, I.; Mecking, S. Monofunctional Hyperbranched Ethylene Oligomers. J. Am. Chem. Soc. 2014, 136, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Redshaw, C.; White, A.J.P.; Williams, D.J. Synthesis and structural characterisation of aluminium imino-amide and pyridyl-amide complexes: Bulky monoanionic N, N chelate ligands via methyl group transfer. J. Organomet. Chem. 1998, 550, 453–456. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Delferro, M.; Marks, T.J. Multinuclear Olefin Polymerization Catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- Sun, W.-H. Novel polyethylenes via late transition metal complex pre-catalysts. Adv. Polym. Sci. 2013, 258, 163–178. [Google Scholar]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- D’Auria, I.; Maggio, M.; Guerra, G.; Pellecchia, C. Efficient Modulation of Polyethylene Microstructure by Proper Activation of (α-Diimine) Ni(II) Catalysts: Synthesis of Well-Performing Polyethylene Elastomers. Macromolecules 2017, 50, 6586–6594. [Google Scholar] [CrossRef]
- Saito, J.; Onda, M.; Matsui, S.; Mitani, M.; Furuyama, R.; Tanaka, H.; Fujita, T. Propylene Polymerization with Bis(phenoxy-imine) Group-4 Catalysts Using iBu3Al/Ph3CB(C6F5)4as a Cocatalyst. Macromol. Rapid Commun. 2002, 23, 1118–1123. [Google Scholar] [CrossRef]
- Yasuhiko, S.; Norio, K.; Fujita, T. Synthesis and Ethylene Polymerization Behavior of a New Titanium Complex Having Two Imine–Phenoxy Chelate Ligands. Chem. Lett. 2002, 31, 358–359. [Google Scholar]
- Vishwa, P.A.; Haruyuki, M.; Junji, S.; Mitsuhiko, O.; Fujita, T. Highly Isospecific Polymerization of Propylene with Bis(phenoxy-imine) Zr and Hf Complexes Using iBu3Al/Ph3CB(C6F5)4 as a Cocatalyst. Chem. Lett. 2004, 33, 250–251. [Google Scholar]
- Lamberti, M.; Consolmagno, M.; Mazzeo, M.; Pellecchia, C. A Binaphthyl-Bridged Salen Zirconium Catalyst Affording Atactic Poly(propylene) and Isotactic Poly (α-olefins). Macromol. Rapid Commun. 2005, 26, 1866–1871. [Google Scholar] [CrossRef]
- Zai, S.; Liu, F.; Gao, H.; Li, C.; Zhou, G.; Cheng, S.; Guo, L.; Zhang, L.; Zhu, F.; Wu, Q. Longstanding living polymerization of ethylene: Substituent effect on bridging carbon of 2-pyridinemethanamine nickel catalysts. Chem. Commun. 2010, 46, 4321–4323. [Google Scholar] [CrossRef]
- Zai, S.; Gao, H.; Huang, Z.; Hu, H.; Wu, H.; Wu, Q. Substituent Effects of Pyridine-amine Nickel Catalyst Precursors on Ethylene Polymerization. ACS Catal. 2012, 2, 433–440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).