Abstract
Background: currently, there is a growing trend toward multifunctional cosmetics, which combine several active ingredients in a single product to enhance efficacy and user convenience. As ingredients may influence one another, it is important to study the behavior of mixing multiple compounds in complex formulations, especially regarding their interaction with the skin. Piceatannol, for instance, is a naturally occurring stilbene recognized for its in vitro potent antioxidant, anti-inflammatory, and anti-aging activities, making it a promising candidate for dermocosmetic use in suncare. But despite its beneficial biological activities, its cutaneous permeation remains poorly understood, particularly when delivered from complex formulations containing multiple ingredients. Objectives: in this sense, this study aimed to evaluate the in vitro skin diffusion profile of piceatannol from a passion fruit seed extract (Pext) incorporated into a topical base (Bem) or an organic sunscreen emulsion (Oem), with or without a spilanthol-rich Acmella oleracea extract (Jext) used as a natural permeation enhancer. Methods: due to ethical and variability issues with human and animal skins, the Strat-M™ synthetic membrane was chosen as a standardized model for the in vitro skin permeation assays. Piceatannol localization within membrane layers was examined by confocal Raman microscopy (CRM), while compound identification in donor and receptor compartments was performed via UHPLC-DAD. Results: piceatannol from Bem was detected up to 140 µm from the Strat-M™ surface and exceeded 180 µm in depth when Jext and organic sunscreens were included in the formulation. Notably, formulations containing Jext and those based on Oem promoted enhanced accumulation in both the stratum corneum and deeper skin layers, suggesting an improved delivery potential in lipid-rich vehicles. Conclusions: even though some instability issues were observed, piceatannol penetration into Strat-M™ from the proposed formulations was confirmed, and the results provide a foundation for further research on its topical delivery, supporting the rational development of formulations capable of harnessing its demonstrated biological properties.
1. Introduction
Stilbenes are natural phenolic compounds which have attracted considerable attention due to their diverse health-promoting properties, including anticancer, anti-inflammatory, anti-aging, antioxidant, and anti-atherogenic effects [1]. Among them, piceatannol (trans-3,3′,4,5′-tetrahydroxystilbene) stands out as a bioactive stilbenoid. In vitro assays have highlighted piceatannol’s dermocosmetic potential, demonstrating its ability to protect skin cells from reactive oxygen species (ROS)-induced apoptosis, inhibit melanogenesis in human melanoma cells, stimulate collagen synthesis in fibroblasts, suppress UVB-induced ROS generation, and reduce matrix metalloproteinase-1 (MMP-1) activity, a key enzyme involved in collagen degradation. Naturally present in foods such as grapes, white tea, and rhubarb, piceatannol is particularly abundant in passion fruit (Passiflora edulis Sims) seeds. Brazil is the world’s largest producer of passion fruit, generating approximately 600,000 tons annually. While the pulp is widely used in the food and beverage industry, the phenolic-rich seeds remain underutilized. Exploring the use of these seeds as sources of bioactive compounds for pharmaceutical or cosmetic purposes may enhance the economic value of passion fruit processing and support sustainability efforts [2,3,4,5,6].
Although piceatannol sources are not currently commercially available for skincare purposes, it has great potential to become a novel dermo-cosmetic ingredient due to its well-documented in vitro effects on skin cells and its environmental compatibility. But despite its reported skin-whitening, anti-aging, photoprotective, and regenerative properties, clinical data on piceatannol’s efficacy via oral administration remain limited. A double-blind study involving 32 women (aged 35–54) who consumed 5 mg of piceatannol extract daily for eight weeks only reported improved skin hydration as an observed effect [7]. Animal and in vitro studies suggest that piceatannol undergoes rapid metabolism and clearance, with limited oral bioavailability due to biotransformation by the gut microbiota and hepatic metabolism [8,9,10]. These findings suggest that topical application may be a more effective route for delivering piceatannol’s skin benefits. However, its cutaneous diffusion properties are poorly characterized. Some predictive and in vitro models indicate it can cross the skin barrier to a certain extent [11], albeit at rates significantly lower—approximately 11-fold less—than resveratrol, an extensively studied stilbene with strong peroxide radical scavenging activity [12].
Given this context, the present study aimed to assess whether piceatannol can reach deeper skin layers when incorporated into a topical vehicle. To that, a passion fruit seed extract (Pext) developed in-house served as the piceatannol source and in vitro diffusion assays were conducted using Franz diffusion cells. While human skin remains the gold standard for such studies, its use presents several limitations, including variability due to anatomical site, donor age, and sex, as well as ethical and logistical challenges, but alternative membranes such as pig skin, rodent skin, bovine tissue, and snake skin also pose issues with variability, preservation, and ethical acceptability. In light of restrictions on animal testing for cosmetic ingredients, the Strat-M™ synthetic membrane was chosen as a skin model alternative, offering standardized and reproducible barrier properties for in vitro skin diffusion studies [13,14].
When active compounds such as piceatannol exhibit poor diffusion through the skin, formulation strategies may employ permeation enhancers to improve their delivery to their site of activity. In this context, we hypothesized that a spilanthol-rich paracress extract (Jext) could enhance piceatannol’s skin penetration. Spilanthol (2E,6Z,8E)(2E,6Z,8E)(2E,6Z,8E)-N-(2-methylpropyl)deca-2,6,8-trienamide), a bioactive amide from paracress (Acmella oleracea (L.) R.K. Jansen, commonly known as jambu in Brazil), demonstrates excellent skin penetration properties and is widely used in cosmetics for its anti-wrinkle and anti-aging actions. It also exhibits antioxidant and anti-inflammatory activities, making it a promising candidate not only as an active ingredient but also as a penetration enhancer for compounds such as piceatannol. Therefore, in this study, Jext was incorporated into topical formulations to assess its effect on piceatannol skin delivery [15,16,17].
And to further enhance the formulation functional benefits in compliance with the multifunctional cosmetics tendency, piceatannol’s behavior was also evaluated in the presence of organic sunscreens, which act primarily by absorbing ultraviolet radiation and converting it into less harmful forms of energy, such as heat. An increasing number of commercial sunscreen products now include natural ingredients or plant extracts, driven by the additional benefits these compounds offer compared to traditional sunscreens. While inorganic and organic UV filters remain the primary active components, certain natural compounds such as flavonoids and polyphenols can also contribute to UV protection due to the presence of aromatic rings in their structures, which enable them to absorb ultraviolet radiation and reduce sunburn. But their main mechanism of action is actually their antioxidant, anti-inflammatory and other beneficial activities which further enhance skin protection. These indirect photoprotective mechanisms play a crucial role in preventing photoinduced oxidative stress, inflammation, and the subsequent degradation of skin components such as collagen and elastin. The merging of classical photoprotective agents with anti-aging ingredients aligns with the current trends in skincare, offering practical, efficient solutions for consumers seeking protection and prevention in a single product [18,19,20].
Nowadays, sunscreens are marketed in various dosage forms, including creams, lotions, gels, sprays, sticks, butters, and oils. Creams and lotions represent about 30% of the global sunscreen market, followed by aerosols and gels at around 20% each. Public health guidelines recommend reapplication every 2–3 h or after sweating or swimming to maintain adequate UV protection [21,22,23,24]. Therefore, to align with these recommendations, the present study evaluated the skin diffusion profile of piceatannol over a 3 h exposure period to assess the behavior of active compounds in the selected model, simulating a realistic sunscreen application scenario. To the best of our knowledge, this is the first study to analyze piceatannol’s skin diffusion profile under such conditions and this approach establishes a framework for the development of advanced dermocosmetic products formulated with piceatannol.
2. Materials and Methods
2.1. Piceatannol-Rich Extract from Passion Fruit Seed Material (Pext)
The extract was prepared following the procedure described by Silva, Rodrigues, and Bottoli [25]. Briefly, residual passion fruit seed cake (Passiflora edulis Sims.), obtained after cold press oil extraction (Extrair Óleos Naturais, Bom Jesus do Itabapoana, RJ, Brazil), was defatted using analytical-grade n-hexane (UNICAMP, Campinas, SP, Brazil) at a 1:10 (w/v) ratio. The defatting process was performed by magnetic stirring for 1 h at room temperature (approximately 25 °C). After defatting, the seed cake was extracted twice with 70% ethanol (EtOH), at 87 °C, for 30 min, using a Start E bench-scale microwave extractor (Milestone, Sorisole, BG, Italy). The extracts from both cycles were collected by vacuum filtration through filter paper, and the solvent was removed under a flow of nitrogen gas (N2). The mass yield of the extraction process was 10%, and the extract contained 27.2 ± 0.9 μg of piceatannol per mg.
2.2. Spilanthol-Rich Extract from Paracress (Jext)
A crude extract of paracress (Acmella oleracea (L.) R.K. Jansen) aerial parts was prepared following the method of Freitas-Blanco et al. [26]. Briefly, the leaves, stems, and flowers were dried and ground, then extracted with 95% analytical-grade ethanol (1:5 w/v) under mechanical stirring in a stainless-steel tank for 1.5 h. The solid residue was removed by filtration, and the extraction was repeated twice.
Due to the dark color of the crude extract, a purification step was performed using a classical liquid–liquid extraction with hexane, a solvent commonly employed for spilanthol recovery [27]. Specifically, 50 mL of the crude extract were mixed with 300 mL of analytical-grade n-hexane (UNICAMP, Campinas, SP, Brazil) in a separatory funnel. The hexane layer was collected, and the solvent was removed under a nitrogen (N2) stream. The resulting oil was then filtered under vacuum through silica using a sintered plate funnel, yielding Jext, a translucent olive-green extract with a spilanthol content of 249.2 ± 22.1 μg mg−1.
2.3. Formulations
The formulations were prepared as described in Table 1 and structured using a nonionic surfactant and a thickening system composed of an acrylate-based gelling agent and lecithin. The formulation termed Oem was obtained by incorporating three organic ultraviolet (UV) filters—Parsol MCX™, Uvinul A Plus™, and Uvinul T150™—into the base emulsion (Bem). These filters were selected based on their proven efficacy, photostability, and broad-spectrum protection, as well as their recognition as safe and legally permitted by major regulatory agencies within their established concentration limits. Their safety profiles have been extensively evaluated through toxicological assessments, and they are widely used in commercial sunscreen formulations, ensuring compliance with current global cosmetic regulations. The concentrations of the UV filters used in the formulations were defined using the BASF Sunscreen Simulator™ to achieve a theoretical sun protection factor (SPF) of 10 [28].

Table 1.
Compositions of the oil-in-water (O/W) emulsions.
Pext (2 mg per gram of emulsion) and Jext (1 mg per gram of emulsion) were first dispersed in propylene glycol (10 mg per gram of emulsion) and then incorporated into the emulsions by manual spatulation. From the base formulations (Bem and Oem), a panel of six different emulsions was developed, each containing Pext, Jext, or a combination of both (Figure 1).
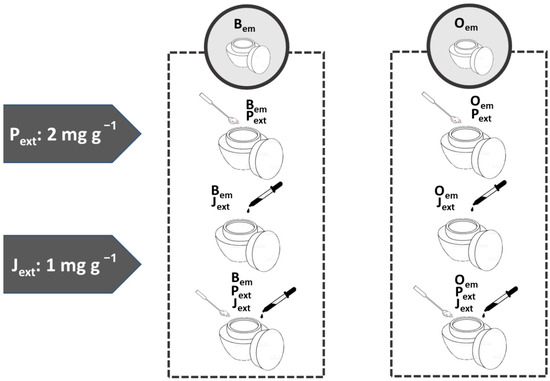
Figure 1.
Schematic representation of the formulation panel. Pext: passion fruit seed extract. Jext: Paracrass extract. Bem: base cream. Oem: base cream added with the organic filters Parsol MCXTM, Uvinul A PlusTM and Uvinul T150TM.
2.4. Piceatannol and Spilanthol Quantification
Piceatannol and spilanthol were quantified in the emulsions using a Waters Acquity UPLC H-Class system with a photodiode array detector (PAD) (Waters, Milford, MA, USA). Quantification curves were constructed using Bem as the matrix. First, 50 mg of Bem and 1.0 mL of 50% ethanol (EtOH, HPLC-grade) were vortexed in Falcon tubes. The suspensions were then centrifuged at 3000 rpm for 5 min, and the supernatants were dried in glass flasks under a nitrogen gas (N2) flow. Standard solutions of piceatannol (>98% purity, ChemScene LLC, Monmouth Junction, NJ, USA) and spilanthol (74% purity, isolated from paracress aerial parts at CPQBA, Paulínia, Brazil) in 50% EtOH (HPLC-grade) were added to the dried supernatants, providing curve points ranging from 2 to 10 mg L−1 for piceatannol and from 6 to 30 mg L−1 for spilanthol. Each curve point was prepared in triplicate. The samples were filtered through 0.22 µm regenerated cellulose membranes (GVS, Bologna, Italy) before injection.
For analysis, 50 mg of each formulation and 1.0 mL of 50% EtOH HPLC-grade were vortexed in Falcon tubes, followed by centrifugation and filtration as described above. Separations were performed on an Acquity UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm) at 30 °C, using a gradient of HPLC-grade 0.1% formic acid (A) and acetonitrile (B) with the following elution program: 0 min—20% B, 7 min—80% B, 8 min—80% B, 8.5 min—20% B, and 10 min—20% B. Piceatannol and spilanthol were detected at 323 nm and 229 nm, respectively. The samples were maintained at 20 °C in the autosampler, and 5 µL of each sample was injected per run. Chromatographic data were collected using Empower 2 software (Waters, Milford, MA, USA). The linearity and the limits of detection (LOD) and quantification (LOQ) of the quantification curves were determined with Action Stat 3.7 software (EstatCamp, São Carlos, Brazil).
2.5. Receptor Fluid Selection for Franz Cell Diffusion Assays
The stabilities of Pext and Jext in hydroalcoholic solutions were evaluated. Two mg of each extract (Pext and Jext) were solubilized in 5 mL of methanol and subsequently diluted with 95 mL of either 30% or 50% ethanol (EtOH, HPLC-grade). The solutions were divided into 5 mL aliquots and placed in screw-cap glass vials equipped with magnetic stirrers. The vials were immersed in a water bath maintained at 36 ± 2 °C, with magnetic stirring. Four vials of each solution type were removed from the bath at time intervals of 0, 1, 2, and 3 h, and 1 mL samples from each vial were dried under a nitrogen gas (N2) flow.
Samples were analyzed using HPLC-MS/MS (Waters, Milford, MA, USA). To each dried sample, 300 µL of a methanol (MeOH): 0.1% formic acid solution (90:10, HPLC-grade) were added. Five microliters of each sample were then injected into a Waters NovaPak C18 column (150 mm × 3.9 mm, 4 μm) and eluted with a mobile phase composed of HPLC-grade 0.1% formic acid (A) and acetonitrile (ACN) (B) at a flow rate of 0.3 mL min−1. The gradient program was as follows: 40% B (0–4 min), 40–100% B (4–7 min), 100% B (7–9 min), and 100–40% B (9–12 min).
Piceatannol (245 > 135 m/z) and spilanthol (222 > 123 m/z) were detected using an ESI-QqQ Micromass Quattro Micro API mass spectrometer (Waters, Milford, MA, USA) in positive MRM mode. The conditions for the analysis were: cone voltage of 30 V, capillary voltage of 3.0 kV, extractor cone voltage of 3.0 V, source temperature of 150 °C, desolvation temperature of 350 °C, desolvation gas flow (N2) at 350 L/h, and cone gas flow (N2) at 100 L/h. Chromatographic peak integration was performed using MassLynx 4.0 software, with Savitzky–Golay smoothing applied.
2.6. Franz Cell Permeation Assays with the Strat-M® Membranes
A schematic representation of the experimental setup is shown in Figure 2. Five milliliters of receptor fluid were added to the receptor chambers of Franz diffusion cells. A magnetic stir bar was placed in each chamber, and the cells were positioned on a closed bench maintained at 33 ± 2 °C using electric heaters. The bench was placed on a magnetic stirring plate, and stirring was set to 350 rpm to ensure homogeneity of the receptor fluid throughout the experiment. Two milligrams of each formulation were evenly applied to the surface of Strat-M™ membranes (Merck Millipore, Burlington, MA, USA), which were then positioned between the donor and receptor compartments without prior hydration and clipped securely in place. The assay was conducted under non-occlusive conditions using a finite dose protocol, simulating a realistic topical application. Each formulation was assayed in triplicate.
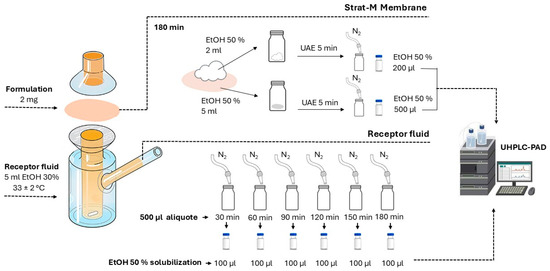
Figure 2.
Schematic representation of the Franz cell permeation assay. Strat-M™ membranes were mounted between donor and receptor compartments filled with 5 mL of receptor fluid, maintained at 33 ± 2 °C under magnetic stirring at 350 rpm. Two milligrams of each formulation were applied to the membrane surface. Receptor fluid samples (500 µL) were collected at predefined intervals up to 180 min and analyzed by UHPLC-DAD. After diffusion, membrane surfaces and membranes were extracted with 50% ethanol using ultrasound-assisted extraction (UAE) and analyzed by UHPLC-DAD.
Receptor fluid aliquots (500 µL) were withdrawn at 30, 60, 90, 120, 150, and 180 min and immediately replaced with an equal volume of fresh fluid. Solvents were evaporated from the collected samples under a stream of nitrogen (N2(g)), and the residues were reconstituted in 100 µL of 50% HPLC-grade ethanol prior to UPLC-DAD analysis. An estimate of the permeated compounds was made by dividing the peak area of each compound detected in the receptor fluid by the peak area corresponding to 2 mg of the cream applied on the membrane. The results were expressed as a percentage (%) from the initial concentration.
Following the 180 min diffusion period, the membrane surfaces were gently wiped with cotton pads, which were then extracted in 2 mL of 50% ethanol under sonication for 5 min. The membranes were subsequently extracted in 5 mL of 50% HPLC-grade ethanol under the same sonication conditions. Both cotton and membrane extracts were dried under N2(g) and reconstituted in 100 µL and 300 µL of 50% HPLC-grade ethanol, respectively, for UHPLC-PAD analysis, as previously described.
2.7. Confocal Raman Microscopy Analyzes
An additional permeation assay was conducted according to 2.6 for each formulation and the membranes were collected after a 180 min exposure. The depth of piceatannol diffusion within the membranes was qualitatively evaluated using a confocal Raman microscope (XploRA Plus, Horiba Scientific, Irvine, CA, USA), with spectral acquisition ranging from 500 to 2500 cm−1. The instrument was configured with the following parameters: 50× objective lens, 1200 lines mm−1 diffraction grating (750 µm), 1% optical filter, 532 nm excitation laser, 50 µm slit width, 100 µm pinhole, 5 s accumulation time, and a total of 15 accumulations. Reference spectra of the standards were acquired under identical conditions. Data analysis was performed in Microcal Origin 2018 (OriginLab Coorporation, Northampton, MA) using the peak analyzer tool, following baseline correction via asymmetric least squares smoothing.
3. Results and Discussion
For piceatannol to exert its biological effects, it must penetrate the stratum corneum and reach the deeper layers of the epidermis, or even the dermis, as suggested by its reported biological activities in the literature. Molecules with favorable skin permeation characteristics generally possess a molecular weight under 500 Da, a melting point below 200 °C, a logP ranging from 1 to 3, and amphiphilic solubility profiles. Both piceatannol (MW = 244.2 Da; logP = 2.12) and spilanthol, the proposed permeation enhancer in this study (MW = 221.3 Da; logP = 3.6), exhibit near-optimal physicochemical profiles for transdermal delivery. Nonetheless, their effective skin diffusion profiles may be influenced by individual factors such as age, sex, and hydration status, as well as by the formulation vehicle, which can modify stratum corneum hydration and lipid organization [13,14,29,30].
The quantified concentrations of piceatannol and spilanthol in each formulation are presented in Table 2. Piceatannol and spilanthol curves and lack-of-fit ANOVA tables are presented in Supplementary Materials Figure S4 and Tables S1 and S2. The R2 values were 0.9994 and 0.9991 for piceatannol and spilanthol, respectively, indicating strong evidence that the data fit the proposed linear models at a significance level of p = 0.05. The LOD and LOQ values were calculated as 0.56 and 1.70 ppm for piceatannol, and of 2.07 and 5.27 ppm for spilanthol, respectively.

Table 2.
Piceatannol and spilanthol content in the formulations and the corresponding amount applied onto the Strat-MTM membranes.
In accordance with the international standard for sunscreen application, which recommends the use of 2 mg of product per cm2 of skin, this same dosage was adopted for the Franz cell permeation assays. Considering the effective diffusion area of 1 cm2 per membrane, the resulting quantities of piceatannol and spilanthol applied in each assay were approximately 0.1 µg and 0.2 µg, respectively.
During Franz cell assays, it is critical to maintain sink conditions, where the analyte’s concentration in the receptor phase remains below one-third of its saturation solubility, to avoid back-diffusion and ensure accurate permeation assessment. Equally important is ensuring the chemical stability of the target compounds in the receptor fluid throughout the assay, as degradation could compromise the reliability of the results.
Receptor fluids are typically water- or buffer-based and may be supplemented with alcohols, proteins, or surfactants to enhance the solubility of poorly water-soluble compounds, provided they do not compromise membrane integrity [13,14]. In this work, since Pext and Jext exhibited low water solubility but high solubility in ethanol or hydroalcoholic solutions, their stabilities were evaluated in 30% or 50% EtOH, as both of which with had pior confirmation not to affect the structural integrity of the Strat-M™ membranes.
Initially, a 50% EtOH solution was considered as a starting point due to the previously known good solubility of both extracts. However, such a high organic content would not represent physiological conditions. Therefore, an attempt to reduce the organic proportion in the solution was considered, given that both extracts are poorly soluble in water. In this context, a receptor fluid with a 30% organic load was evaluated. The extract concentrations used in the experiments were set at 500 times the maximum theoretical permeation of piceatannol and spilanthol from the emulsions, allowing any insoluble particles to be more easily observed. As no visible particles were detected and clear solutions were obtained, it can be inferred that piceatannol and spilanthol from the formulations would permeate freely into the receptor chamber, thereby ensuring true sink conditions.
Nonetheless, compound stability over time is essential to ensure reliable analyses. Therefore, since the experiments were designed to last 180 min to mimic sunscreen reapplication recommendations, the stability of piceatannol and spilanthol in each receptor fluid was assessed over this period. Surprisingly, as shown in Figure 3, both piceatannol and spilanthol exhibited greater stability over 180 min in 30% EtOH compared to 50% EtOH. Under the same analytical conditions, piceatannol peak areas (245 > 135 m/z) in 30% EtOH were consistently higher with lower standard deviations, and spilanthol peak areas (222 > 123 m/z) were also more stable over 180 min, presenting less variation among the replicates. Therefore, 30% EtOH was chosen as the receptor fluid for the diffusion assays, providing both improved consistency and a lower organic modifier content.
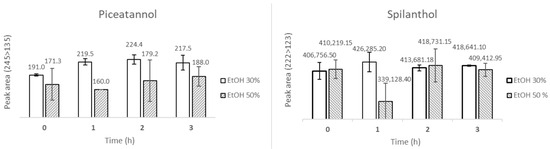
Figure 3.
Peak areas of piceatannol (245 > 135) and spilanthol (222 > 123) in 30% (white bars) and 50% EtOH (hatched bars) at 0, 1, 2 and 3 h, measured by HPLC-MS/MS. The assay conditions are described at Section 2.5.
Each Franz cell was then filled with 5 mL of 30% EtOH solution, and a Strat-M™ membrane loaded with 2 mg of the test formulation was secured between the donor and receptor chambers of each cell. The Strat-M™ membrane, a synthetic skin simulant, consists of dual polyether sulfone layers designed to replicate the structural properties of the epidermis and dermis. The membrane is supported by a polyolefin base that simulates subcutaneous tissue, resulting in a total thickness of approximately 300 µm [13].
Receptor fluid samples were collected over a 180 min period, and piceatannol was not detected in any of the fluids from experiments with formulations containing Pext. Based on this result alone, neither the presence of sunscreens nor the addition of Jext, which was intended to enhance piceatannol penetration to its dermal sites of action, appears to promote its passage into the bloodstream. This is desirable, as piceatannol’s intended effect is local, at the dermal level, rather than systemic.
It is important to highlight, however, that the presence of sunscreen agents, particularly Parsol MCX™, was noticed even in formulations lacking the extracts in the Oem formulation series (Figure 4A). The addition of Pext (Figure 4B) and the combination of Pext with Jext (Figure 4C) led to a relative increase (2–3 times) in Parsol MCX™ permeation through the Strat-M™ membrane from the proposed emulsions. Furthermore, Uvinul A Plus™ and Uvinul T150™ were also detected in the receptor fluid under these conditions. Data from the Oem-Jext assays, however, were inconclusive as sunscreen agents were detected in only one of the replicates, indicating an instability of this formulation under the test conditions (Figure 4D).
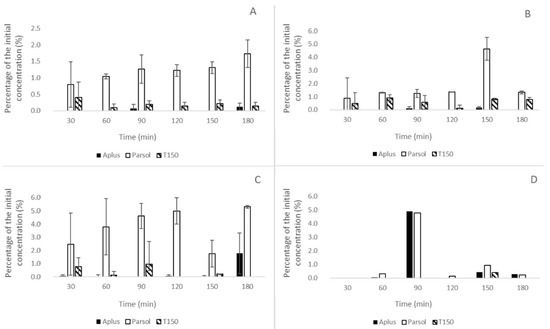
Figure 4.
Estimated percentage of the initial sunscreen concentration in receptor fluids over time during in vitro permeation assays using the Strat-M™ membrane. Formulations tested: (A) Oem (organic sunscreen emulsion only), (B) Oem-Pext (with passion fruit seed extract), (C) Oem-Pext-Jext (with passion fruit and paracress extracts), and (D) Oem-Jext (with paracress extract only). Bars represent mean values ± standard deviation for each compound: Uvinul A Plus™ (Aplus, black), Parsol MCX™ (Parsol, white), and Uvinul T150™ (T150, hatched). Results indicate variable permeation of the sunscreen agents, particularly increased permeation of Parsol MCX™ in the presence of botanical extracts.
A possible explanation for the predominance of Parsol MCX™ in the receptor chambers may be related to its more prone-to-permeation physicochemical properties in comparison with the other active compounds of the proposed formulations. Parsol MCX™ (MW = 290.4 Da; LogP = 5.3) is the smallest and least lipophilic of the three sunscreens evaluated. In contrast, Uvinul A Plus™ and T150™ have MWs and LogP values of 397.5 Da and 823.1 Da, and 6.93 and 15.5, respectively, which fall outside the ideal parameters for skin permeation. The molecular structures of these sunscreens (Figure 5) also feature larger resonance areas compared to Parsol MCX™, resulting in more rigid, planar structures with less flexibility to rearrange their conformations as they pass through the lipidic barrier of the Strat-M™ membrane [13,31]. Therefore, based on the in vitro findings, it is advisable to reformulate the Oem emulsions to limit the dermal penetration of Parsol MCX™. Alternatively, the use of other UV filters with lower skin permeability profiles may be considered.
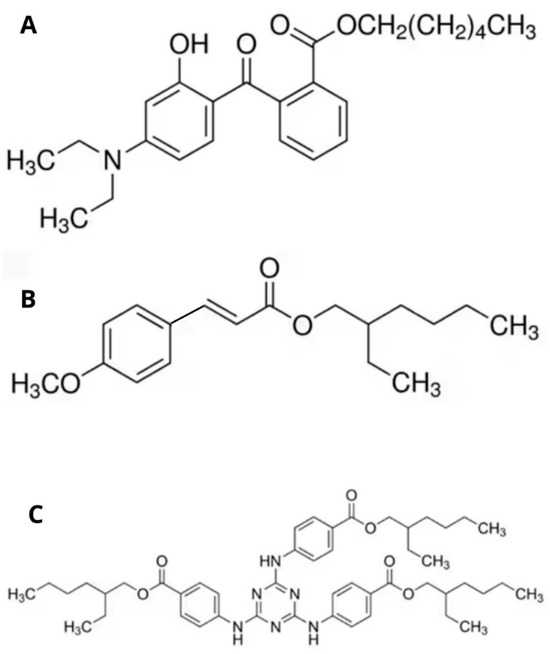
Figure 5.
Molecular structures of the organic UV filters used in the formulations. (A) Uvinul A Plus™ (Diethylamino hydroxybenzoyl hexyl benzoate): UVA filter that absorbs long-wave ultraviolet radiation (320–400 nm). (B) Parsol MCX™ (Ethylhexyl methoxycinnamate): UVB filter that absorbs short-wave UVB radiation (280–320 nm). (C) Uvinul T150™ (Ethylhexyl triazone): a photostable UVB filter with a peak absorption at 314 nm, offering strong protection in the 280–320 nm range.
Although piceatannol was not detected in the receptor fluid during the experiments, complete retention on the stratum corneum surface was also undesirable. Analysis of the cotton pads used to clean the membrane surfaces from experiments with Pext revealed residual piceatannol in the donor chamber, accompanied by some signs of degradation (Supplementary Materials Figure S2). The presence of non-permeated piceatannol was expected, as previous studies suggest limited diffusion in in vitro assays [12]. The sunscreens Uvinul A Plus™ and Parsol MCX™ were also detected in the cotton extracts from the Oem series, while Uvinul T150™ appeared sporadically. All three UV filters showed signs of degradation after 180 min of permeation. The same compounds were also detected in the membrane extracts of the Oem series, displaying indications of degradation as well. Piceatannol was also present in the membrane extracts of the Bem series, which shows that it could penetrate the skin to some extent even in the absence of Jext, but some sort of molecular protection would be required for a more stable formulation (Supplementary Materials Figure S3).
Concerning Jext, although it remained stable in the receptor fluid assay, spilanthol was not detected in the receptor fluids of the Bem and Oem formulations. The possible degradation of Jext at the membrane could explain the observed non-reproducibility of the Oem-Jext formulation results. However, there were indications that spilanthol may have been internalized into the membrane. The standard spilanthol chromatogram (Supplementary Materials Figure S1) presented four peaks, with the major peak attributed to spilanthol, based on its UV spectral peak at λmax = 229 nm. A small peak with a retention time of 1.8 min (λmax = 222.5 and 276.1 nm) was found in the receptor fluids of emulsions containing Jext, indicating its permeation through the Strat-MTM membrane. No spilanthol or Jext signal was detected in the cotton extracts, but spilanthol was identified in one of the membranes from the Bem-Pext-Jext emulsion, as a broad peak at TR = 1.852 min (Supplementary Materials Figure S3).
Regarding spilanthol’s stability, Grymel et al. [27] observed that spilanthol, when dissolved in dichloromethane or CDCl3 solutions and exposed to air, gradually disappeared, forming an oxidized product, especially in the presence of photosensitizing agents. Chlorophyll, for instance, is considered a photosensitizing agent, and the chlorophyll in Jext, coupled with the organic sunscreens in Oem, may have contributed to the depletion of spilanthol in this assay. These findings indicate that, if the inclusion of spilanthol is desired to harness its beneficial properties, strategies to protect it from degradation should be considered as well. Otherwise, alternative permeation enhancers may be explored for the formulation if needed.
Even though these instabilities were observed over a 180 min period, the membrane analysis via confocal Raman microscopy allowed for the visualization of the extent to which piceatannol diffused in each scenario.
The Raman spectra of the standard compounds are shown in Figure 6. Neither Bem nor spilanthol exhibited intense signals and their penetration through the membrane could not be observed in this experiment. The region between 1580 and 1700 cm−1 revealed Raman shift signals corresponding to piceatannol, Oem, and the membrane itself. The presence of piceatannol into the membranes was observed by searching for the characteristic signals at 1600.33 cm−1 (λemission = 581.51 nm) and 1632.4 cm−1 (λemission = 582.52 nm) which did not overlap with the signals of other formulation components.
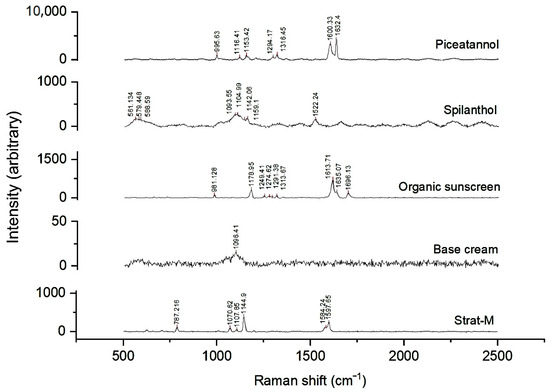
Figure 6.
Raman spectra of reference standards (piceatannol and spilanthol), formulation components (organic sunscreens and base cream), and the surface of the Strat-M™ membrane. Characteristic peaks are observed for each compound, with piceatannol displaying intense signals near 1600 cm−1, spilanthol showing distinct bands in the 1000–1600 cm−1 range, and the Strat-M™ membrane exhibiting its own fingerprint spectrum.
Scans of the membrane surfaces after a three-hour permeation assay with Pext containing emulsions revealed peaks corresponding to Strat-MTM. A piceatannol signal at 1600.33 cm−1 (λemission = 581.51 nm) was also found. The second piceatannol signal at 1632.4 cm−1 (λemission = 582.52 nm) was likely not observed due to the low piceatannol concentration and/or the presence of degradation products, extracts, and other formulation components which may have suppressed the signal.
In the depth analysis, piceatannol signal could be observed up to 140 µm from the Bem-Pext formulation as indicated by the presence of a 1600.33 cm−1 signal in this assay (Figure 7A,B). This depth corresponds to the dermal region in the Strat-M™ membrane, suggesting that, despite potential degradation, piceatannol would be able to reach a level sufficient to potentially influence fibroblast activity and collagen regulation. When Jext was added to the formulation, piceatannol observable signal reached up to 220 µm from the surface, demonstrating a synergistic effect on its diffusion (Figure 7C,D).
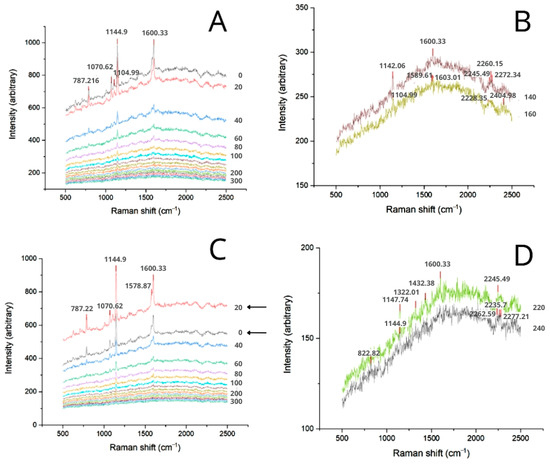
Figure 7.
Transversal Raman spectra of the membranes 180 min after Bem application. (A) In-depth spectra of Bem-Pext. (B) Bem-Pext spectra at 140 and 160 µm from the surface. (C) In-depth spectra of Bem-Pext-Jext. (D) Bem-Pext-Jext spectra at 220 and 240 µm from the surface.
Another significant observation in the spectral map of Figure 7C was the inversion of peak intensities between 0 (surface) and 20 µm from the surface. This suggests that piceatannol accumulated in deeper layers of the stratum corneum equivalent, rather than just being retained at the membrane surface. This inversion was also observed for Oem, indicating that formulations with higher lipophilic loads may facilitate the entry of piceatannol into the stratum corneum (Figure 8A,C). In these cases, the 1600.33 cm−1 signal was detected up to 180 µm and 220 µm for the formulations with and without Jext, respectively (Figure 8B,D). These results align with those obtained with Pext plus Jext in Bem and corroborate that components which exhibit lower polarities enhance piceatannol’s penetration through the skin layers.
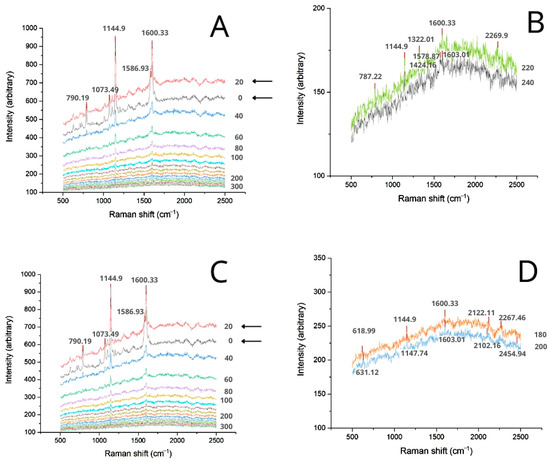
Figure 8.
Transversal Raman spectra of the membranes 180 min after Oem application. (A) In-depth spectra of Oem-Pext. (B) Oem-Pext spectra at 220 and 240 µm from the surface. (C) In-depth spectra of Oem-Pext-Jext. (D) Oem-Pext-Jext spectra at 180 and 200 µm from the surface.
In all cases, the diffusibility of the formulation components through the Strat-MTM membrane, as assessed in this study, was consistent with the in silico predictions from the Frasch [32], Potts and Guy [33], and modified Robinson [34] models, as determined by using the skin permeation calculator from the United States Centers for Disease Control and Prevention (CDC) [35]. Table 3 displays the permeation coefficients (kp) and log kp values for piceatannol, spilanthol, and Parsol MCXTM, the latter being the only sunscreen with a logP value within the acceptable range of the calculator. Regardless of the selected model, piceatannol exhibited the lowest diffusibility among the three compounds, followed by spilanthol, which had kp values approximately 10 times greater, and Parsol MCXTM, which had a kp value around 100 times higher than that of piceatannol. Therefore, the absence of piceatannol and the presence of Parsol MCXTM in the receptor fluid align with the predictions, although this behavior is undesirable in the context of safe and effective formulations.

Table 3.
Skin permeation parameters of Oem active ingredients and piceatannol analogues calculated by CDC Skin permeation calculator [35].
The observed diffusibility of piceatannol was consistent with theoretical predictions and aligned with what is expected for structurally related pharmacologically active stilbenes. Among them, resveratrol is the most extensively studied due to its wide range of biological, pharmacological, and clinical applications. Compared to piceatannol, the theoretical calculation for resveratrol predicts higher skin permeation capacity, likely owing to its lower molecular weight and the absence of one hydroxyl group. These features result in a higher logP value, which enhances its lipophilicity and thereby promoting more efficient diffusion across the skin barrier [1,12].
Oxyresveratrol, a positional isomer of piceatannol, is already incorporated into commercial skincare products, exhibits activity against post-inflammatory hyperpigmentation by inhibiting melanogenesis, blocking melanosome transfer, and offering photoprotective mechanisms that are comparable to those reported for piceatannol. Theoretical calculations (Table 3) suggest that shifting a hydroxyl group from the 3′ position (in piceatannol) to the 2′ position (in oxyresveratrol) slightly enhances its skin permeation, possibly by optimizing hydrogen bonding and molecular conformation [36].
Although the CDC skin permeation calculator provides reliable theoretical predictions for the diffusibility of isolated molecules based on their physicochemical properties, it is important to recognize that actual skin permeation is influenced by a variety of additional factors. While the intrinsic characteristics of the molecule, including reduced steric hindrance and higher molecular flexibility, do favor diffusion through the skin, external conditions also play a decisive role. Factors such as the anatomical origin and condition of the skin, the presence or absence of occlusion, and the physicochemical characteristics of the vehicle, including viscosity, solubility of the active compound in the formulation, and the degree of skin contact, can significantly alter the permeation profile. Therefore, while in silico tools offer valuable baseline estimations under idealized conditions, actual skin permeation in real-world formulations may vary depending on these critical formulation and application parameters [37].
In this sense, this study shows the first evidence that piceatannol is capable of achieving the dermis region of the skin when incorporated into an emulsion-based formulation, and that its skin diffusion is enhanced in the presence of lipophilic ingredients such as organic sunscreens and spilanthol, even though its physicochemical properties are less favorable to skin diffusion compared to other well-known stilbenes. These findings highlight the potential of piceatannol and piceatannol-rich extracts such as Pext to serve as novel active ingredients in suncare products, provided they are adequately protected from degradation through modulation of the emulsion components or by micro- or nanoencapsulation of Pext or isolated piceatannol.
Receptor fluid analyses also indicated that the extracts increased the permeation of organic sunscreens through the skin model, underscoring the importance of evaluating potential interactions between botanical extracts and UV filters during formulation development. In a safe and effective formulation, sunscreens should ideally remain on the skin’s surface, confined to the stratum corneum, as systemic absorption raises concerns regarding their potential endocrine-disrupting effects [38]. Therefore, skin permeation studies of novel formulations derived from those presented in this work are strongly recommended.
4. Conclusions
Despite the noticed degradation of the compounds during the tests, the results demonstrated that piceatannol was capable of reaching the dermis-equivalent region of the Strat-MTM membrane when incorporated into both the Bem and Oem formulations. In the Bem formulation, the addition of paracress extract (Jext) enhanced the depth at which piceatannol was detected in the membrane, compared to Pext alone. The organic sunscreens exhibited a similar effect, regardless of the presence of Jext, suggesting that a more lipophilic environment may facilitate piceatannol diffusion through the skin. Both the organic sunscreens and the paracress extract promoted higher concentrations of active ingredients within the membrane’s stratum corneum equivalent, instead of on the surface. Additionally, the organic sunscreens used in this study, particularly Parsol MCXTM, were able to permeate the Strat-MTM membrane to varying degrees. Notably, Parsol MCXTM, the smallest and most flexible sunscreen among those evaluated, exhibited the greatest permeation. The inclusion of the extracts in the Oem formulation increased the relative amount of Parsol MCXTM detected in the receptor fluids of the Franz cell assays. This finding should be carefully considered when formulating topically applied products which combine sunscreens with agents which must permeate the skin to exert their biological activities.
In conclusion, based on the observed instability and permeation limitations, several reformulation strategies can be considered to enhance the performance of Pext or piceatannol itself. As a more lipophilic environment may facilitate piceatannol diffusion through the skin and potentially improves its bioavailability at the target sites, modifying the emulsion system to favor lipophilic phases or incorporating suitable penetration enhancers can be strategic to formulate an effective product. In addition, addressing the compounds’ instability is crucial. Strategies such as adjusting the emulsion components to minimize degradation pathways, or employing micro- and nanoencapsulation techniques, may provide improved ingredient protection. These approaches could enhance the chemical stability and modify the skin permeation profile of the active compounds. Finally, if effectively stabilized through formulation adjustments or encapsulation strategies, both Pext and piceatannol hold promise for skincare applications with adequate skin diffusion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dermato5040019/s1. Figure S1: Chromatograms of the standards (left) and corresponding UV spectra of the peaks (right) are shown. The spilanthol peak is marked with a square, which also encompasses three smaller adjacent peaks. These minor peaks, located immediately to the left of the spilanthol signal, were also detected in the receptor fluid during the Franz cell permeation assay. (A) Chromatogram of the spilanthol standard at 30 µg·L−1 (229 nm). B. UV spectrum of the peak in A, showing λmax at 222.5 and 266.7 nm. (C) Chromatogram of the receptor fluid from the Bem-Pext-Jext sample after 30 min. (D) UV spectrum of the peak in (C), with λmax at 222.5 and 267.1 nm. Figure S2: Bem-Pext-Jext membrane extract (50% EtOH). (A) Chromatogram at 323 nm, piceatannol highlighted. (B) Spectrum corresponding to piceatannol peak. (C) Chromatogram at 229 nm, highlighting spilanthol. (D) spectrum corresponding to spilanthol peak. Figure S3: Chromatograms of Oem-Pext-Jext piece of cotton extract at 323 nm (A), at 229 nm (B), and peak spectra of piceatannol and its degradation products (C). Figure S4: Calibration curves for the quantification of piceatannol (top) and spilanthol (bottom) in the formulations. Table S1: Lack-of-fit analysis of variance (ANOVA) tables for piceatannol model. Table S2: Lack-of-fit analysis of variance (ANOVA) tables for spilanthol model.
Author Contributions
G.C.d.S.: Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Writing—original draft; R.A.F.R.: Supervision, Writing—review & editing; C.B.G.B.: Funding acquisition, Project administration, Resources, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Science and Technology (INCTs) [grant numbers: FAPESP/INCT 2014/50867-3, CNPq 465389/2014-7]. C. B. G. Bottoli acknowledges CNPq for a research fellowship [CNPq 313185/2021-2].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge LabFarQui, of the State University of Campinas Institute of Chemistry (IQ-Unicamp), for performing the Waters Acquity UPLC system experiments, and the National Nanotechnology Laboratory—(LNNANO,) of the National Center for Research in Energy and Materials—(CNPEM,) for the confocal Raman microscopy assays.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849. [Google Scholar] [CrossRef]
- Cordova-Gomez, M.; Galano, A.; Alvarez-Idaboy, J.R. Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 2013, 3, 20209–20218. [Google Scholar] [CrossRef]
- Krambeck, K.; Santos, D.; Sousa Lobo, J.M.; Amaral, M.H. Benefits of skin application of piceatannol—A minireview. Australas. J. Dermatol. 2023, 64, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.A.R.; Xavier, J.A.; da Silva, F.C.; Jose Merlin, J.P.; Goulart, M.O.F.; Vasantha Rupasinghe, H.P. Antidiabetic, Antiglycation, and Antioxidant Activities of Ethanolic Seed Extract of Passiflora edulis and Piceatannol In Vitro. Molecules 2022, 27, 4064. [Google Scholar] [CrossRef] [PubMed]
- Maruki-Uchida, H.; Morita, M.; Yonei, Y.; Sai, M. Effect of Passion Fruit Seed Extract Rich in Piceatannol on the Skin of Women: A Randomized, Placebo-Controlled, Double-Blind Trial. J. Nutr. Sci. Vitaminol. 2018, 64, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lim, J.X.; Yeo, S.C.M.; Xiang, X.; Tan, K.S.; Fu, J.H.; Huang, L.; Lin, H.S. Biotransformation of Piceatannol, a Dietary Resveratrol Derivative: Promises to Human Health. Mol. Nutr. Food Res. 2020, 64, e1900905. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of stilbenoids by human faecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef]
- Eskandari, M.; Rembiesa, J.; Startaitė, L.; Holefors, A.; Valančiūtė, A.; Faridbod, F.; Ganjali, M.R.; Engblom, J.; Ruzgas, T. Polyphenol-hydrogen peroxide reactions in skin: In vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta 2019, 1075, 91–97. [Google Scholar] [CrossRef]
- Hung, C.-F.; Lin, Y.-K.; Huang, Z.-R.; Fang, J.-Y. Delivery of Resveratrol, a Red Wine Polyphenol, from Solutions and Hydrogels in the Skin. Biol. Pharm. Bull. 2008, 31, 955–962. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Rodrigues Leite-Silva, V.; Mandelli De Almeida, M.; Fradin, A.; Grice, J.E.; Roberts, M.S. Delivery of drugs applied topically to the skin. Expert Rev. Dermatol. 2014, 7, 383–397. [Google Scholar] [CrossRef]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, I.R.; Huang, S.J.; Hu, C.Y.; Han, S.Y. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.F.; de Carvalho, M.G.; Smith, R.E.; Sabaa-Srur, A.U.O. Spilanthol: Occurrence, extraction, chemistry and biological activities. Braz. J. Pharmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and controversies in studying sunscreen delivery and toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86. [Google Scholar] [CrossRef]
- ABIHPEC. A Indústria de Higiene Pessoal, Perfumaria e Cosméticos; Essencial para o Brasil FEV; ABIHPEC: São Paulo, Brazil, 2023. [Google Scholar]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- How to Apply Sunscreen. Available online: https://www.aad.org/public/everyday-care/sun-protection/shade-clothing-sunscreen/how-to-apply-sunscreen (accessed on 1 December 2024).
- Diffey, B.L. When should sunscreen be reapplied? J. Am. Acad. Dermatol. 2001, 45, 882–885. [Google Scholar] [CrossRef]
- Formulações Cosméticas: Tendências, Desafios e Inovação. Available online: https://www.talkscience.com.br/industria-cosmetica/formulacoes-cosmeticas-tendencias-desafios-e-inovacao (accessed on 1 December 2024).
- FDA. Sunscreen: How to Help Protect Your Skin from the Sun. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun (accessed on 1 December 2024).
- Silva, G.C.; Rodrigues, R.A.F.; Bottoli, C.B.G. Passion fruit seed extract enriched in piceatannol obtained by microwave-assisted extraction. Sustain. Chem. Pharm. 2021, 22, 100472. [Google Scholar] [CrossRef]
- Freitas-Blanco, V.S.; Franz-Montan, M.; Groppo, F.C.; De Carvalho, J.E.; Figueira, G.M.; Serpe, L.; Sousa, I.M.O.; Damasio, V.A.G.; Yamane, L.T.; Paula, E.; et al. Development and Evaluation of a Novel Mucoadhesive Film Containing Acmella oleracea Extract for Oral Mucosa Topical Anesthesia. PLoS ONE 2016, 11, e0162850. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Mazurkiewicz, R.; Bajkacz, S.; Bilik, J.; Kowalczyk, S. Extraction, Purification, Quantification, and Stability of Bioactive Spilanthol from Acmella oleracea. Planta Medica 2023, 89, 551–560. [Google Scholar] [CrossRef]
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef]
- Parhi, R.; Mandru, A. Enhancement of skin permeability with thermal ablation techniques: Concept to commercial products. Drug Deliv. Transl. Res. 2020, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, B.; Tutone, M.; Hoffman, E.; Hutter, V.; Almerico, A.M.; Traynor, M. Predicting Skin Permeability by Means of Computational Approaches: Reliability and Caveats in Pharmaceutical Studies. J. Chem. Inf. Model. 2019, 59, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef]
- Frasch, H.F. A random walk model of skin permeation. Risk Anal. 2002, 22, 265–276. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Wilschut, A.; ten Berge, W.F.; Robinson, P.J.; McKone, T.E. Estimating skin permeation. The validation of five mathematical skin permeation models. Chemosphere 1995, 30, 1275–1296. [Google Scholar] [CrossRef]
- NIOSH; CDC. Skin Permeation Calculator. Available online: https://www.cdc.gov/niosh/topics/skin/skinpermcalc.html (accessed on 1 July 2023).
- Wang, H.; Chen, X.; Li, J.; Chen, Z.; Zhou, A.; Ye, L. Oxyresveratrol from mulberry (Morus alba L.) ameliorates post-inflammatory hyperpigmentation in vitro by anti-melanogenesis, inhibiting melanosome transfer, and providing photoprotection. J. Funct. Foods 2024, 122, 106557. [Google Scholar] [CrossRef]
- Silva, G.C.; Rodrigues, R.A.F.; Bottoli, C.B.G. In vitro diffusion of plant phenolics through the skin: A review update. Int. J. Cosmet. Sci. 2024, 46, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Just-Sarobé, M. Sunscreens and Their Impact on Human Health and the Environment: A Review. Int. J. Dermatol. 2025, 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).