Safety Concern and Regulatory Status of Chemicals Used in Cosmetics and Personal Care Products
Abstract
1. Introduction
1.1. Range of Personal Care or Topical Products
1.2. Market Size and Growth
1.3. Harmful Chemicals Present in the Products
2. Major Health Concerns
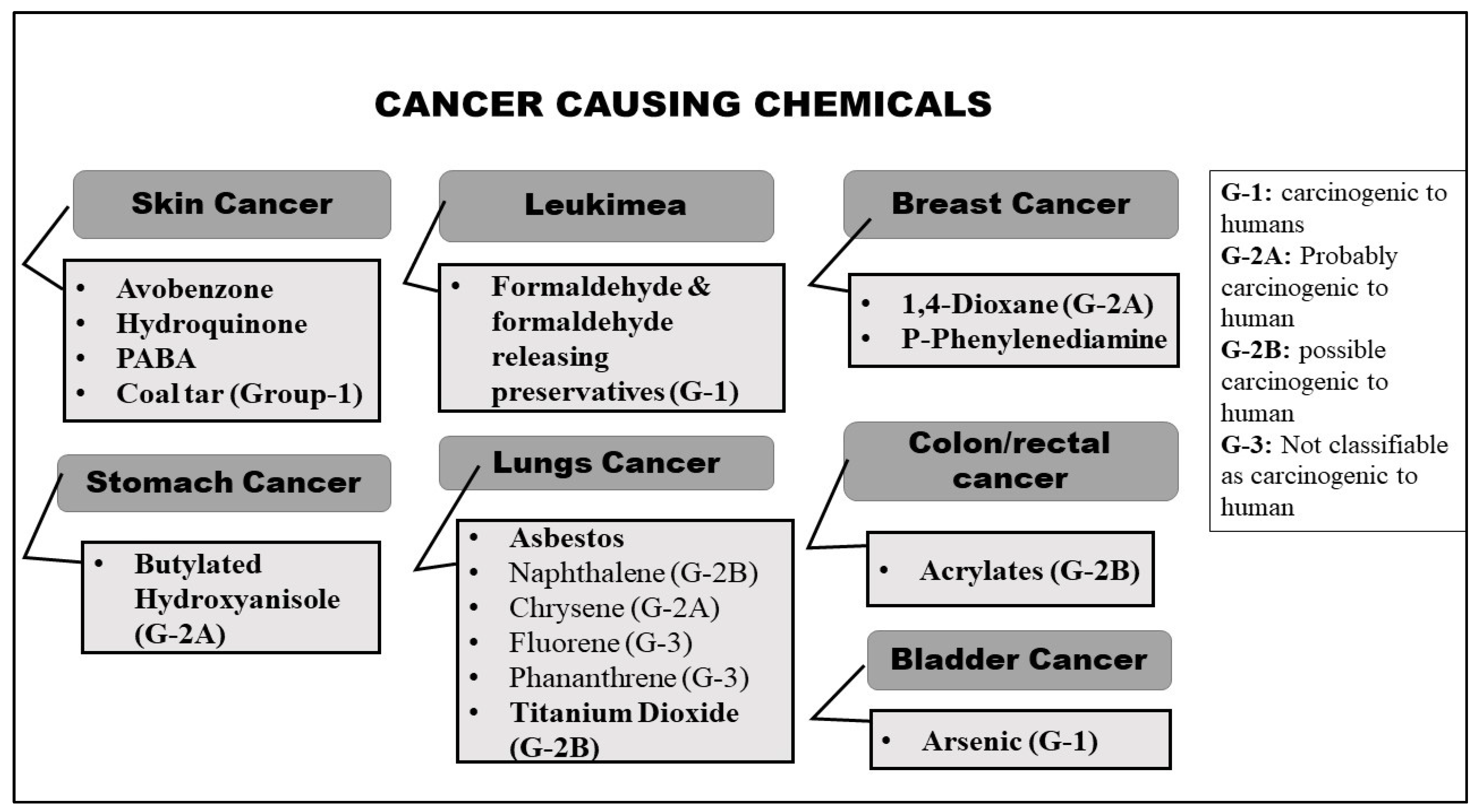
2.1. Cancer
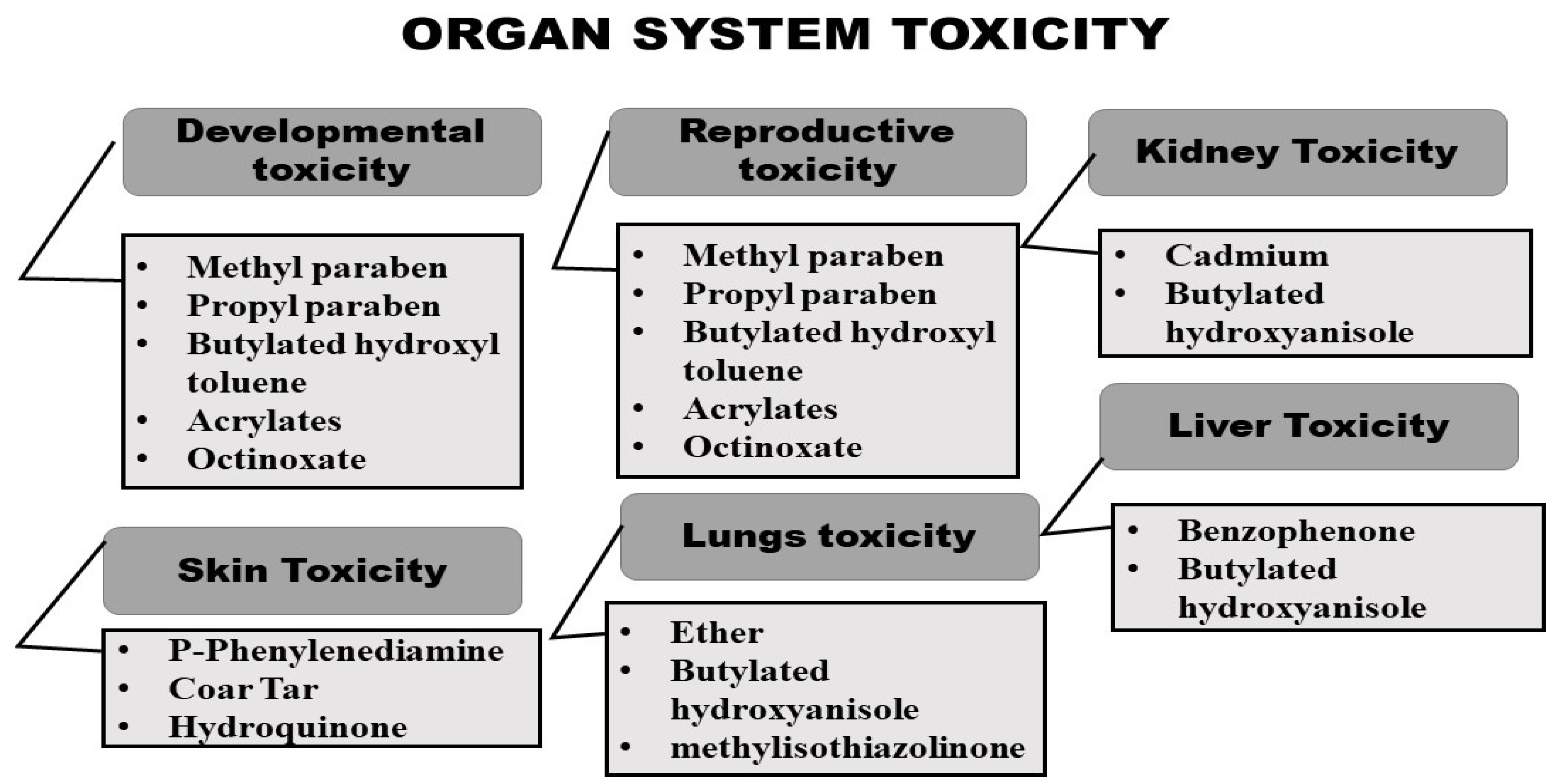
2.2. Organ System Toxicity
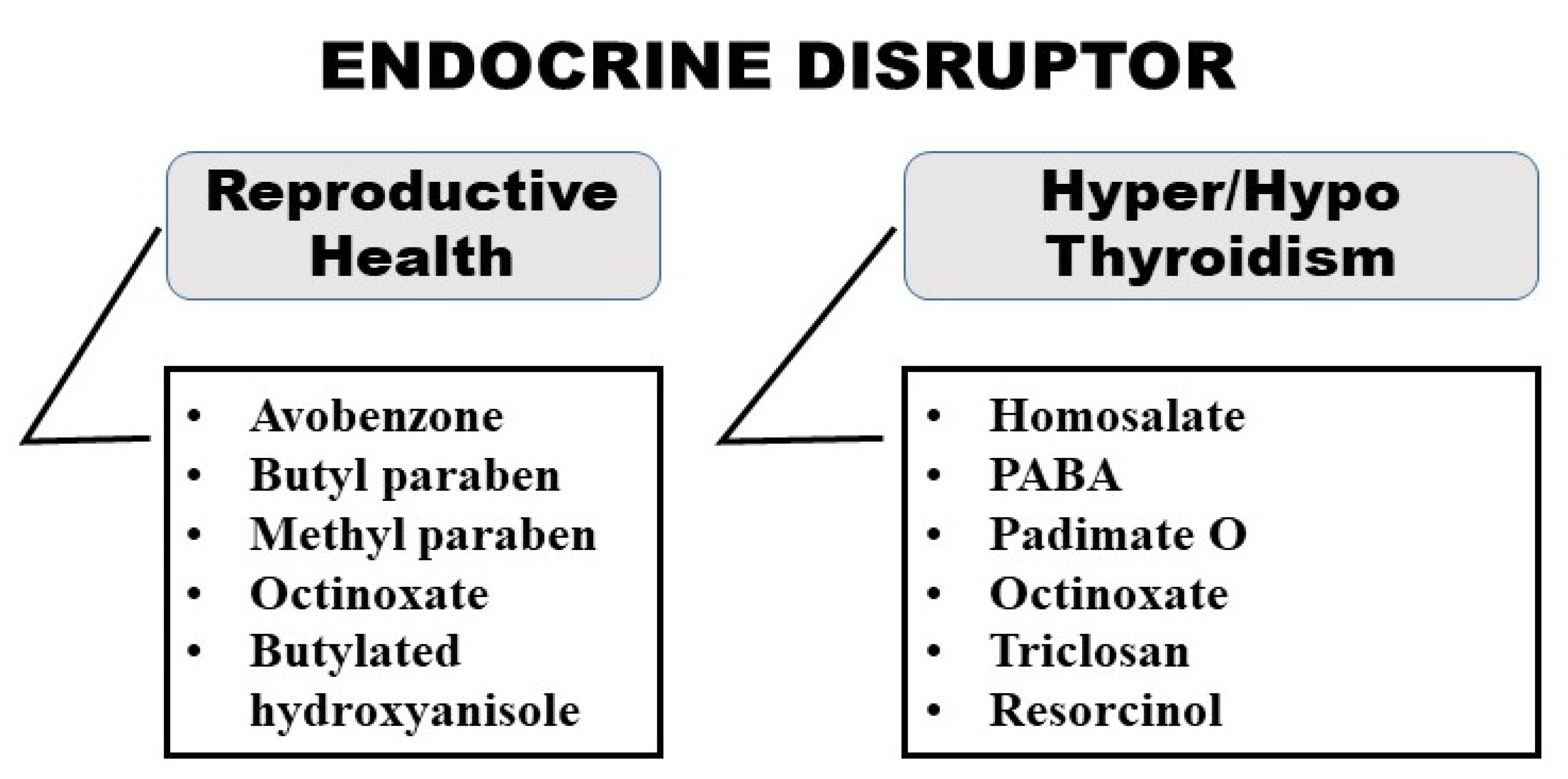
2.3. Endocrine Disruption
2.4. Allergies, Irritation, and Asthma
2.5. Other (Cellular and Neurological Damage, Ochronosis, Sensitization)
3. Regulatory Issues
4. Safety-Based Global Clinical Trials of Personal Care Products
| S.No. | Category and Name of the Ingredients, Types of Cosmetic Products and Functions | The Amount Used in PCP, Other Pharmaceutical Products and Major Health Concerns | References |
|---|---|---|---|
| 1. | Acrylates: Ethyl acrylate, ethyl methacrylate and methyl methacrylate Used in: Artificial nail products Functions: Acrylic nails, nail modifying polish | The amount used in PCP: No regulation prohibits the use of 100% concentration of acrylates in cosmetic products, but acrylates in cosmetics are banned in some states of the USA Major health concerns: Cancer, toxic effect on various organs, cellular damage, and neurological disruption. Developmental and reproductive, toxicity, cellular and neurological injuries Regulatory Status:
| [46] |
| 2. | Aromatic amine: Diazolidinyl urea, p-phenylenediamine Used in: Permanent hair dyes Function: Hair colorant | The amount used in PCP: 0.5% w/w (US) 6% w/w in hair dyes Major health concerns: Skin sensitization, cancer, mutagenicity, and organ system toxicity | [47,48,49] |
| 3. | Asbestos: Chrysotile, Amosite, crocidolite, tremolite, actinolite and anthophylite Used in: Baby powder, lotion, foundation | The amount limit: There is no federal law in the USA requiring Talscum to be asbestos free Major health concerns: Cancer, irritation, organ system toxicity | [50] |
| 4. | Benzophenone: Oxybenzone, avobenzone, and di-oxybenzone. Used in: Sunscreen, hair spray Function: UV-A rays protection | The amount used in PCPs: 3–6% w/w Major health concerns: Bioaccumulative, Heptotoxic, Endocrine disruptor, increase risk of skin cancer, irritation and sensitization Regulatory Status:
| [51,52,53] |
| 5. | Butylated Compounds: Butylatedhydroxyanisole Used in: Hair products, make up, sunscreen | The amount used in PCP: 0.1% w/w in cream, 0.1% w/w in gel, 0.1% w/w in shampoo, 0.1% w/w in spray Amount used in other pharmaceutical products: Buccal Gum—0.21 mg, Nasal Spray—0.1 mg, Oral Capsule—0.4 mg, Oral Tablet—0.81 mg. Major health concern: Organ-system toxicity and irritation | [54] |
| 6. | Coal tar Used in: Shampoos, soaps, hair dyes, lotion Function: Control psoriasis, dandruff | The amount used in PCP: 0.5% to 5% (US) Major health concerns: Cancer, organ toxicity, bioaccumulation | [55] |
| 7. | Ethanolamine: Diethanolamine and Triethanolamine Used in: Soaps and hair products Eye makeup Function: Emulsifier, pH adjuster | The amount used in PCP: ≤5% in products applied for longer time (US) and ≤2.5% in non-rinse off products(EU) Major health concern: Carcinogenic/hepatocarcinogenic due to formation of nitrosamine on contamination | [56,57] |
| 8. | Ether alcohol: Phenoxyethanol Used in: Moisturizer, sunscreen, lotion, shampoo Function: Preservative and stabilizer | The amount used in PCP: 1.5% w/w in aerosol, 1% w/w in cream, 0.7% w/w in gel, 1% w/w in lotion Major health concerns: Allergies, nervous system effects (infants) | [58,59] |
| 9. | Ether: 1.4-dioxane Used in: Hair products Function: Impurity | The amount use in PCP: There is no maximum potency as it is a by-product of the chemicals used Major health concern: Breast cancer, organ toxicity, irritation | [60] |
| 10. | Formaldehyde and formaldehyde-releasing preservatives (imidazolidinyl Urea) [59] Used in: Nail polish, hair gel, nail adhesive, shampoo for babies Function: Preservatives | The amount used in PCP: 0.6% w/w (EU) and 0.28%w/w Amount used in other pharmaceutical products: Intramuscular injection—3 mg, intravenous injection—3 mg, subcutaneous injection—3 mg Major health concerns: Human carcinogen Regulatory Status:
| [59,61] |
| 11. | Heavy metals: Lead, arsenic, mercury, chromium, and cadmium Used in: Colorant Function: Color additive, impurity | Amount limit: 10 ppm are allowed as impurity and 20 ppm in color additive for lead 3 ppm for arsenic 1 ppm for mercury 50 ppm for chromium 3 ppm (impurity), 15 ppm as color additive for cadmium. Major health concerns: Neurotoxin, developmental and reproductive toxicity. Cancer, mercury poisoning Respiratory and carcinogenic effects Toxic effects on kidneys and skeletal and respiratory system | [62,63,64] |
| 12. | Isomeric benzenediol: Resorcinol Used in: Hair dyes, shampoo, acne products Function: preservative | The amount used in PCP: 2% when combined with sulfur The amount used in other pharmaceutical products: Anorectal drug—1–3% Major health concerns: Endocrine Disruptor, Organ system, toxicity, irritant and sensitizer | [65,66,67] |
| 13. | Isothiazolinones: Methylisothiazolinone (MI) Methylchloro-isothiaz-olinone (CMIT) Used in: Hair color/cleaner, sunscreen, makeup remover Body wash, lotion, mascara, detergents Function: Preservatives | The amount used: 100 ppm for rinse off product 15 ppm for rinse off product 0.05% w/w in cream Major health concerns: Toxicity/neurotoxicity and allergies | [68,69,70] |
| 14. | Octinoxate: Octylmethoxycinnamate Used in: Shampoo, sunscreen, lipstick Function: UV filter | The amount used: 7.5% w/w in sunscreen and lip balm Major health concerns: Endocrine disruptor, reproductive organs, and development toxicity | [52,71] |
| 15. | PABA (Para amino benzoic acid) and its derivatives): Padimate O Used in: Suncreen Function: Absorb UV-B radiation | The amount used in PCP: In 2019 FDA restricted use of PABA in OTC sunscreen; prohibited in the cosmetic products (EU). 7% w/w in sunscreen The amount used in other pharmaceutical products: 300 to 400 mg daily for the treatment of Peyronie’s disease Major health concerns: Endocrine disruptor, sensitization (increased risk of skin cancer) | [72,73,74] |
| 16. | Parabens: Butylparaben, Methylparaben and Propylparaben Used in: Shampoos and conditioners Function: Preservatives | The amount used in PCP: 0.4%w/w cream, 0.15% w/w lotion, 0.18% w/w ointment 0.16% w/w in aerosol, 0.5% w/w in cream, 0.15% w/w in shampoos 5.25% w/w in cream, 0.2% w/w in lotion, 0.03% w/w in shampoo The amount used in other pharmaceutical products: Ophthalmic Solution—0.02% w/w, oral suspension—8 mg/5 mL, oral tablet coated—0.08 mg Auricular formulation (0.01% w/w), Buccal Film (1 mg), and Nasal Drops (26 mg) Oral syrups (100 mg), tablet (0.2% mg), and soft tissue injection (0.01% w/w) Major health concerns: Endocrine disruptors, developmental and reproductive toxicity | [59,75] |
| 17. | Phenol: Hydroquinone Used in: Skin lightener, facial moisturizer Function: Decreases melanine, make skin appear whiter | The amount used in PCP: 2% w/w OTC, higher in prescription only products The amount used in other pharmaceutical products: Vaginal cream—0.02% w/w Major health concerns: Increase risk of skin cancer, ochronosis | [76,77] |
| 18. | Polyacrylic aromatic hydrocarbons: naphthalene, chrysene, fluorene, anthracene, phenanthrene and acenaphthene Used in: Lotions, cosmetics Function: Impurity in petrolatum a moisturizing agent | The amount used: 5 ppm (US), petrolatum should be free of any carcinogenic impurities (EU) and 500 ppm as color additives (US) Major health concern: Cancer | [78,79] |
| 19. | Quaternary ammonium salts: Quaternium-15 Used in: Hair conditioners, cream, lotions, shaving products Function: Preservative | The amount used in PCP: 0.2% in beauty products (EU), there is no regulation on quaternion-15 in cosmetic products (US) The amount used in other pharmaceutical products: 0.02% w/w in cream and 0.1% w/w in cream, augmented (US) Major health concerns: Irritation, sensitization | [80] |
| 20. | Salicylate: homosalate Used in: Sunscreen, Sun protection Function: Convert UV radiation into Infrared radiation | Amount used: 15% w/w in sunscreen Major health concern: Endocrine disruptor, impact hormones | [52,81] |
| 21. | Titanium dioxide Used in: Sunscreen, makeup powder Function: UV filter, colorant | The amount used in PCP: 25% in sunscreen (EU, US) Amount limit in food: 1% of the weight of the food as colorant Major health concern: Carcinogen | [82,83] |
| 22. | Triclosan Used in: Antibacterial soaps, toothpaste, antiperspirant Function: Antibacterial | Amount use: Banned for OTC Major health concern: Endocrine disruptor, bioaccumulative | [84,85] |
| S. No. | Title | Status | Invention | Proof of Concept/Evaluation Parameters | Location | NCT Number |
|---|---|---|---|---|---|---|
| (a) | ||||||
| 1. | Systemic Absorption evaluation of Sunscreen components [86] | Completed (Phase 1) | Products (part-1): Cream, lotion, and spray Products (Part-2): Lotion, aerosol spray, non-aerosol spray, and pump spray Excipients (part-1): Avobenzone, octocrylene, ecamsule Excipients (part-2): Avobenzone, oxybenzone, octocrylene | Concentration optimization of active drugs | Spaulding Clinical Research, West Bend, WI, USA | NCT03582215 |
| 2. | Safety assessment of a Sunscreen C-Spray on Sport persons [87] | Completed | Coded products | Assessment of erythema as graded on a 5 point scale. | Saint Petersburg, FL, USA | NCT02857478 |
| 3. | Safety assessment of Sunscreen Product [88] | Completed | Coded products | Dermatologist’s subjective and objective assessments of potential Adverse events. | Saint Petersburg, FL, USA | NCT02803320 |
| 4. | Irritation studies of Sunscreen Products in Human Eyes [89] | Completed | Coded products |
| Saint Petersburg, FL, USA | NCT02854137 |
| 5. | Phototoxicity study of Sunscreen Products [90] | Completed | Coded products and Drug: Sodium chloride |
| Pinellas Park, FL, USA | NCT02802930 |
| 6. | Sun Protection Factor (SPF) analysis of Defense Skin Cream [91] | Completed | Physiogel Daily Defense Protective Day Cream Light | Skin pH, transepidermal water loss, intercellular lipid lamellae length are measured. | Pro-derm Institute for Applied Dermatological Research, Schenefeld, Germany | NCT03136107 |
| 7. | Analysis of sun protective product according to sunscreen formulas (Study SR09-15) (P08236) (COMPLETED) (PFA and SPF) [92] | Active, not recruiting | Group discussions | Frequency of sunscreen preference differences between ideal and willingness to pay | Northwestern University, Chicago, IL, USA | NCT01001975 |
| 8. | Repeat insult patch study of Daily Facial Moisturizer and Sunscreen with SPF 50+ (Cetaphil®) on human [93] | Completed | ISO 24444:2010 P3 Standard Sunscreen | Arithmetic mean of individual sun protection factor (SPFi) value are calculated. | GlaxoSmithKline (GSK) Investigational Site, Schenefeld, Schleswig-Holstein, Germany | NCT01892657 |
| 9. | Evaluate the sun protection factor of sunscreen products [94] | Completed | Based of SPF used in three sunscreen lipcare products | Minimum effective concentration has analysed for protected and unprotected sites. | GSK investigational sites, Winston-Salem, North Carolina, USA | NCT05085327 |
| 10. | Assay of SPF and UVA Protection Factor (UVAPF) [95] | Completed | Fresh coconut oil, jojoba oil, almond oil, and ointment of white petrolatum | Product satisfaction of the following test articles A, B, and C: Lindi Skin Soothing Balm (Product A), Lindi Skin Face Serum (Product B), and Lindi Skin Face Wash (Product C). | Northwestern University Department of Dermatology, Chicago, IL, USA | NCT02872246 |
| 11. | Sun Protection Factor (SPF) Efficacy Assay [96] | Completed | Lindi skin care products | Evaluation of water resistant SPF | Union, NJ, USA | NCT02885805 |
| 12. | Clinical study of general skin care product for analyze their effect on the Structural Strength of the Skin [97] | Completed (Phase 3) | Survey |
| No Contacts or Locations Provided | NCT03625167 |
| 13. | Clinical assessment of sunscreen products on adult [98] | Completed | Coded products and SPF 15 Control |
| Union, NJ, USA | NCT02877511 |
| 14. | Clinical assessment of sunscreen products on women [99] | Completed | Coded products and SPF 15 Control | Skin response to sun exposure according to the Skin Evaluation Response Scale from 0 to 3 | Saint Petersburg, FL, USA | NCT02779270 |
| (b) | ||||||
| 1. | An evaluation of tolerability study of three wash products in infants [100] | Completed | Test shampoo, Test bath foam and Test head to toe wash | Observation of baseline in Corneometer Values for 8 hrs. | GSK Investigational Site, Valinhos, Brazil | NCT02403999 |
| 2. | Determination of Tolerability of cleanser and moisturizing product with SPF 30 on kids [101] | Completed | Coded products and Standard cleanser soap | Frequency of combined dermatologist score and ophthalmologist score | GSK Investigational Site, Campinas, São Paulo, Brazil | NCT01909713 |
| 3. | Efficacy of Baby Talcum in Prevention of Pruritus Associated With Cast [102] | Recruiting | Treatment with petrolatum | Schirmer test is performed. | ErolOlcokCorumWEducatin and Research Hospital, Corum, Turkey | NCT01017315 |
| 4. | UK Baby Study using a baby products (body wash and lotion regimen) [103] | Completed | Coded products | Skin response to sun exposure according to the skin evaluation response scale from 0 to 3. | Saint Petersburg, FL, USA | NCT03142984 |
| 5. | An in-vivo study of skin hydration effect using bath product and moisturizers on human skin in atopic dermatitis [104] | Active, not recruiting | Coded products |
| Saint Mary’s Hospital, Manchester, Machester, UK) | NCT02028546 |
| (c) | ||||||
| 1. | Irritation studies of skin care Product on human [105] | Completed | Drug: Coppertone and Coded product. | Number of subjects showing growth in papules, erythema, dryness, telangiectasia, tactile exterior roughness, and irritation. | The Education & Research Foundation, Inc. Lynchburg, VA, USA | NCT03841032 |
| 2. | Clinical Study of cosmetic products for the assessment of efficacy and safety for facial line treatment [106] | Completed | Combination Product: Facial cleanser Combination Product: Facial moisturizer Combination Product: Sunscreen |
| Ablon Skin Institute Research Center Manhattan Beach, CA, USA | NCT04545970 |
| 3. | Clinical Study of cosmetic products for the assessment of efficacy and safety for Arm Firming [107] | Completed | Topical Body Firming Moisturizer Other: Body Cleanser Other: Sunscreen Other: Placebo Moisturizer | Change from baseline in skin creepiness score, elasticity score, firmness score, sagging score, skin roughness (visual and tactile), overall photo damage score, evenness of skin tone score, evenness of skin redness score and in objective erythema parameters. | Stephens and Associates Richardson, TX, USA | NCT04065035 |
| 4. | A Clinical Study to analyze the Dermal and Ocular Tolerance of cosmetic Facial Serum (Healthy Females With Sensitive Skin) [108] | Completed | Developmental Serum and Physiogel Calming Relief Anti-Redness Serum | Eczema Area Severity Index (EASI) is used. | Stephens & Associates, Inc Richardson, TX, USA | NCT03719742 |
| 5. | A Clinical Study (Controlled) of two different dry skin care moisturizing products [109] | Completed | Coded products | Number of participants with a unit difference of greater than 1 in Symptoms and signs of cutaneous irritation total result from baseline to 21 days of article use | GSK Investigational Site Schenefeld, Schleswig-Holstein, Germany | NCT03640832 |
| 6. | Local cutaneous and ocular tolerance study for assessment of three developmental facial skin-care products [110] | Completed | Serum, Lotion and Cream | Average difference from baseline for 42 days observation in clinical grading of skin dryness | Thomas J. Stephens & Associates, Inc. Colorado Springs, CO, USA | NCT04510103 |
| 7. | Characterization of a moisturizing cream on human with blemish prone skin [111] | Recruiting | Washout/Standard Cleanser, Test product and Positive control | Local cutaneous tolerance assessment of participants for irritation determined by a dermatologist | Schenefeld, Schleswig-Holstei, Germany | NCT03093181 |
| 8. | Tolerance study of Cosmetic Product on facial acne for one year regular follow-up [112] | Completed | Coded products | Tolerability assessment of test products | GSK Investigational Site, Edinburgh, UK | NCT04301063 |
| 9. | Analysis of Efficacy and Barrier Protection of Two Cosmetic Products [113] | Active, not recruiting | Coded products |
| France-Centre de SantéSabouraud—Hôpital Saint Louis, Paris, France; Cabinet Médical, Sèvres, France; Skin Research Centre, Toulouse, France | NCT03629405 |
| 10. | Clinical trial on characterization of the de-pigmentation activity of a cosmetics on pigmented human skin [114] | Completed (Phase 4) | Emulsion and Gel | Surface area of erythema and increased responses of skin while using product | DermIng SRL, Monza, Italy | NCT02204436 |
| 11. | Clinical study of anti-ageing Facial Gel [115] | Completed (Phase 3) | Facial Moisturizer with SPF 50+ | Skin spots image analysis: change from baseline | DermIng SRL, Monza, Italy | NCT01948531 |
| 12. | Cosmetic Dermatology Study [116] | Completed (Phase 4) | Emulsion and gel | Wrinkles profilometry (Ra—micrometers) test is done. | Derming S.r.l. Single Member Company, Monza, MB, Italy | NCT02200471 |
| 13. | Clinical study of cosmetic product to improve the appearance of human skin affliction with low to optimum atopic dermatitis. [117] | Completed | Gynomunal® gel | Patient perception of the use of the device and physician perception of the use of the device | Dermatology Cosmetic Laser Medical Associates of La Jolla, Inc., La Jolla, CA, USA | NCT03268174 |
| 14. | Cosmetics and Pregnancy (PERICOS) [118] | Completed | Device: VeinViewer | Determination of cosmetics by the physical examination on volunteers | Medical Dermatology Associates of Chicago, Chicago, IL, USA | NCT03283189 |
| 15. | Effect of Eye Make up on Ocular Surface [119] | Completed | Sunscreen and Standard SPF 4 Sunscreen | Outcome Measures- (1) Blistering time on day 28 (buffer time of 2 days) and at Day 56 (buffer time of 2 days) (2) Difference from baseline in epidermal thickness, hydration, and difference starting from baseline in stratum corneum hydration (SCH), observed in arbitrary units (AU) | Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, ChariteUnive Berlin, Germany | NCT04478955 |
| 16. | Epicutaneous Testing of Cosmetics [120] | Unknown (Phase 3) | Diagnostic Test: Schirmer test | Pruritus score, satisfactory score, complication rate and number of antihistamine drugs used are recorded and measured. | Prince of Songkla University, Hatyai, Songkhla, Thailand | NCT03024671 |
| 17. | Epidermal Delivery of Ani-Aging Ingredients [121] | Recruiting | Baby talcum | Number of patients with positive patch test reactions to cosmetics | Inselspital Bern, Bern, Switzerland | NCT01847066 |
| 18. | Determination of whitening cosmetic product for the lightening effect [122] | Unknown | Patch test application | Clinical photographs to determine improvement of appearance | NY Derm LLC, New York City, NY, USA | NCT01249469 |
| 19. | Determination of skin irritation study/Allergic Sensitivity by skin patch test intended to applied natural personal-care products [123] | Unknown | Device: Erbium 2940 plus cosmetics plus Impact Device: Erbium 2940 plus cosmetics | The average decrease of darkness from baseline in target surface after treatment is observed and noted. | National Taiwan University Hospital, Taipei, Taiwan | NCT01816542 |
| 20. | Ocular stinging potential study of shampoo components on human [124] | Unknown | Sunscreen Agents | Eye irritation was assessed by tearing/inflammation/discomfort/post installation effect on eye | Department of Dermatology, Hadassah University Hospital, Jerusalem, Israel | NCT02869113 |
| 21. | Facial Cosmetic Acupuncture on Skin Rejuvenation [125] | Completed | patch tests on healthy skin | Subjective discomfort in the eye, tearing/lacrimation, objective inflammation, post installation eye effects were measured by an ophthalmologist. | Saint Petersburg, FL, USA | NCT01486303 |
| 22. | Hair Care Product Use Among Women of Color [126] | Unknown | Coded products and Standard shampoo mixture (Control) | Criteria for evaluating Moire’s topography are used. | Kyung Hee University Hospital at Gangdong, Seoul, Korea | NCT04493892 |
| 23. | Human Photoallergy Test [127] | Recruiting | Procedure: Facial Cosmetic Acupuncture | Change in internal dose of urinary phthalate metabolites is measured. | Community Engagement Core CommunitySpace, NY, USA | NCT02750449 |
| 24. | Clinical trial of relative effect of single nucleotide polymorphisms (SNP) to Skin Care Product [128] | Completed | Behavioral: Educational Intervention | Potency of skin interaction is measured by 5 point grading scale | Piscataway, NJ, USA | NCT03446079 |
| 25. | Impact of Exposure to Cosmetics on Sensitive Skin (SENSICOS) [129] | Enrolling by invitation | Drug: Sun screening Sport Lotion (Coded products) and Untreated skin | Genetic Profile & Product Response are assessed. | Halcyon Dermatology, Laguna Hills, CA, USA | NCT03958968 |
| 26. | In-Use Test With a Cosmetic Product [130] | Recruiting | Topical Anti Aging Cream | Comparison of cosmetics use between subjects with and without sensitive skin | Laboratory Interaction Neurones Keratinocytes, Brest, France | NCT03252730 |
| 27. | Determination of retinal toxicity of hair color products containing Aromatic Amines (CAPITOX) [131] | Completed | Cosmetic Exposure | Tolerance of the test product on the scalp | SIT Skin Investigation and Technology Hamburg GmbH, Hamburg, Germany | NCT04222387 |
| 28. | Skin irritation study and sensitivity study through patch test of facial moisturizer with SPF 50 (Cetaphil) [132] | Recruiting | Coded products | Percentage of patients with MEKAR retinopathy noticed on OCT-B scan. | Fondation A de Rothschild, Paris, France | NCT01887860 |
| 29. | Prospective Evaluation of Facial Cosmetic Procedures [133] | Completed | Device: OCT-B scan | Area of erythema and edema measured | AMA Laboratories, New City, NY, USA | NCT03460158 |
| 30. | Quaternium-15, Use Test [134] | Recruiting (Phase 4) | Other: Cetaphil Daily Facial Moisturizer SPF 50 | Patient satisfaction with validated FACE-Q survey | University of Pennsylvania Health System, Philadelphia, PA, USA, | NCT00311454 |
| 31. | Repeat Insult Patch Test of Skin Irritation/Sensitization for CetaphilDermacontrol Oil Control Moisturizer SPF 30 [135] | Completed | Drug: T.R.U.E.Test |
| Pinellas Park, FL, USA | NCT01887808 |
| 32. | Tissue augmentation study on human for safety analysis of color products [136] | Completed | Coded products and Drug: Sodium chloride | Surface of erythema and edema to test interaction of skin to article | Galderma Laboratories, L.P., Fort Worth, TX, USA | NCT01147172 |
| 33. | Barrier of skin, biophysical effect and clinical appearance of moisturizer on human dry skin [137] | Completed | Cetaphil Derma Control Oil and Control Moisturizer SPF 30 |
| Vitiligo and Pigmentation Inst of Southern California, Los Angeles, CA, USA; Skin Care Research, Inc., Boca Raton, FL, USA | NCT03093597 |
| 34. | Dermal effect of cosmetic product on cancer patient: A Survey [138] | Completed | Device: Elevess | Observe the appearance of xerosis | University of Arizona, Banner-University Medical Center, Tucson, AZ, USA | NCT00871429 |
| 35. | Clinical study of OTC product dermally [139] | Completed | Coded products | Hydration capacity of skin using corneometer and loss of water trans-epidermally, value of water loss using Tewameter at different regimens of bath product and moisturizer on trans-epidermal. | Skin Center, Faculty of Medicine, SrinkharinwirotUniversity, Bangkok, Thailand | NCT03641430 |
| 36. | Functional activity of skin care regimen on human skin [140] | Not yet Recruiting | Procedure: bathing and moisturizer application | Photoaging (time frame: 1 year) | Cutaneous Translational Research Program, Department of Dermatology, Johns Hopkins University Schoo Baltimore, MD, USA | NCT03497130 |
| 37. | Assessment of facial product by the potency of photosensitivity and photo allergic reaction on healthy volunteers [141] | Completed | Supportive care with OCT product |
| Cutaneous Translational Research Program, Baltimore, MD, USA | NCT03183518 |
| 38. | Clinical studies of dermal irritation/skin sensitivity by using facial products [142] | Completed | skin care regimen | Observation of superficial irritation scores and skin irritation score at different time interval | GSK Investigational Site, Valinhos, Brazil | NCT04007159 |
| 39. | Effect of moisturizing creams on the barriers of skin [143] | Completed | micellar cleansing cream and saline solution | Percentage of participants with potential sensitization reactions as assessed by dermatologist on day 40 | GSK Investigational Site, Campinas, São Paulo, Brazil | NCT03804710 |
| 40. | Clinical studies of cleanser by cutaneous/ocular tolerance on healthy women with dermal sensitivity [144] | Completed | Serum, Lotion, Cream and Normal Saline | Loss of water from dermal skin at time interval | GSK Investigational Site, Schenefeld, Schleswig-Holstein, Germany | NCT03172364 |
| 41. | Tolerance and Efficacy Evaluation of 3 Face Creams (FILLER) [145] | Completed (Phase 4) | Micellar cleanser and Micellar foaming cleanser | Cutaneous tolerability based on visual inspection—erythema, edema, dryness, and roughness. | RCTS, Inc., Irving, TX, USA | NCT02063971 |
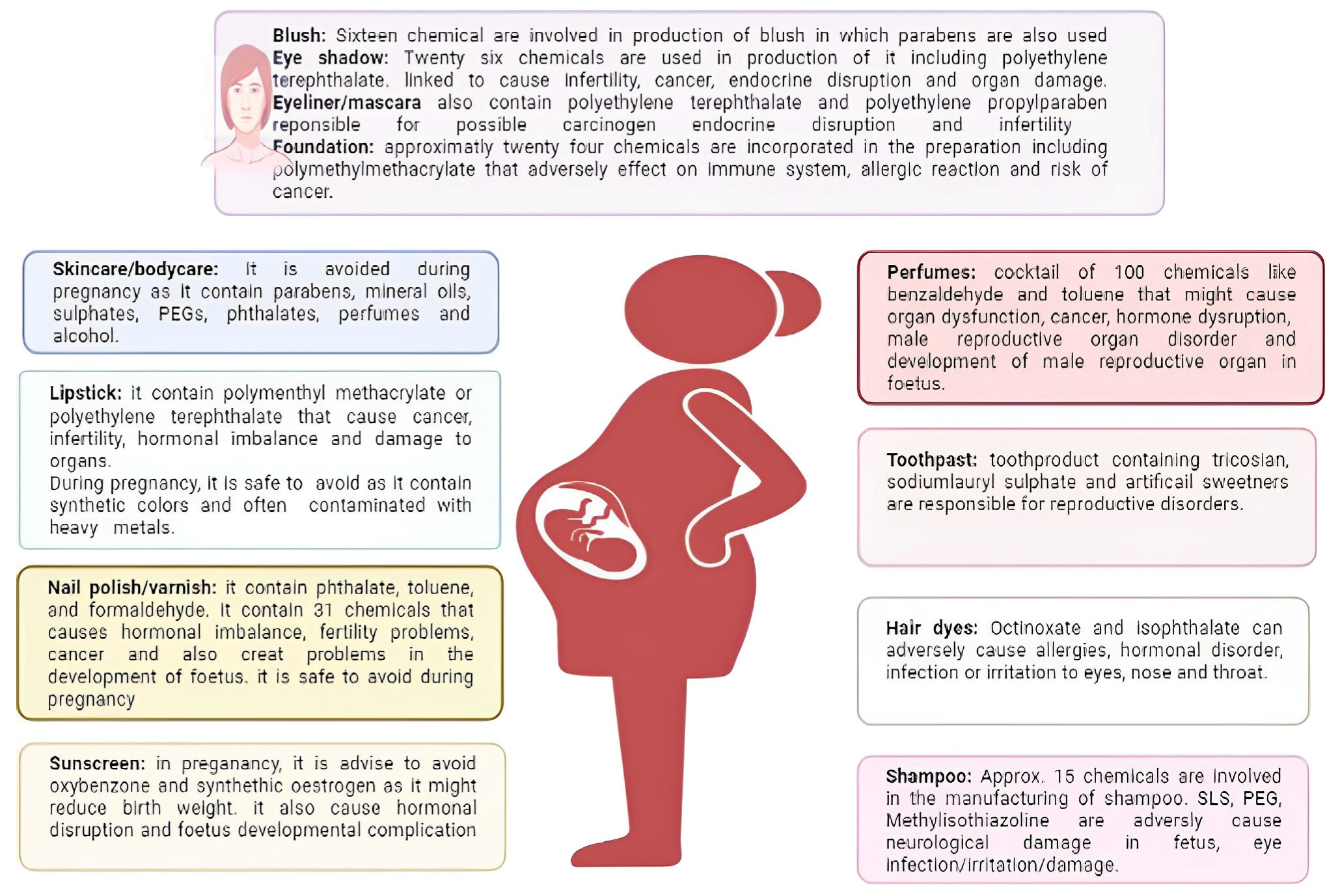
5. Safety Assessment of Excipient Used in Cosmetic/Personal Care Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in cosmetics and cosmeceuticals—A review of latest advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Dureja, H. Cosmetics: Regulatory Scenario in USA, EU and India. J. Pharm. Technol. Res. Manag. 2015, 3, 127–139. [Google Scholar] [CrossRef]
- Lintner, K. Global Regulatory Issues for the Cosmetics Industry; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, Natural and Organic Cosmetics: Consumer, Products, Efficacy, Toxicological and Regulatory Considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2014; p. 9. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-safety-nanomaterials-cosmetic-products (accessed on 20 December 2022).
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Danziger, P.N. 6 Trends Shaping the Future of the $532 B Beauty Business. Forbes Magazine. 2019. Available online: https://www.forbes.com/sites/pamdanziger/2019/09/01/6-trends-shaping-the-future-of-the-532b-beauty-business/?sh=474ef85d588d (accessed on 23 May 2022).
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin Safety and Health Prevention: An Overview of Chemicals in Cosmetic Products. J. Prev. Med. Hyg. 2019, 60, E50. [Google Scholar]
- Chauhan, P.; Mendonica, M. Role of Regulatory Authorities on the Working of Contract Research Organization and Pharmaceutical Company’s Clinical Trials in India: A Study of Strategic Alliances between Pharmaceutical Companies and Clinical Research Organisations in India. Asia Pac. J. Health Manag. 2021, 16, 87–91. [Google Scholar] [CrossRef]
- Malik, A.; Claoué, C. Transport and Interaction of Cosmetic Product Material within the Ocular Surface: Beauty and the Beastly Symptoms of Toxic Tears. Contact Lens Anterior Eye 2012, 35, 247–259. [Google Scholar] [CrossRef]
- Semwal, S.; Rani, E.; Verma, V. Impact Assessment of Goods and Services Tax (GST) on Shopkeepers. Adv. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Navabhatra, A.; Maniratanachote, R.; Yingngam, B. Antiphotoaging Properties of Zingiber Montanum Essential Oil Isolated by Solvent-Free Microwave Extraction against Ultraviolet B-Irradiated Human Dermal Fibroblasts. Toxicol. Res. 2021, 38, 1–14. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Iqbal, H. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. [Google Scholar] [CrossRef]
- Okereke, J.; Udebuani, A.; Ezeji, E.; Obasi, K.; Nnoli, M. Possible Health Implications Associated with Cosmetics: A Review. Sci. J. Public Health 2015, 3, 58. [Google Scholar]
- Adepoju-Bello, A.A.; Oguntibeju, O.O.; Adebisi, R.; Okpala, N.; Coker, H. Evaluation of the Concentration of Toxic Metals in Cosmetic Products in Nigeria. Afr. J. Biotechnol. 2012, 11, 16360–16364. [Google Scholar]
- Nasto, B. Biotech at the Beauty Counter. Nat. Biotechnol. 2007, 25, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Klaschka, U. Trust, but Verify! Personal Care Products in the Rapid Alert System Database RAPEX. Sustain. Chem. Pharm. 2017, 5, 30–41. [Google Scholar] [CrossRef]
- Kala, C.P. Medicinal and Aromatic Plants: Boon for Enterprise Development. J. Appl. Res. Med. Aromat. Plants 2015, 2, 134–139. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Abrar, M.; Iqbal, T.; Fahad, M.; Andleeb, M.; Farooq, Z.; Afsheen, S. Determination of Hazardous Ingredients in Personal Care Products Using Laser-Induced Breakdown Spectroscopy. Laser Phys. 2018, 28, 056002. [Google Scholar] [CrossRef]
- Siddanthi, P. Analysis of Indian Cosmetic Brands over International Cosmetic Brands; Politecnico: Milano, Italy, 2019. [Google Scholar]
- Kajapriya, R.; Surya, R. An Analysis on Insight of Women Consumer’s towards Cosmetic Products. Int. J. Manag. Res. Rev. 2015, 5, 246. [Google Scholar]
- Hashim, P. and Hashim, D.. A review of cosmetic and personal care products: Halal perspective and detection of ingredient. Pertanika J. Sci. Technol. 2013, 21, 281–292. [Google Scholar]
- Gao, P.; Lei, T.; Jia, L.; Yury, B.; Zhang, Z.; Du, Y.; Feng, Y.; Xing, B. Bioaccessible Trace Metals in Lip Cosmetics and Their Health Risks to Female Consumers. Environ. Pollut. 2018, 238, 554–561. [Google Scholar] [CrossRef]
- Gao, P.; Liu, S.; Zhang, Z.; Meng, P.; Lin, N.; Lu, B.; Xing, B. Health impact of bioaccessible metal in lip cosmetics to female college students and career women, northeast of China. Environ. Pollut. 2015, 197, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Just, A.C.; Williams, P.L.; Smith, K.W.; Calafat, A.M.; Hauser, R. Personal Care Product Use and Urinary Phthalate Metabolite and Paraben Concentrations during Pregnancy among Women from a Fertility Clinic. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A. Healing Beauty? More Biotechnology Cosmetic Products That Claim Drug-like Properties Reach the Market. EMBO Rep. 2008, 9, 1073–1077. [Google Scholar] [CrossRef]
- Harp, B.; Barrows, J. US Regulation of Color Additives in Foods. In Colour Additives for Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; pp. 75–88. [Google Scholar]
- Alam, M.; Akhter, M.; Mazumder, B.; Ferdous, A.; Hossain, M.; Dafader, N.; Ahmed, F.; Kundu, S.; Taheri, T.; Ullah, A.A. Assessment of Some Heavy Metals in Selected Cosmetics Commonly Used in Bangladesh and Human Health Risk. J. Anal. Sci. Technol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, G. Current Trends in Sample Preparation for Cosmetic Analysis. J. Sep. Sci. 2017, 40, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic Metals Contained in Cosmetics: A Status Report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Oishi, S. Effects of Propyl Paraben on the Male Reproductive System. Food Chem. Toxicol. 2002, 40, 1807–1813. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Bellavia, A.; Gaskins, A.; Chavarro, J.; Ford, J.; Souter, I.; Calafat, A.; Hauser, R.; Williams, P.; EARTH Study Team. Paternal mixtures of urinary concentrations of phthalate metabolites, bisphenol A and parabens in relation to pregnancy outcomes among couples attending a fertility center. Environ. Int. 2021, 146, 106171. [Google Scholar] [CrossRef]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Hepatotoxicity of Di-(2-Ethylhexyl) Phthalate Is Attributed to Calcium Aggravation, ROS-Mediated Mitochondrial Depolarization, and ERK/NF-ΚB Pathway Activation. Free Radic. Biol. Med. 2010, 49, 1779–1791. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Butyl Paraben Promotes Apoptosis in Human Trophoblast Cells through Increased Oxidative Stress-induced Endoplasmic Reticulum Stress. Environ. Toxicol. 2018, 33, 436–445. [Google Scholar] [CrossRef]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef]
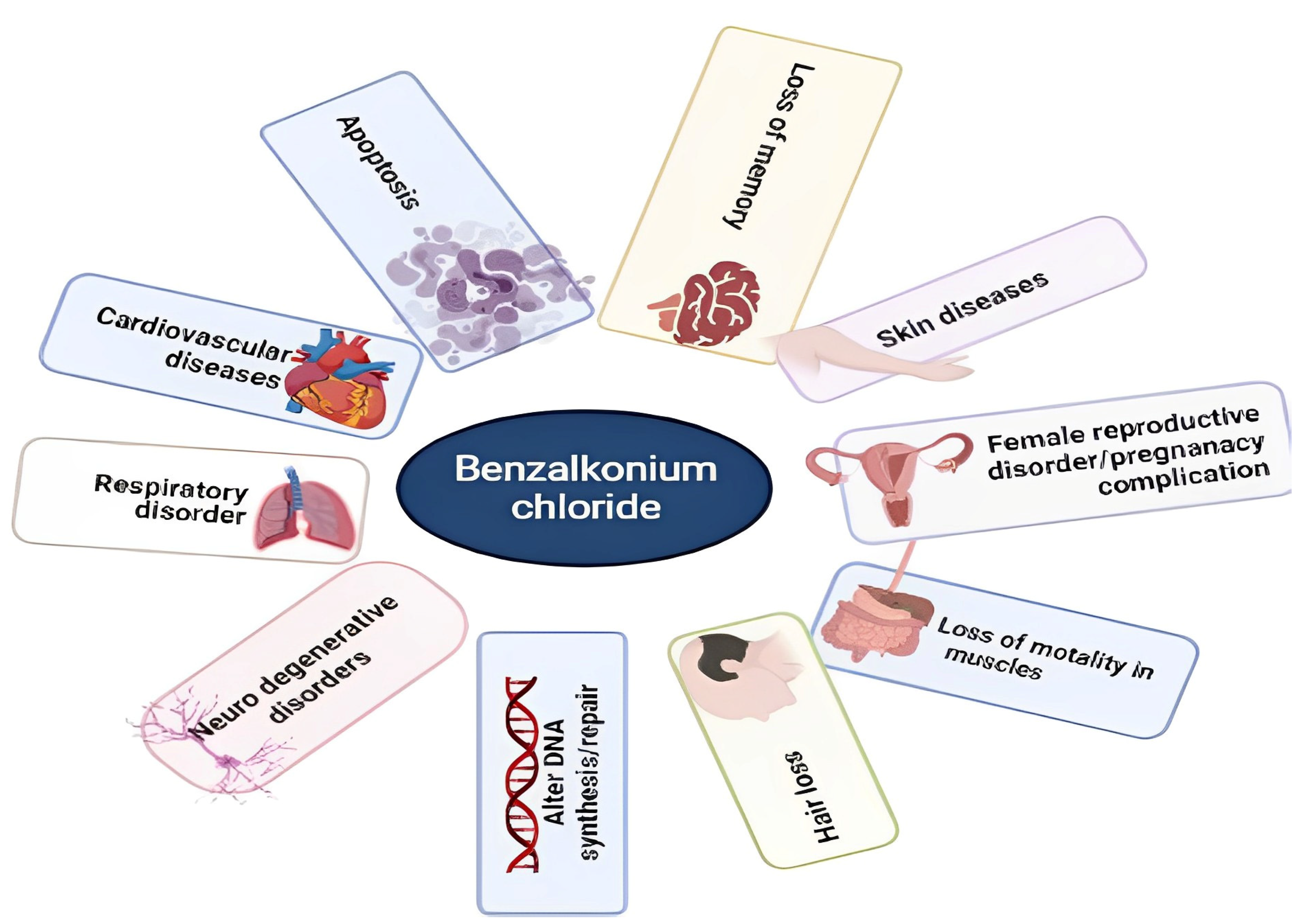
- Choi, S.M.; Roh, T.H.; Lim, D.S.; Kacew, S.; Kim, H.S.; Lee, B.-M. Risk Assessment of Benzalkonium Chloride in Cosmetic Products. J. Toxicol. Environ. Health Part B 2018, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Son, A.; Her, N.; Zoh, K.-D.; Cho, J.; Yoon, Y. Removal of Endocrine Disrupting Compounds, Pharmaceuticals, and Personal Care Products in Water Using Carbon Nanotubes: A Review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Pop, A.; Kiss, B.; Loghin, F. Endocrine Disrupting Effects of Butylated Hydroxyanisole (BHA-E320). Clujul Med. 2013, 86, 16. [Google Scholar]
- Jones, G. Globalization and Beauty: A Historical and Firm Perspective. EurAmerica 2011, 41, 885–916. [Google Scholar]
- Brody, T. Food and Dietary Supplement Package Labeling—Guidance from FDA’s Warning Letters and Title 21 of the Code of Federal Regulations. Compr. Rev. Food Sci. Food Saf. 2016, 15, 92–129. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.T.K.; Reddy, G.N.K. Significance of Pharmaceutical Regulatory Bodies-a Review. PharmaTutor 2017, 5, 15–22. [Google Scholar]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and Practical Challenges in Classifying Nanomaterials According to Regulatory Definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Abellán, E.F.G.; Pérez, D.M. Quality Control of Cosmetic Products: Specific Legislation on Ingredients. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–53. [Google Scholar]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- Acrylates. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/2978/ (accessed on 16 September 2021).
- P-Phenylenediamine. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/p-phenylenediamine/ (accessed on 16 September 2021).
- Diazolidinyl Urea|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/diazolidinyl-urea-0 (accessed on 16 September 2021).
- p-Phenylenediamine|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/p-phenylenediamine (accessed on 16 September 2021).
- Talc. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/talc/ (accessed on 16 September 2021).
- Benzophenone & Related Compounds-Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/benzophenone/ (accessed on 12 September 2021).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352&showFR=1 (accessed on 16 September 2021).
- Commission Regulation (EU) 2017/238-of 10 February 2017-Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products 2. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02009R1223-20220301&qid=1653495150566&from=en (accessed on 16 September 2021).
- Butylated Compounds. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/butylated-compounds/ (accessed on 16 September 2021).
- Coal Tar. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/coal-tar/ (accessed on 16 September 2021).
- Ethanolamine Compounds (MEA, DEA, TEA and Others). Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/ethanolamine-compounds/ (accessed on 16 September 2021).
- Triethanolamine|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/triethanolamine (accessed on 16 September 2021).
- Phenoxyethanol. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/phenoxyethanol/ (accessed on 16 September 2021).
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.Cfm?event=BasicSearch.page (accessed on 16 September 2021).
- 1,4-dioxane. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/14-dioxane/ (accessed on 16 September 2021).
- Formaldehyde And Formaldehyde-Releasing Preservatives. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/formaldehyde/ (accessed on 16 September 2021).
- Lead And Other Heavy Metals. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/lead-and-other-heavy-metals/ (accessed on 16 September 2021).
- Michalek, I.M.; Benn, E.K.T.; dos Santos, F.L.C.; Gordon, S.; Wen, C.; Liu, B. A Systematic Review of Global Legal Regulations on the Permissible Level of Heavy Metals in Cosmetics with Particular Emphasis on Skin Lightening Products. Environ. Res. 2019, 170, 187–193. [Google Scholar] [CrossRef]
- FDA’s Testing of Cosmetics for Arsenic, Cadmium, Chromium, Cobalt, Lead, Mercury, and Nickel Content. FDA 2020. Available online: https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/fdas-testing-cosmetics-arsenic-cadmium-chromium-cobalt-lead-mercury-and-nickel-content (accessed on 20 December 2022).
- Resorcinol. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/resorcinol/ (accessed on 16 September 2021).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310 (accessed on 16 September 2021).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=346.20 (accessed on 16 September 2021).
- Methylisothiazolinone and Methylchloroisothiazolinone. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/methylisothiazolinone/ (accessed on 16 September 2021).
- Burnett, C.L.; Boyer, I.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Amended Safety Assessment of Methylisothiazolinone as Used in Cosmetics. Int. J. Toxicol. 2019, 38 (Suppl. S1), 70S–84S. [Google Scholar] [CrossRef] [PubMed]
- FDA to Set Limits on Use of MCI/MI, MI Preservatives—Taipei Times. Available online: http://www.taipeitimes.com/News/taiwan/archives/2013/11/19/2003577227 (accessed on 16 September 2021).
- Octinoxate—Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/octinoxate/ (accessed on 16 September 2021).
- PABA. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/paba/ (accessed on 16 September 2021).
- PABA|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/paba-0 (accessed on 16 September 2021).
- PABA (Para-Aminobenzoic Acid)—Health Library. Available online: https://healthlibrary.epnet.com/GetContent.aspx?token=b07cead8-3508-47b4-a855-614f3d8eb5ee&chunkiid=21831 (accessed on 16 September 2021).
- Parabens. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/parabens/ (accessed on 16 September 2021).
- Hydroquinone—Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/hydroquinone/ (accessed on 16 September 2021).
- Hydroquinone: Uses, Safety, Side Effects, OTC Products, Alternatives. Available online: https://www.healthline.com/health/beauty-skin-care/hydroquinone (accessed on 16 September 2021).
- Polyacrylamide. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/polyacrylamide-2/ (accessed on 16 September 2021).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=74.2053 (accessed on 16 September 2021).
- Quaternium-15—Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/quaternium-15/ (accessed on 16 September 2021).
- Homosalate—Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/homosalate/ (accessed on 16 September 2021).
- Titanium Dioxide. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/titanium-dioxide-2/ (accessed on 16 September 2021).
- Titanium Dioxide|Cosmetics Info. Available online: https://cosmeticsinfo.org/ingredient/titanium-dioxide (accessed on 16 September 2021).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.575 (accessed on 16 September 2021).
- Triclosan—Safe Cosmetics. Available online: https://www.safecosmetics.org/get-the-facts/chemicals-of-concern/triclosan/ (accessed on 16 September 2021).
- Food and Drug Administration (FDA). Assessment of the Human Systemic Absorption of Sunscreen Ingredients. Clinical Trial Registration NCT03582215. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Supervised Outdoor-Use Test to Assess the Safety of a Sunscreen C-Spray, Bayer Sponsored, CC Products. Clinical Trial Registration NCT02857478. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. To Assess the Safety of a Sunscreen Product, BAY 987519, Bayer Sponsored, CC Products. Clinical Trial Registration NCT02803320. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Evaluation of the Irritation Potential of Sunscreen Products in Human Eyes. Clinical Trial Registration NCT02854137. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. A Randomized Study to Assess the Potential for Phototoxicity of SPF 50 Y65 110, SPF 50 Y51 002 and SPF 15 V27-104 in Human Subjects. Clinical Trial Registration NCT02802930. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. Determination of the Sun Protection Factor of a Cosmetic Daily Defence Skin Cream. Clinical Trial Registration NCT03136107. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Determination of Sun Protection Factors (PFA and SPF) in Sunscreen Formulas Containing Combinations of Zinc Oxide and Avobenzone. Clinical Trial Registration NCT01001975. 2015. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Galderma Laboratories, L.P. Cetaphil® Daily Facial Moisturizer with Sunscreen SPF 50+—Human Repeat Insult Patch Test Skin Irritation/Sensitization Evaluation (Semi-Occlusive Patch). Clinical Trial Registration NCT01892657. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline, Clinical Study to Evaluate the Sun Protection Factor (SPF) of Sunscreen Products. Clinical Trial Registration number NCT05085327. 2021. Available online: clinicaltrials.gov (accessed on 4 November 2021).
- Bayer. Sun Protection Factor (SPF)/UVA Protection Factor (UVAPF) Assay. Clinical Trial Registration NCT02872246. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Sun Protection Factor (SPF) Efficacy Assay. Clinical Trial Registration NCT02885805. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Kottner, P.D.J. An Exploratory Randomized Controlled Study to Evaluate the Effect of a Basic Skin Care Product on the Structural Strength of the Dermo-Epidermal Junction. Clinical Trial Registration NCT03625167. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Supervised Outdoor-Use Test for Sunscreen Products in Adults. Clinical Trial Registration NCT02877511. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Supervised Outdoor-Use Test for Sunscreen Products in Female Adults. Clinical Trial Registration NCT02779270. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Clinical Study in Infants to Assess the Tolerability of Three Wash Products. Clinical Trial Registration NCT02403999. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Galderma Laboratories, L.P. Tolerability of a Foaming Facial Cleanser and Moisturizer SPF 30 in a Pediatric Population (7–11) with Acne Prone Skin. Clinical Trial Registration NCT01909713. 2014. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Tangtrakulwanich, B. Efficacy of Baby Talcum in Prevention of Pruritus Assosiatedwith Cast. A Randomized Controlled Trial. Clinical Trial Registration NCT01017315. 2012. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Johnson & Johnson Consumer Inc. (J & JCI). A Randomized Controlled Trial of Gentle Touch/Early Massage with a New Wash and Lotion Regimen for Improved Skin Barrier Strength, Parental Bonding, and Physical Development in Newborn Babies: The Barrier Optimizing Skincare for Newborn Development (BOND) Trial. Clinical Trial Registration NCT03142984. 2021. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Arisa Kaewkes. The Effect of Bathing and Moisturizers on Skin Hydration in Atopic Dermatitis: An in vivo study. Clinical Trial Registration NCT02028546. 2014. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. 4 Week In-Use Study Evaluating the Tolerability of a Skin Care Product on Adults with Rosacea. Clinical Trial Registration NCT03841032. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Revision Skincare. A Randomized, Double-Blind, Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of an Anti-Aging Serum in the Treatment of Facial Lines. Clinical Trial Registration NCT04545970. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Revision Skincare. A Double-Blind Split-Body Placebo-Controlled Clinical Study to Evaluate the Efficacy of a Cosmetic Product for Arm Firming. Clinical Trial Registration NCT04065035. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Burt’s Bees Inc. A Clinical Study to Evaluate the Safety and Efficacy of a Baby Cleanser and a Moisturizer Under the Supervision of Dermatologist and Pediatrician. Clinical Trial Registration NCT03719742. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Clinical Study to Assess the Local Cutaneous and Ocular Tolerance of a Developmental Cosmetic Facial Serum Formulation in Healthy Females with Sensitive Skin. Clinical Trial Registration NCT03640832. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Johnson & Johnson Consumer Inc. (J & JCI). A Bilateral, Controlled Clinical Trial to Evaluate 2 Different Moisturizer Chassis Formulas for the Relief of Dry Skin. Clinical Trial Registration NCT04510103. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Randomized, Parallel-Group, Evaluator-Blind, No-Treatment and Positive Controlled, Single-Site, Proof of Concept Clinical Study to Evaluate the Cosmetic Benefit Provided by 8 Weeks of Twice-Daily Topical Application of a Developmental Moisturizing Cream with Niacinamide in Healthy Subjects with Sensitive, Oily, Blemish-Prone Skin. Clinical Trial Registration NCT03093181. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Pierre Fabre Dermo Cosmetique. An International Multicentric Open-Label Descriptive Study to Assess the Effects and Evaluate the Tolerance of the Cosmetic Care Product RV3278A in the Follow-up Phase of Facial Acne in Teenagers and Young Adults for 1 Year. Clinical Trial Registration NCT04301063. 2021. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Dr. August Wolff GmbH & Co. KG Arzneimittel. Assessment of the Long-Term Efficacy and Barrier Protection of Two Skin Care Products. Clinical Trial Registration NCT03629405. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Derming SRL. Clinical Study for the Evaluation of the Depigmenting Activity of a Cosmetic Product on Spotted Hand Skin. Clinical Trial Registration NCT02204436. 2014. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Derming SRL. Clinical and Instrumental Evaluation of a Topical “Antiage” Formulation for the Face. Clinical Trial Registration NCT01948531. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Christie Medical Holdings, Inc. Evaluation of VeinViewer in Cosmetic Dermatology. Clinical Trial Registration NCT02200471. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- AOBiome LLC. A Placebo-Controlled, Double-Blind, Bilateral Cosmetic Study with an Open Label Extension to Evaluate the Performance of a Cosmetic Product Designed to Improve the Appearance of Skin Afflicted with Mild to Moderate Atopic Dermatitis. Clinical Trial Registration NCT03268174. 2017. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- University Hospital, Clermont-Ferrand. Perception of the Risk Associated with the Use of Cosmetics Products During Pregnancy—A Qualitative Study. Clinical Trial Registration NCT03283189. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Ercan, Z.E. Effect of Pencil Eyeliners and Waterproof Mascara on Tear Stability Tests and Meibomography. Clinical Trial Registration NCT04478955. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- University Hospital Inselspital, Berne. Epicutaneous Testing of Cosmetic Products to Determine Skin Compatibility. Clinical Trial Registration NCT03024671. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Alma Lasers Inc. Fractional Erbium 2940 nm Laser & Impact US for Trans-Epidermal Delivery of Cosmetic Anti-Aging Ingredients for Wrinkles, Acne Scars and Pigmented Skin: A Randomized Split Face & Dorsal Hand Side by Side Study. Clinical Trial Registration NCT01847066. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- National Taiwan University Hospital. Evaluate the Lightening Effect of the Whitening Cosmetic Product BEX-2011 on Solar Lentigines by Harmonic Generation Microscopy. Clinical Trial Registration NCT01249469. 2010. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Mother’s Choice Ltd. Dermatologically Tested-Patch Tests-to Assess the Irritancy/Allergic Sensitivity of the Natural Personal-Care Products Developed by “Mother’s Choice”. Clinical Trial Registration NCT01816542. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Evaluation of the Stinging Potential of Sunscreen Products in Human Eyes. Clinical Trial Registration NCT02869113. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Choi, I. Efficacy and Safety of Facial Cosmetic Acupuncture on Skin Rejuvenation. Clinical Trial Registration NCT01486303. 2011. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Columbia University. Hair Care Product Use Among Women of Color: A Northern Manhattan Intervention. Clinical Trial Registration NCT04493892. 2021. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Bayer. Human Photoallergy Test. Clinical Trial Registration NCT02750449. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Helical Code, M.D. Relationship of Skin Related Single Nucleotide Polymorphisms to Clinical Response to a Topical Skin Care Product. Clinical Trial Registration NCT03446079. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Emilie, B. Impact of Exposure to Cosmetics on Sensitive Skin. Clinical Trial Registration NCT03958968. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Dr. August Wolff GmbH & Co. KG Arzneimittel. In-Use Test with a Cosmetic Product (WO 4260) for Topical Use on the Scalp. Clinical Trial Registration NCT03252730. 2017. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- FondationOphtalmologique Adolphe de Rothschild. Investigation of Potential Retinal Toxicity Associated with Hair Dye Products Containing PPD (Para-Phenylenediamine) Type Aromatic Amines. Clinical Trial Registration NCT04222387. 2021. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Galderma Laboratories, L.P. Subject Repeat Insult Patch Test Skin Irritation/Sensitization Evaluation (Occlusive Patch). Clinical Trial Registration NCT01887860. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- University of Pennsylvania. Prospective Evaluation of Minimally Invasive Facial Cosmetic Procedures through Measured Volumetric Changes and Patient Reported Outcomes. Clinical Trial Registration NCT03460158. 2021. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Mekos Laboratories, A.S. An Open Single Centre Evaluation of the Reactivity of the T.R.U.E. Test Quaternium-15 Patch and a Real Use Exposure in Subjects Known to Be Allergic to Quaternium-15. Clinical Trial Registration NCT00311454. 2006. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Galderma Laboratories, L.P. Cetaphil Dermacontrol Oil Control Moisturizer SPF 30–100 Human Subject Repeat Insult Patch Test Skin Irritation/Sensitization Evaluation (Semi-Occlusive Patch). Clinical Trial Registration NCT01887808. 2013. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Anika Therapeutics, Inc. A Post Approval, Multicenter, Open-Label, Longitudinal, Uncontrolled Safety Study of Cosmetic Tissue Augmentation Product (ELEVESSTM) in the Treatment of Nasolabial Folds in People of Color. Clinical Trial Registration NCT01147172. 2017. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- University of Arizona. Evaluating Skin Barrier Biophysical Properties and Clinical Appearance After Moisturizer Use in Patients with Dry Skin. Clinical Trial Registration NCT03093597. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Northwestern University. Selected Skin Products for Cancer Patients: A Product Satisfaction Survey. Clinical Trial Registration NCT00871429. 2015. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Johns Hopkins University. The Effects of Over-the-Counter Products on the Skin. Clinical Trial Registration NCT03641430. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Johns Hopkins University. The Role of Skin Care Regimen in Skin Health. Clinical Trial Registration NCT03497130. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Clinical Study to Assess the Photosensitisation and Photoallergy Potential of a Cosmetic Facial Product in Healthy Subjects. Clinical Trial Registration NCT03183518. 2018. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Human Repeat Insult Patch Test (HRIPT) in Healthy Subjects to Assess the Cutaneous Irritation and Sensitization Potential of Three Developmental Cosmetic Facial Products. Clinical Trial Registration NCT04007159. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Clinical Study to Investigate the Effects of Two Developmental Cosmetic Moisturizing Cream Formulations on the Barrier Function of Human Skin on the Face and Legs. Clinical Trial Registration NCT03804710. 2020. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- GlaxoSmithKline. A Clinical Study to Assess the Cutaneous and Ocular Local Tolerance of Two Cosmetic Facial Cleansers in Healthy Females with Sensitive Skin Under Normal Conditions of Use. Clinical Trial Registration NCT03172364. 2019. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Sparavigna, A. Tolerance and Efficacy Evaluation of Three Face Creams in Subjects Undergoing to Injection Procedure with Hyaluronic Acid (Intradermal Implant). Clinical Trial Registration NCT02063971. 2014. Available online: clinicaltrials.gov (accessed on 20 December 2022).
- Rodu, B.; Cole, P.; Mandel, J.S. Evaluation of the National Toxicology Program Report on Carcinogens. Regul. Toxicol. Pharmacol. 2012, 64, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Thierse, H.-J.; Luch, A. Consumer Protection and Risk Assessment: Sensitising Substances in Consumer Products. Allergo J. Int. 2019, 28, 167–182. [Google Scholar] [CrossRef]
- Koo, H.J.; Lee, B.M. Estimated Exposure to Phthalates in Cosmetics and Risk Assessment. J. Toxicol. Environ. Health A 2004, 67, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Radawski, C.; Morrato, E.; Hornbuckle, K.; Bahri, P.; Smith, M.; Juhaeri, J.; Mol, P.; Levitan, B.; Huang, H.; Coplan, P. Benefit–Risk Assessment, Communication, and Evaluation (BRACE) throughout the Life Cycle of Therapeutic Products: Overall Perspective and Role of the Pharmacoepidemiologist. Pharmacoepidemiol. Drug Saf. 2015, 24, 1233–1240. [Google Scholar] [CrossRef]
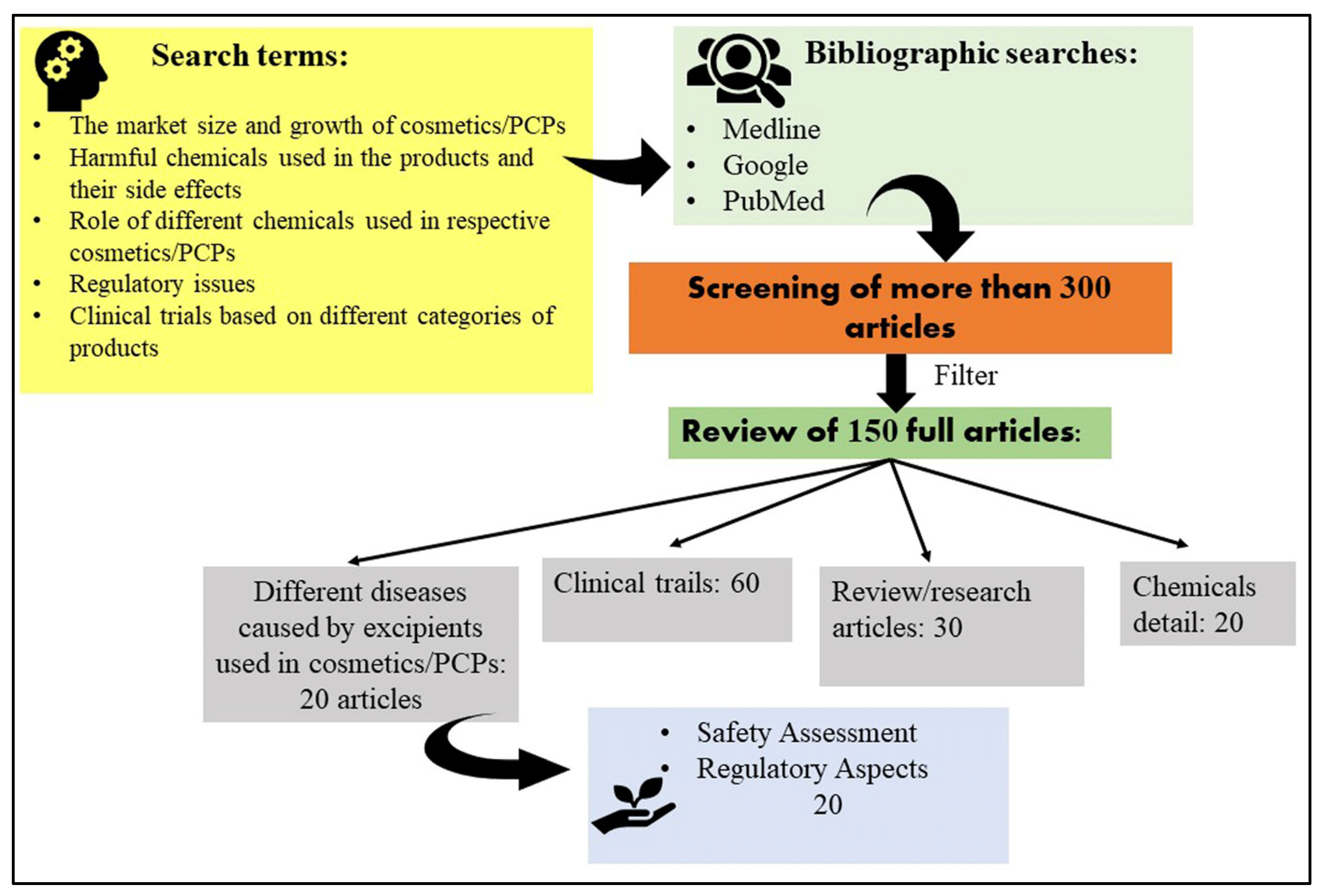
| Regulatory Agencies | Definition of Cosmetics |
|---|---|
| USFDA | “A product (except pure soap) that is used to be applied dermally for cleansing, whitening, and beautifying purposes” |
| CDSCO (India) | “An article anticipated for coating, pouring, and sprinkling or spraying on, or incorporated into, or otherwise applied on the human skin or any part thereof for cleansing, embellishing, enhancing attractiveness, or improve appearance, and includes any article used for a component of cosmetic.” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, M.; Farooq, U.; Ali, M.S.; Ansari, M.J.; Iqbal, Z.; Mirza, M.A. Safety Concern and Regulatory Status of Chemicals Used in Cosmetics and Personal Care Products. Dermato 2023, 3, 131-157. https://doi.org/10.3390/dermato3020011
Kaushik M, Farooq U, Ali MS, Ansari MJ, Iqbal Z, Mirza MA. Safety Concern and Regulatory Status of Chemicals Used in Cosmetics and Personal Care Products. Dermato. 2023; 3(2):131-157. https://doi.org/10.3390/dermato3020011
Chicago/Turabian StyleKaushik, Manthan, Uzma Farooq, Mohd Shoab Ali, Mohammad Javed Ansari, Zeenat Iqbal, and Mohd Aamir Mirza. 2023. "Safety Concern and Regulatory Status of Chemicals Used in Cosmetics and Personal Care Products" Dermato 3, no. 2: 131-157. https://doi.org/10.3390/dermato3020011
APA StyleKaushik, M., Farooq, U., Ali, M. S., Ansari, M. J., Iqbal, Z., & Mirza, M. A. (2023). Safety Concern and Regulatory Status of Chemicals Used in Cosmetics and Personal Care Products. Dermato, 3(2), 131-157. https://doi.org/10.3390/dermato3020011







