Establishing an Electron FLASH Platform for Preclinical Research in Low-Resource Settings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
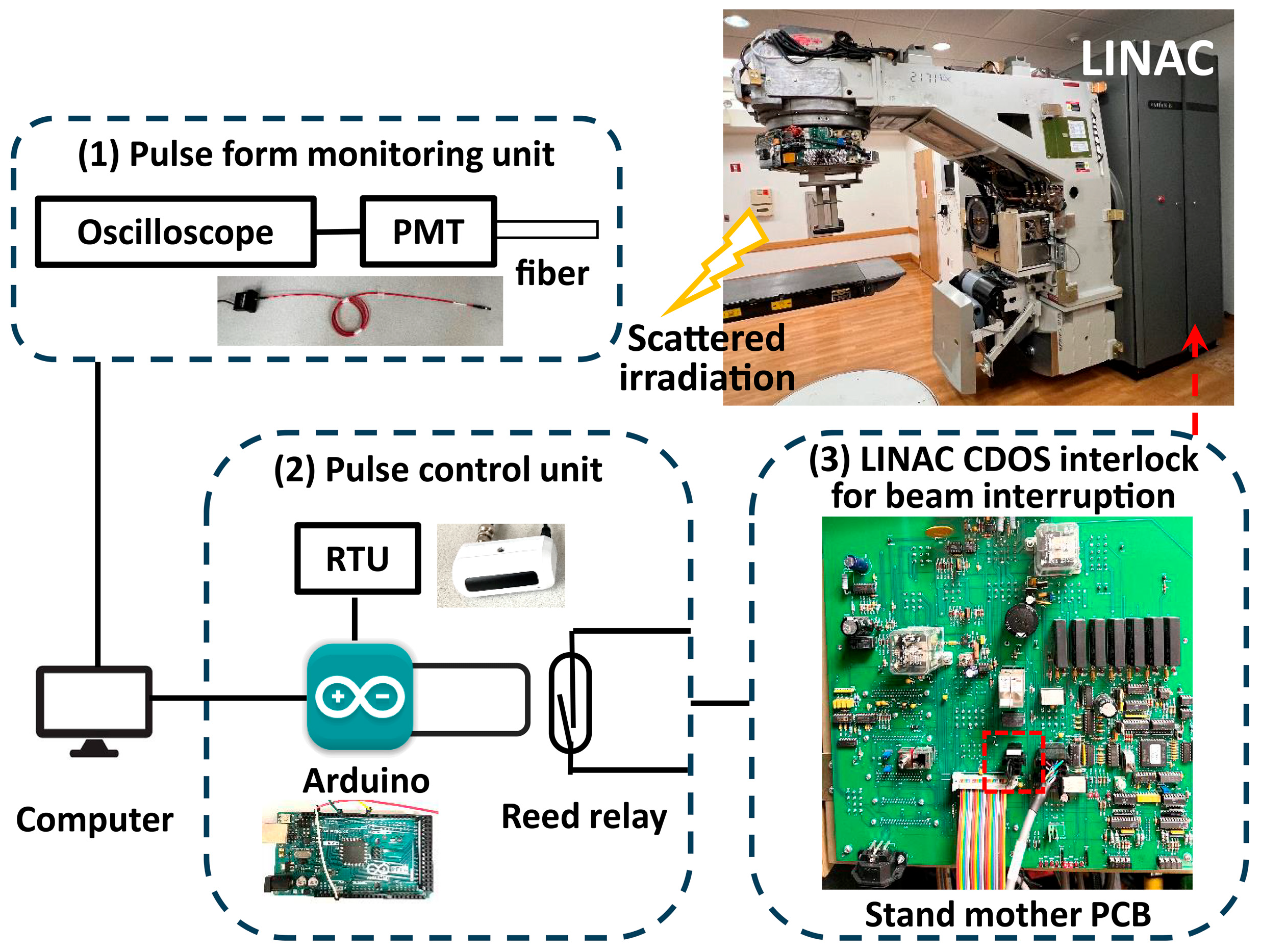
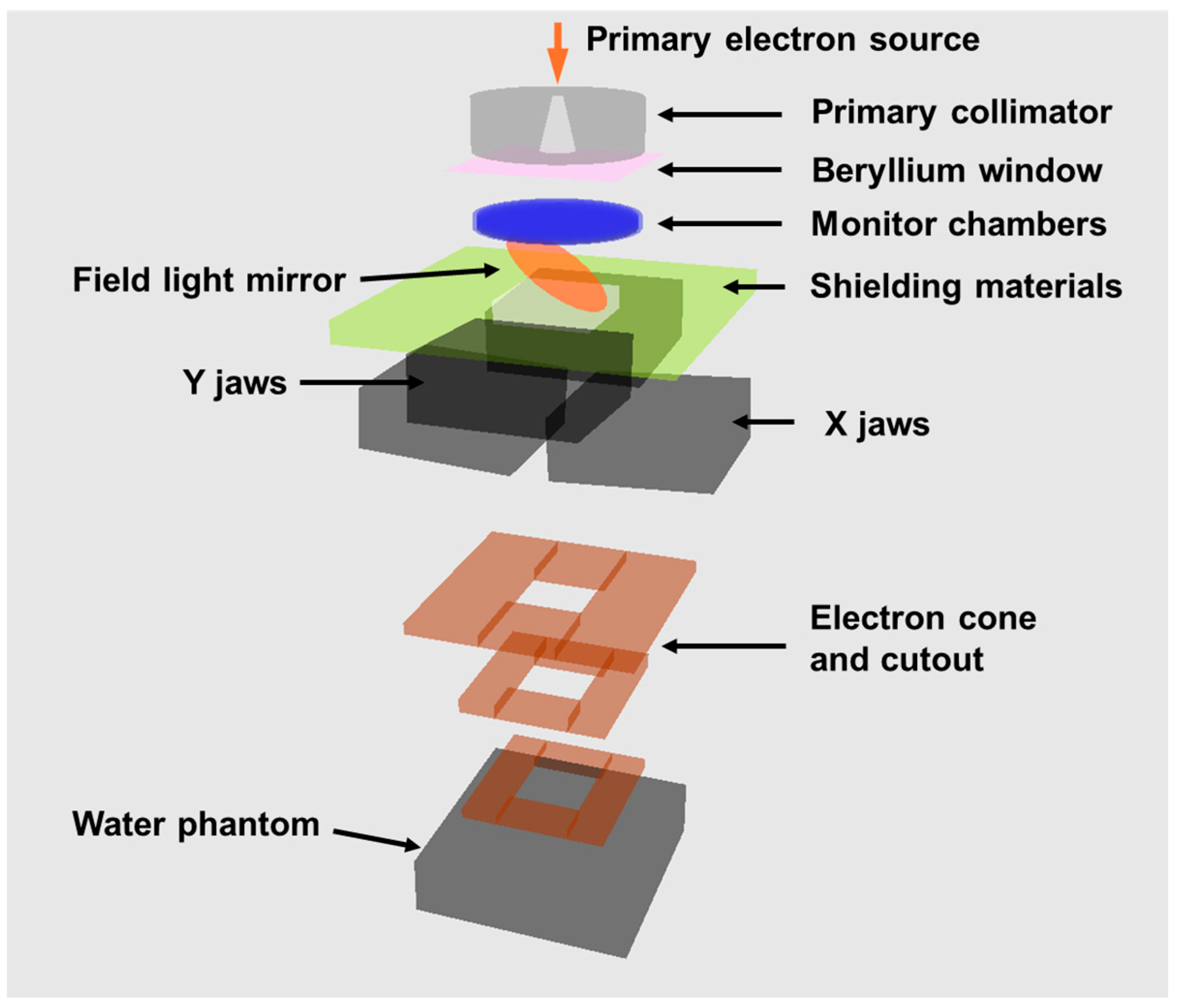
2.1. Machine Modification
2.2. Pulse Monitoring and Control
2.2.1. Pulse Form Monitoring
2.2.2. Monitor Units (MU) and External Pulse Control
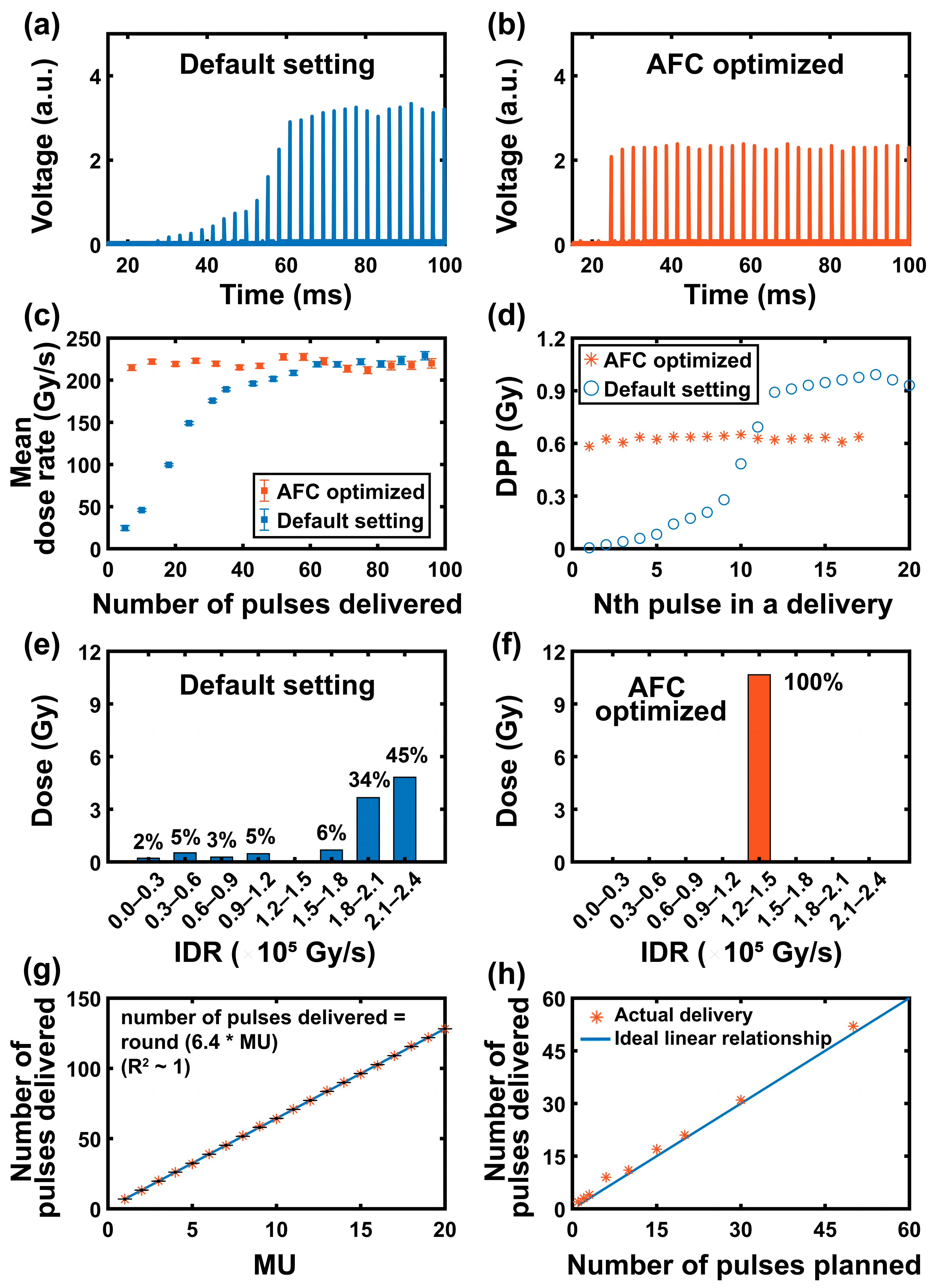
2.3. Pulse Form Optimization
2.4. Pulse Form Analysis
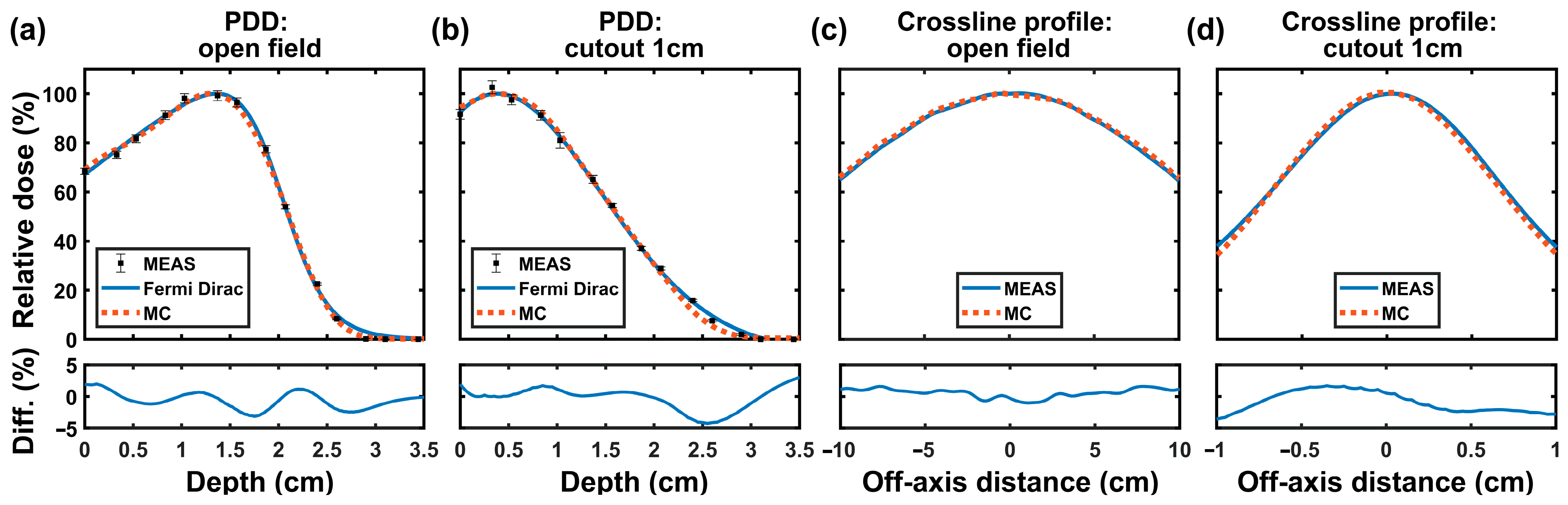
2.5. Beam Data Measurement
2.6. MC Beam Modeling and Dose Calculation
2.6.1. MC Beam Modeling
2.6.2. MC Dose Calculation
2.7. FLASH Preclinical Planning Studies
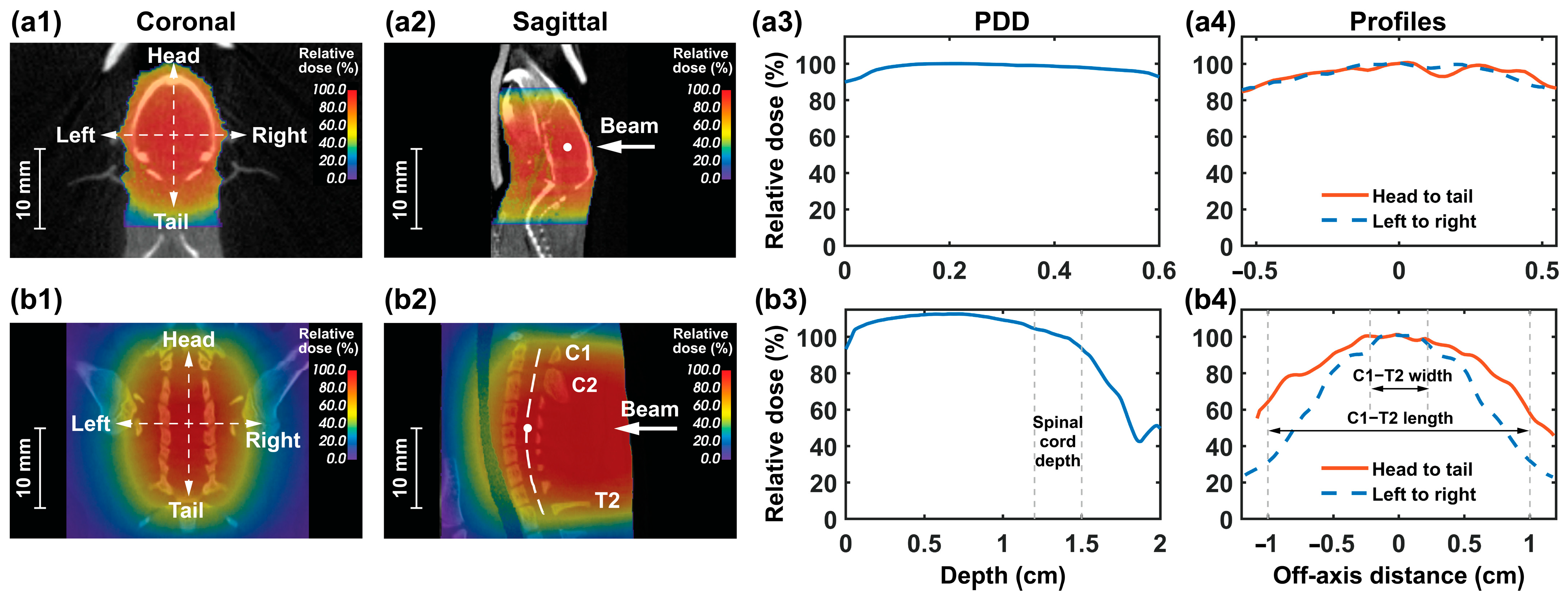
2.7.1. Mouse Whole-Brain Irradiation
2.7.2. Rat Spinal Cord Irradiation
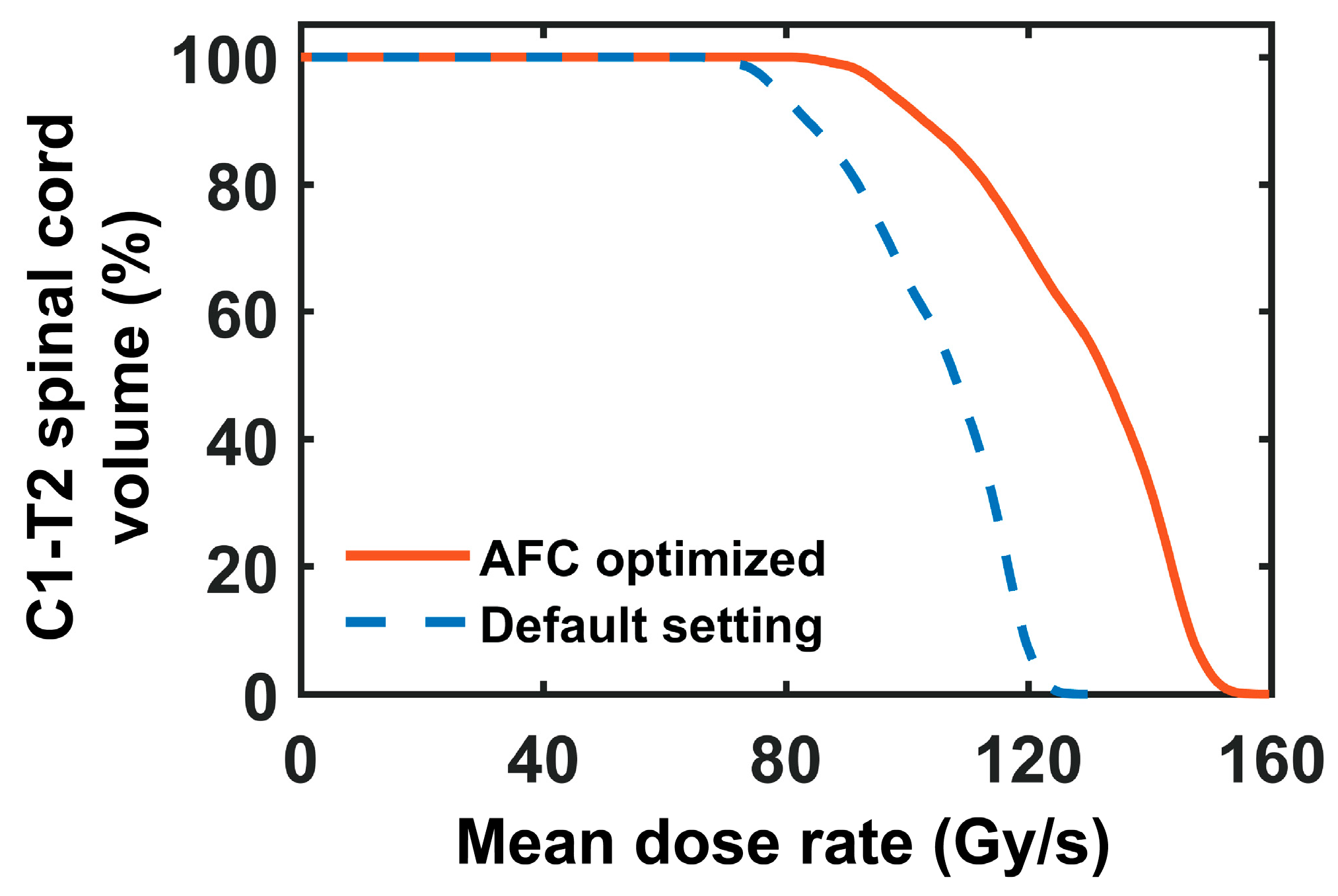
2.8. Assess the Impact of AFC Optimization on FLASH Animal Studies
3. Results
3.1. Pulse Control and Optimization
3.2. Beam Data
3.3. MC Beam Modeling
3.4. FLASH Preclinical Planning Studies
3.4.1. Mouse Whole-Brain Irradiation
3.4.2. Rat Spinal Cord Irradiation
3.5. Impact of AFC Optimization on FLASH Animal Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFC | automatic frequency control |
| AUC | area under the curve |
| AAD | average absolute difference |
| CDOS | customer-defined dosimetry interlock |
| CAX | central axis |
| CT | computed tomography |
| CBCT | cone beam computed tomography |
| CONV | conventional |
| CV | coefficient of variation |
| C1-T2 | cervical 1 to thoracic 2 |
| dmax | the depth of maximum dose |
| DPP | dose per pulse |
| DRVH | dose-rate volume histogram |
| eFLASH | electron FLASH |
| ED50 | median effective dose |
| FLASH-RT | FLASH radiotherapy |
| GAMOS | Geant4-based architecture for medicine-oriented simulations |
| IDR | instantaneous dose rate |
| LINAC | linear accelerators |
| MC | Monte Carlo |
| MU | monitor unit |
| OSLD | optically stimulated luminescence dosimeters |
| OAD | off-axis distance |
| PA | posterior–anterior |
| PET | positron emission tomography |
| PCB | printed circuit board |
| PDD | percentage depth dose |
| PS | phase-space |
| PMT | photomultiplier tube |
| PRF | pulse repetition frequency |
| RTU | remote trigger unit |
| RF | radiofrequency |
| SSD | source-to-surface distance |
| SARRP | small animal radiation research platform |
| UHDR | ultra-high dose rate |
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W.; Vozenin, M.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Chang, C.; Charyyev, S.; Zhou, J.; Bradley, J.D.; Liu, T.; Yang, X. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy. J. Appl. Clin. Med. Phys. 2022, 23, e13790. [Google Scholar] [CrossRef]
- Giannini, N.; Gadducci, G.; Fuentes, T.; Gonnelli, A.; Di Martino, F.; Puccini, P.; Naso, M.; Pasqualetti, F.; Capaccioli, S.; Paiar, F. Electron FLASH radiotherapy in vivo studies. A systematic review. Front. Oncol. 2024, 14, 1373453. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gaide, O.; Herrera, F.; Sozzi, W.J.; Jorge, P.G.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.-F.; Gondré, M.; et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, D.D.; Therriault-Proulx, F.; Lambert-Girard, S.; Hart, A.; Macdonald, A.; Pfleger, M.; Lenckowski, M.; Bazalova-Carter, M. Characterization of an x-ray tube-based ultrahigh dose-rate system for in vitro irradiations. Med. Phys. 2021, 48, 7399–7409. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Iordachita, I.; Wong, J.W. Ultrahigh dose-rate (FLASH) x-ray irradiator for pre-clinical laboratory research. Phys. Med. Biol. 2021, 66, 095006. [Google Scholar] [CrossRef]
- Miles, D.; Sforza, D.; Wong, J.; Rezaee, M. Dosimetric characterization of a rotating anode x-ray tube for FLASH radiotherapy research. Med. Phys. 2023, 51, 1474–1483. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother. Oncol. 2022, 166, 44–50. [Google Scholar] [CrossRef]
- Patriarca, A.; Fouillade, C.; Auger, M.; Martin, F.; Pouzoulet, F.; Nauraye, C.; Heinrich, S.; Favaudon, V.; Meyroneinc, S.; Dendale, R.; et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. Int. J. Radiat. Oncol. 2018, 102, 619–626. [Google Scholar] [CrossRef]
- Darafsheh, A.; Hao, Y.; Zwart, T.; Wagner, M.; Catanzano, D.; Williamson, J.F.; Knutson, N.; Sun, B.; Mutic, S.; Zhao, T. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med. Phys. 2020, 47, 4348–4355. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Zhang, Q.; Cascio, E.; Li, C.; Yang, Q.; Gerweck, L.E.; Huang, P.; Gottschalk, B.; Flanz, J.; Schuemann, J. FLASH Investigations Using Protons: Design of Delivery System, Preclinical Setup and Confirmation of FLASH Effect with Protons in Animal Systems. Radiat. Res. 2020, 194, 656–664. [Google Scholar] [CrossRef]
- Weber, U.A.; Scifoni, E.; Durante, M. FLASH radiotherapy with carbon ion beams. Med. Phys. 2021, 49, 1974–1992. [Google Scholar] [CrossRef]
- Lempart, M.; Blad, B.; Adrian, G.; Bäck, S.; Knöös, T.; Ceberg, C.; Petersson, K. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother. Oncol. 2019, 139, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Y.; Ma, S.; Yang, X.; Lan, R.; Chen, A.; Zhu, H.; Mei, Y.; Peng, L.; Li, Z.; et al. Electron ultra-high dose rate FLASH irradiation study using a clinical linac: Linac modification, dosimetry, and radiobiological outcome. Med. Phys. 2022, 49, 6728–6738. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W.; Maxim, P.G. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int. J. Radiat. Oncol. 2017, 97, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ashraf, M.R.; Zhang, R.; Bruza, P.; Dexter, C.A.; Thompson, L.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Pogue, B.W.; et al. Electron FLASH Delivery at Treatment Room Isocenter for Efficient Reversible Conversion of a Clinical LINAC. Int. J. Radiat. Oncol. 2021, 110, 872–882. [Google Scholar] [CrossRef]
- Byrne, K.E.; Poirier, Y.; Xu, J.; Gerry, A.; Foley, M.J.; Jackson, I.L.; Sawant, A.; Jiang, K. Technical note: A small animal irradiation platform for investigating the dependence of the FLASH effect on electron beam parameters. Med. Phys. 2024, 51, 1421–1432. [Google Scholar] [CrossRef]
- Sloop, A.; Ashraf, M.R.; Rahman, M.; Sunnerberg, J.; Dexter, C.A.; Thompson, L.; Gladstone, D.J.; Pogue, B.W.; Bruza, P.; Zhang, R. Rapid Switching of a C-Series Linear Accelerator Between Conventional and Ultrahigh-Dose-Rate Research Mode With Beamline Modifications and Output Stabilization. Int. J. Radiat. Oncol. 2024, 119, 1317–1325. [Google Scholar] [CrossRef]
- Oh, K.; Gallagher, K.J.; Hyun, M.; Schott, D.; Wisnoskie, S.; Lei, Y.; Hendley, S.; Wong, J.; Wang, S.; Graff, B.; et al. Initial experience with an electron FLASH research extension (FLEX) for the Clinac system. J. Appl. Clin. Med. Phys. 2023, 25, e14159. [Google Scholar] [CrossRef]
- Cetnar, A.J.; Jain, S.; Gupta, N.; Chakravarti, A. Technical note: Commissioning of a linear accelerator producing ultra-high dose rate electrons. Med. Phys. 2023, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Bello, R.D.; von der Grün, J.; Fabiano, S.; Rudolf, T.; Saltybaeva, N.; Stark, L.S.; Ahmed; Bathula, M.; Dogan, S.K.; McNeur, J.; et al. Enabling ultra-high dose rate electron beams at a clinical linear accelerator for isocentric treatments. Radiother. Oncol. 2023, 187, 109822. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Allen, B.D.; Acharya, M.M.; Montay-Gruel, P.; Jorge, P.G.; Bailat, C.; Petit, B.; Vozenin, M.-C.; Limoli, C. Maintenance of Tight Junction Integrity in the Absence of Vascular Dilation in the Brain of Mice Exposed to Ultra-High-Dose-Rate FLASH Irradiation. Radiat. Res. 2020, 194, 625–635. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Markarian, M.; Allen, B.D.; Baddour, J.D.; Giedzinski, E.; Jorge, P.G.; Petit, B.; Bailat, C.; Vozenin, M.-C.; Limoli, C.; et al. Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain. Radiat. Res. 2020, 194, 636–645. [Google Scholar] [CrossRef]
- Alaghband, Y.; Cheeks, S.N.; Allen, B.D.; Montay-Gruel, P.; Doan, N.-L.; Petit, B.; Jorge, P.G.; Giedzinski, E.; Acharya, M.M.; Vozenin, M.-C.; et al. Neuroprotection of Radiosensitive Juvenile Mice by Ultra-High Dose Rate FLASH Irradiation. Cancers 2020, 12, 1671. [Google Scholar] [CrossRef]
- Ursino, S.; Gadducci, G.; Giannini, N.; Gonnelli, A.; Fuentes, T.; Di Martino, F.; Paiar, F. New insights on clinical perspectives of FLASH radiotherapy: From low- to very high electron energy. Front. Oncol. 2023, 13, 1254601. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Greene, D.; Williams, P. Linear Accelerators for Radiation Therapy; Taylor & Francis: London, UK, 2017. [Google Scholar]
- Karsch, L.; Beyreuther, E.; Burris-Mog, T.; Kraft, S.; Richter, C.; Zeil, K.; Pawelke, J. Dose rate dependence for different dosimeters and detectors: TLD, OSL, EBT films, and diamond detectors. Med. Phys. 2012, 39, 2447–2455. [Google Scholar] [CrossRef]
- Jaccard, M.; Petersson, K.; Buchillier, T.; Germond, J.; Durán, M.T.; Vozenin, M.; Bourhis, J.; Bochud, F.O.; Bailat, C. High dose-per-pulse electron beam dosimetry: Usability and dose-rate independence of EBT3 Gafchromic films. Med. Phys. 2017, 44, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Villoing, D.; Koumeir, C.; Bongrand, A.; Guertin, A.; Haddad, F.; Métivier, V.; Poirier, F.; Potiron, V.; Servagent, N.; Supiot, S.; et al. Technical note: Proton beam dosimetry at ultra-high dose rates (FLASH): Evaluation of GAFchromic™ (EBT3, EBT-XD) and OrthoChromic (OC-1) film performances. Med. Phys. 2022, 49, 2732–2745. [Google Scholar] [CrossRef]
- Micke, A.; Lewis, D.F.; Yu, X. Multichannel film dosimetry with nonuniformity correction. Med. Phys. 2011, 38, 2523–2534. [Google Scholar] [CrossRef]
- Christensen, J.B.; Togno, M.; Nesteruk, K.P.; Psoroulas, S.; Meer, D.; Weber, D.C.; Lomax, A.J.; Yukihara, E.G.; Safai, S. Al2O3:C optically stimulated luminescence dosimeters (OSLDs) for ultra-high dose rate proton dosimetry. Phys. Med. Biol. 2021, 66, 085003. [Google Scholar] [CrossRef]
- Liu, K.; Velasquez, B.; Schüler, E. Technical note: High-dose and ultra-high dose rate (UHDR) evaluation of Al2O3:C optically stimulated luminescent dosimeter nanoDots and powdered LiF:Mg,Ti thermoluminescent dosimeters for radiation therapy applications. Med. Phys. 2023, 51, 2311–2319. [Google Scholar] [CrossRef]
- Motta, S.; Bello, R.D.; Christensen, J.B.; Bossin, L.; Yukihara, E.G. Dosimetry of ultra-high dose rate electron beams using thermoluminescence and optically stimulated luminescence detectors. Phys. Med. Biol. 2024, 69, 035022. [Google Scholar] [CrossRef] [PubMed]
- Kry, S.F.; Alvarez, P.; Cygler, J.E.; DeWerd, L.A.; Howell, R.M.; Meeks, S.; O’Daniel, J.; Reft, C.; Sawakuchi, G.; Yukihara, E.G.; et al. AAPM TG 191: Clinical use of luminescent dosimeters: TLDs and OSLDs. Med. Phys. 2019, 47, E19–E51. [Google Scholar] [CrossRef] [PubMed]
- Meigooni, A.S.; Das, I.J. Parametrisation of depth dose for electron beams. Phys. Med. Biol. 1987, 32, 761–768. [Google Scholar] [CrossRef]
- Jiang, S.B.; Kapur, A.; Ma, C. Electron beam modeling and commissioning for Monte Carlo treatment planning. Med. Phys. 2000, 27, 180–191. [Google Scholar] [CrossRef]
- Arce, P.; Lagares, J.I.; Harkness, L.; Pérez-Astudillo, D.; Cañadas, M.; Rato, P.; de Prado, M.; Abreu, Y.; de Lorenzo, G.; Kolstein, M.; et al. Gamos: A framework to do Geant4 simulations in different physics fields with an user-friendly interface. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 735, 304–313. [Google Scholar] [CrossRef]
- Ma, C.M.C.; Chetty, I.J.; Deng, J.; Faddegon, B.; Jiang, S.B.; Li, J.; Seuntjens, J.; Siebers, J.V.; Traneus, E. Beam modeling and beam model commissioning for Monte Carlo dose calculation-based radiation therapy treatment planning: Report of AAPM Task Group 157. Med. Phys. 2019, 47, E1–E18. [Google Scholar] [CrossRef]
- Rahman, M.; Ashraf, M.R.; Gladstone, D.J.; Bruza, P.; Jarvis, L.A.; Schaner, P.E.; Cao, X.; Pogue, B.W.; Hoopes, P.J.; Zhang, R. Treatment Planning System for Electron FLASH Radiation Therapy: Open-Source for Clinical Implementation. Int. J. Radiat. Oncol. 2022, 112, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, E.C.; A Faddegon, B. Sensitivity of large-field electron beams to variations in a Monte Carlo accelerator model. Phys. Med. Biol. 2005, 50, 769–778. [Google Scholar] [CrossRef]
- A Faddegon, B.; Perl, J.; Asai, M. Monte Carlo simulation of large electron fields. Phys. Med. Biol. 2008, 53, 1497–1510. [Google Scholar] [CrossRef]
- EL Bakkali, J.; EL Bardouni, T. Validation of Monte Carlo Geant4 code for a 6 MV Varian linac. J. King Saud Univ.-Sci. 2017, 29, 106–113. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Germond, J.-F.; Bourhis, J.; Vozenin, M.-C.; Ozsahin, E.M.; Bochud, F.; Bailat, C.; Moeckli, R. Normal Tissue Sparing by FLASH as a Function of Single-Fraction Dose: A Quantitative Analysis. Int. J. Radiat. Oncol. 2022, 114, 1032–1044. [Google Scholar] [CrossRef]
- Bijl, H.P.; van Luijk, P.; Coppes, R.P.; Schippers, J.M.; Konings, A.W.; van der Kogel, A.J. Dose-volume effects in the rat cervical spinal cord after proton irradiation. Int. J. Radiat. Oncol. 2002, 52, 205–211. [Google Scholar] [CrossRef]
- No, H.J.; Wu, Y.; Dworkin, M.L.; Manjappa, R.; Skinner, L.; Ashraf, M.R.; Lau, B.; Melemenidis, S.; Viswanathan, V.; Yu, A.S.-J.; et al. Clinical Linear Accelerator-Based Electron FLASH: Pathway for Practical Translation to FLASH Clinical Trials. Int. J. Radiat. Oncol. 2023, 117, 482–492. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Psoroulas, S.; Aylward, J.D.; Beddar, S.; Douralis, A.; Delpon, G.; Garibaldi, C.; Gasparini, A.; Schüler, E.; Stephan, F.; et al. Recording and reporting of ultra-high dose rate “FLASH” delivery for preclinical and clinical settings. Radiother. Oncol. 2024, 200, 110507. [Google Scholar] [CrossRef]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, L.; van der Kogel, A.; Wong, J.W.; Iordachita, I.; Zhang, R.; Chirayath, V.A.; Timmerman, R.; Lu, W.; Ahn, C.; et al. Establish a Flash Research Platform Optimized for Rat Spinal Cord Study. In Proceedings of the AAPM 66th Annual Meeting & Exhibition, AAPM, Los Angeles, CA, USA, 21–25 July 2024. [Google Scholar]
- Zhou, B.; Guo, L.; Tsai, Y.-C.; van der Kogel, A.; Wong, J.; Iordachita, I.; Zhang, R.; Chirayath, V.A.; Timmerman, R.; Lu, W.; et al. Abstract 1820: Investigating whether FLASH radiotherapy spares late-responding organs using a rat spinal cord model. Cancer Res. 2025, 85, 1820. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Guo, L.; Lu, W.; Rahman, M.; Zhang, R.; Chirayath, V.A.; Park, Y.K.; Stojadinovic, S.; Garza, M.; Wang, K.K.-H. Establishing an Electron FLASH Platform for Preclinical Research in Low-Resource Settings. Radiation 2025, 5, 33. https://doi.org/10.3390/radiation5040033
Zhou B, Guo L, Lu W, Rahman M, Zhang R, Chirayath VA, Park YK, Stojadinovic S, Garza M, Wang KK-H. Establishing an Electron FLASH Platform for Preclinical Research in Low-Resource Settings. Radiation. 2025; 5(4):33. https://doi.org/10.3390/radiation5040033
Chicago/Turabian StyleZhou, Banghao, Lixiang Guo, Weiguo Lu, Mahbubur Rahman, Rongxiao Zhang, Varghese Anto Chirayath, Yang Kyun Park, Strahinja Stojadinovic, Marvin Garza, and Ken Kang-Hsin Wang. 2025. "Establishing an Electron FLASH Platform for Preclinical Research in Low-Resource Settings" Radiation 5, no. 4: 33. https://doi.org/10.3390/radiation5040033
APA StyleZhou, B., Guo, L., Lu, W., Rahman, M., Zhang, R., Chirayath, V. A., Park, Y. K., Stojadinovic, S., Garza, M., & Wang, K. K.-H. (2025). Establishing an Electron FLASH Platform for Preclinical Research in Low-Resource Settings. Radiation, 5(4), 33. https://doi.org/10.3390/radiation5040033





