An “Older Old” Woman with Large Squamous Cell Carcinoma of the Nasal Pyramid: Excellent Response to Ultra-Hypofractionated Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
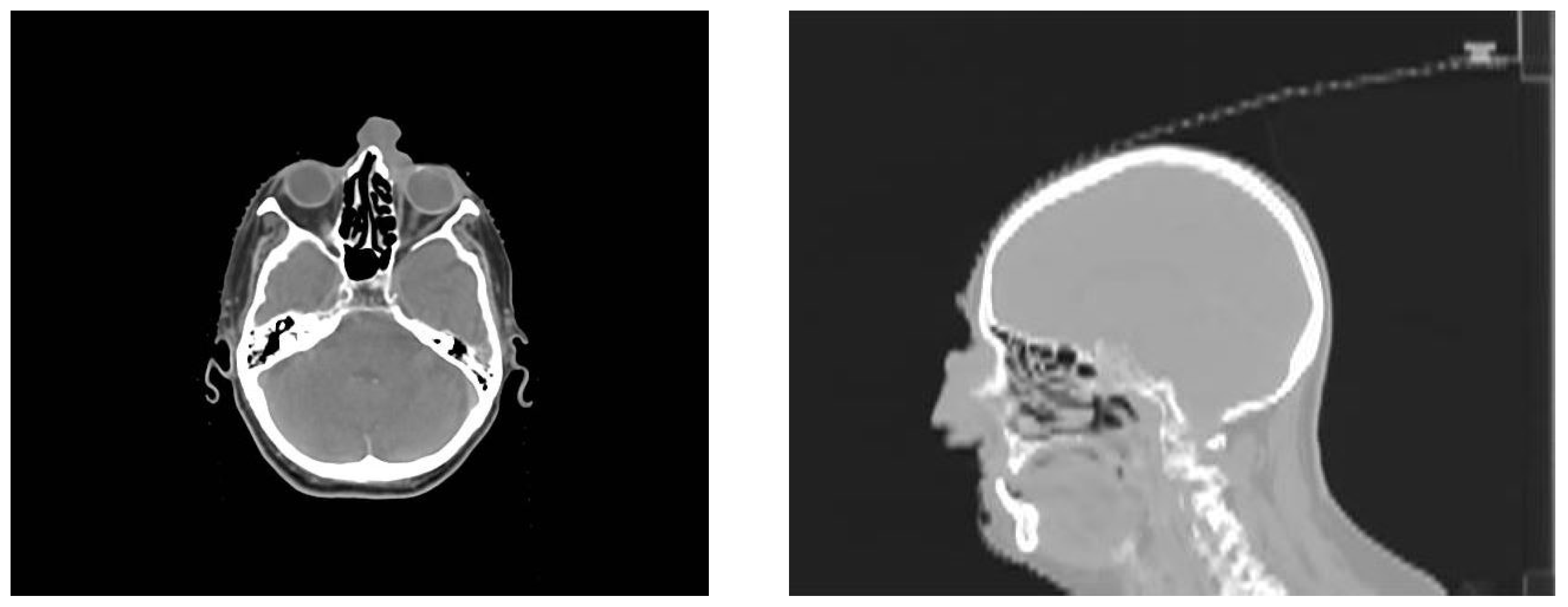
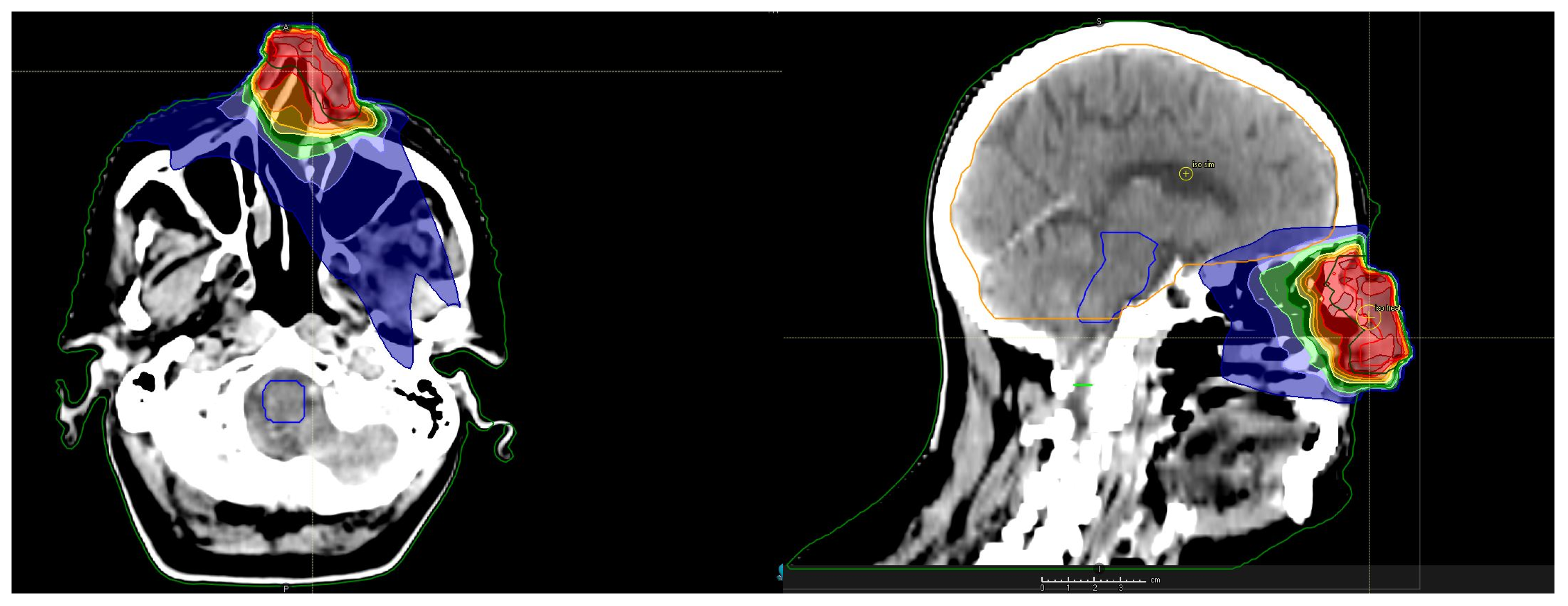
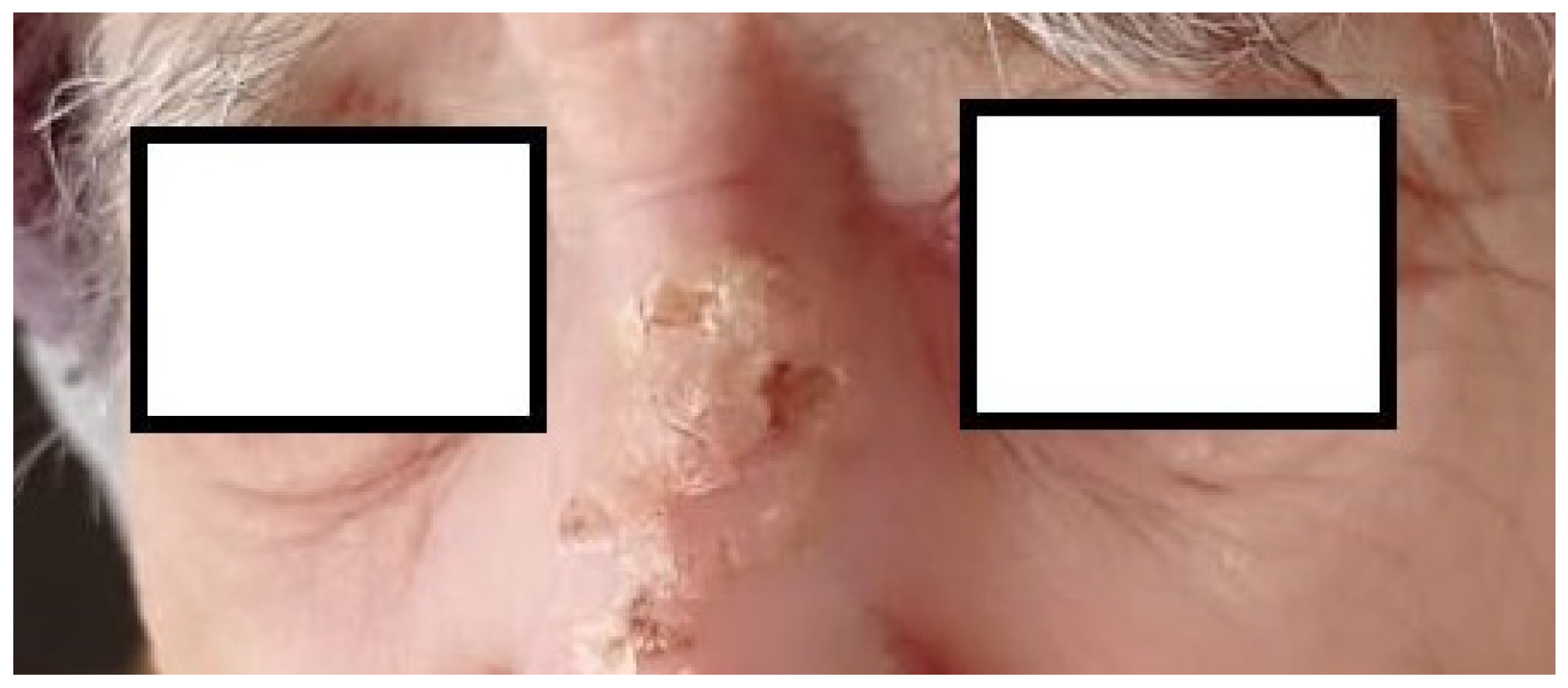
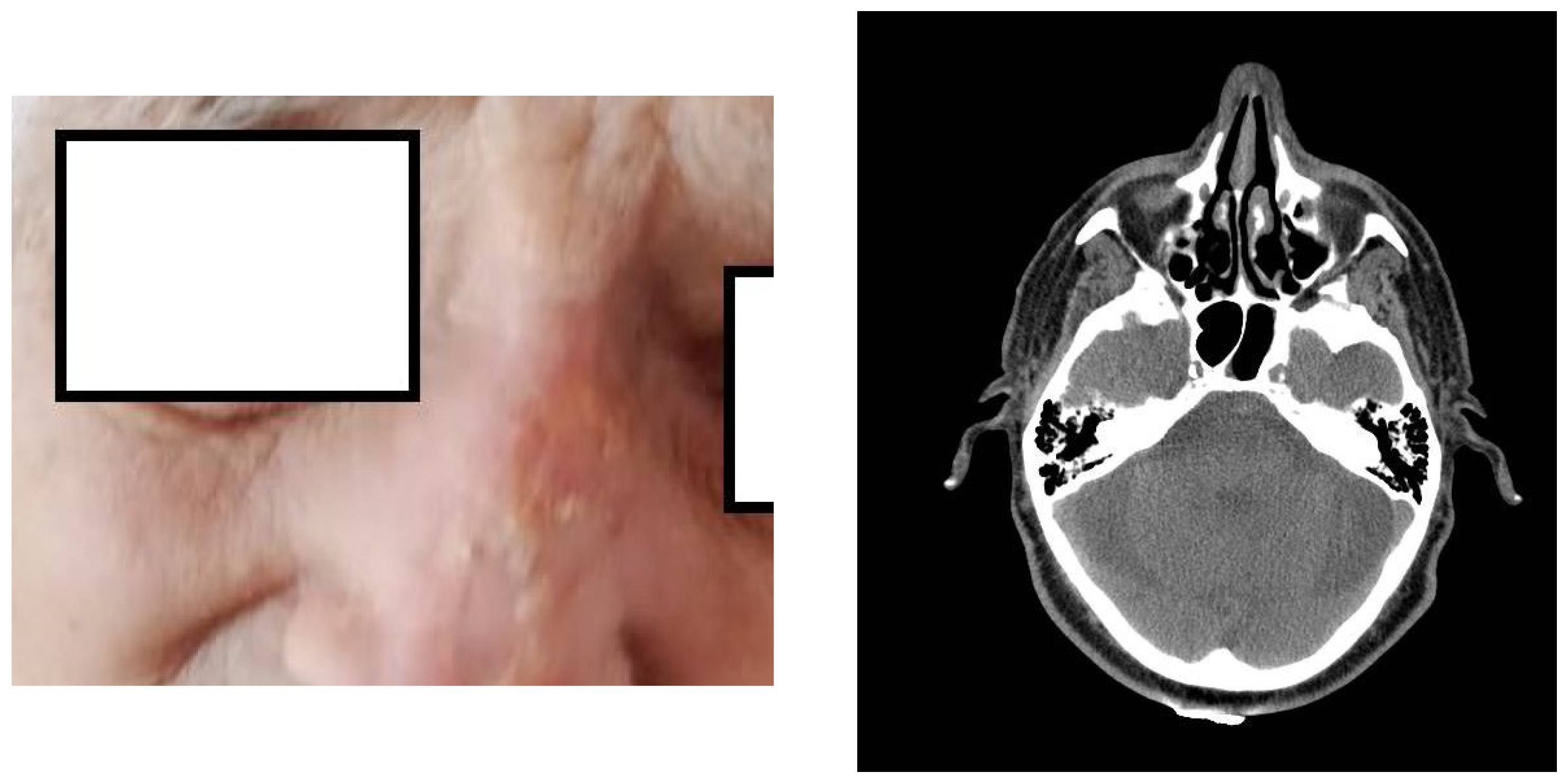
2. Presentation of the Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | radiotherapy |
| VMAT | volumetric modulated arc therapy |
| cSCC | cutaneous squamous cell carcinoma |
| WHO | World Health Organization |
| Gy | Gray |
| CT | computed tomography |
| ECOG | Eastern Cooperative Oncology Group |
| CASP-12 | Control, Autonomy, Self-Realization, and Pleasure scale, 12 items |
| MV | mega voltage |
| IMRT | intensity-modulated radiotherapy |
| BED | biological equivalent dose |
| ASTRO | American Society for Radiation Oncology |
| 3D | three-dimensional |
| QoL | quality of life |
| NRS | Numeric Rating Scale |
References
- Nakamura, A.; Kataoka, K.; Takatsuka, S.; Takenouchi, T. Aging trends in skin cancer: A long-term observational study in Japan. JAAD Int. 2023, 13, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Leus, A.J.G.; Haisma, M.S.; Terra, J.B.; Diercks, G.F.H.; Van Kester, M.S.; Halmos, G.B.; Rácz, E.; Van Dijk, B.A.C.; Plaat, B.E.C. Age-related Differences in Tumour Characteristics and Prognostic Factors for Disease Progression in Cutaneous Squamous Cell Carcinoma of the Head and Neck. Acta Derm. Venereol. 2022, 102, 347. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bajorek, B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm. Pract. 2014, 12, 489. [Google Scholar] [CrossRef]
- Mountzios, G. Optimal management of the elderly patient with head and neck cancer: Issues regarding surgery, irradiation and chemotherapy. World J. Clin. Oncol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Montégut, L.; López-Otín, C.; Kroemer, G. Aging and cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Alattas, A.; Shuweihdi, F.; Best, K.; Nikolova, S.; West, R. Measurement Invariance of a Quality-of-life Measure, CASP-12, within the English Longitudinal Study of Ageing (ELSA). Appl. Res. Qual. Life 2024. [Google Scholar] [CrossRef]
- Oliver, A.; Sentandreu-Mañó, T.; Tomás, J.M.; Fernández, I.; Sancho, P. Quality of Life in European Older Adults of SHARE Wave 7: Comparing the Old and the Oldest-Old. J. Clin. Med. 2021, 10, 2850. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, C.P.; Heijmer, A.; Van Harten-Krouwel, D.G.; Van Den Bos, F.; De Bree, R.; Witteveen, P.O.; Emmelot-Vonk, M.H. Validation of the G8 screening tool in older patients with cancer considered for surgical treatment. J. Geriatr. Oncol. 2021, 12, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Hughley, B.B.; Schmalbach, C.E. Cutaneous Head and Neck Malignancies in the Elderly. Clin. Geriatr. Med. 2018, 34, 245–258. [Google Scholar]
- Ishii, R.; Ohkoshi, A.; Katori, Y. Treatment of elderly patients with head and neck cancer in an aging society: Focus on geriatric assessment and surgical treatment. Auris Nasus Larynx 2024, 51, 647–658. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef]
- Cuperus, E.; Leguit, R.; Albregts, M.; Toonstra, J. Post radiation skin tumors: Basal cell carcinomas, squamous cell carcinomas and angiosarcomas. A review of this late effect of radiotherapy. Eur. J. Dermatol. 2013, 23, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kiafi, P.; Kouri, M.A.; Patatoukas, G.; Kougioumtzopoulou, A.; Chalkia, M.; Nicolatou-Galitis, O.; Kouloulias, V.; Kyrodimos, E.; Platoni, K. Unravelling Quality of Life for Head and Neck Cancer Patients after VMAT Radiation Therapy: Insights from Toxicity, Dosimetry and Symptoms Correlation. Clin. Pract. 2024, 14, 1085–1099. [Google Scholar] [CrossRef]
- Ferini, G.; Palmisciano, P.; Forte, S.; Viola, A.; Martorana, E.; Parisi, S.; Valenti, V.; Fichera, C.; Umana, G.E.; Pergolizzi, S. Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: The Current and Future Role of Radiation Therapy in the Era of Immunotherapy. Cancers 2022, 14, 1871. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lebas, E.; Marchal, N.; Rorive, A.; Nikkels, A.F. Cemiplimab for locally advanced cutaneous squamous cell carcinoma: Safety, efficacy, and position in therapy panel. Expert. Rev. Anticancer. Ther. 2021, 21, 355–363. [Google Scholar] [CrossRef]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.A.; Smith, J.; Ainslie, J.; McEwan, L. Treatment of nonmelanoma skin cancer at a large australian center. Cancer 1990, 63, 1863–1871. [Google Scholar] [CrossRef]
- Chan, S.; Dhadda, A.S.; Swindell, R. Single Fraction Radiotherapy for Small Superficial Carcinoma of the Skin. Clin. Oncol. 2007, 19, 256–259. [Google Scholar] [CrossRef]
- Haseltine, J.M.; Parker, M.; Wernicke, A.G.; Nori, D.; Wu, X.; Parashar, B. Clinical comparison of brachytherapy versus hypofractionated external beam radiation versus standard fractionation external beam radiation for non-melanomatous skin cancers. J. Contemp. Brachyther. 2016, 3, 189–194. [Google Scholar] [CrossRef]
- Levendag, P.C.; Nijdam, W.M.; Van Moolenburgh, S.E.; Tan, L.; Noever, I.; Van Rooy, P.; Mureau, M.A.M.; Jansen, P.P.; Munte, K.; Hofer, S.O.P. Interstitial radiation therapy for early-stage nasal vestibule cancer: A continuing quest for optimal tumor control and cosmesis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Olek, D.; El-Ghamry, M.N.; Deb, N.; Thawani, N.; Shaver, C.; Mutyala, S. Custom mold applicator high-dose-rate brachytherapy for nonmelanoma skin cancer—An analysis of 273 lesions. Brachytherapy 2018, 17, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Tsang, R.W.; Liu, F.-F.; Panzarella, T.; Rotstein, L. Radiotherapy management for squamous cell carcinoma of the nasal skin: The Princess Margaret Hospital experience. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Kadah, B.A.; Niewald, M.; Papaspyrou, G.; Dzierma, Y.; Schneider, M.; Schick, B. Customized individual applicators for endocavitary brachytherapy in patients with cancers of the nasal cavity, sinonasal region and nasopharynx. Eur. Arch. Otorhinolaryngol. 2016, 273, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Musio, D.; Tombolini, V. Weekly hypofractionated radiation therapy in elderly non-resectable cutaneous squamous cell carcinoma of the head and neck region. Radiol. Med. 2021, 126, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Roberson, J.; Patel, R.; Slutsky, J.B.; Ryu, S.; Xu, Z.; Valentine, E. Tumor control and cosmetic outcome of weekly iridium-192 high-dose-rate brachytherapy for nonmelanoma skin cancers in the elderly. Brachytherapy 2021, 20, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Likhacheva, A.; Awan, M.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Zaky, S.; Devlin, P.M. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.; Lutz, S.T. The role of hypofractionated radiation in the management of non- osseous metastatic or uncontrolled local cancer. Ann. Palliat. Med. 2014, 3, 291–303. [Google Scholar]
- Piras, A.; Boldrini, L.; Menna, S.; Venuti, V.; Pernice, G.; Franzese, C.; Angileri, T.; Daidone, A. Hypofractionated Radiotherapy in Head and Neck Cancer Elderly Patients: A Feasibility and Safety Systematic Review for the Clinician. Front. Oncol. 2021, 11, 761393. [Google Scholar] [CrossRef]
- Fryen, A.; Brandes, I.; Wichmann, J.; Christiansen, H.; Tavassol, F.; Durisin, M.; Merten, R. Moderately Hypofractionated Radiotherapy without Chemotherapy in Elderly or Frail Patients with Head and Neck Cancer. In Vivo 2022, 36, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.-L.; Domschikowski, J.; Krug, D.; Sonnhoff, M.; Nitsche, M.; Hoffmann, W.; Becker-Schiebe, M.; Bock, F.; Hoffmann, M.; Schmalz, C.; et al. The impact of palliative radiotherapy on health-related quality of life in patients with head and neck cancer—Results of a multicenter prospective cohort study. Clin. Transl. Radiat. Oncol. 2023, 41, 100633. [Google Scholar] [CrossRef] [PubMed]
- Desideri, I.; Becherini, C.; Belgioia, L.; Merlotti, A.; Ciccone, L.P.; Franzese, C.; Loi, M.; De Felice, F.; Mazzola, R.; Caini, S.; et al. Palliative radiotherapy in older adults with head and neck squamous cell carcinoma: A systematic review. Oral. Oncol. 2021, 119, 105355. [Google Scholar] [CrossRef] [PubMed]
- Cothran, A.; Martin, J.C. Hypofractionated Radiation: Understanding the Modality and Impact on Patient Outcomes. Clin. J. Oncol. Nurs. 2022, 26, 23–26. [Google Scholar] [PubMed]
- Zingeta, G.T.; Worku, Y.T.; Awol, M.; Woldetsadik, E.S.; Assefa, M.; Chama, T.Z.; Feyisa, J.D.; Bedada, H.F.; Adem, M.I.; Mengesha, T.; et al. Outcome of Hypofractionated Palliative Radiotherapy Regimens for Patients with Advanced Head and Neck Cancer in Tikur Anbessa Hospital, Ethiopia: A Prospective Cohort Study. JCO Glob. Oncol. 2024, 10, e2300253. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Song, J.H.; Kim, B.H.; Kwak, Y.-K.; Kim, K.S.; Yoo, G.S.; Byun, H.K.; Kim, Y.J.; Kim, Y.-S. Evidence-based clinical recommendations for hypofractionated radiotherapy: Exploring efficacy and safety—Part 1. Brain and head and neck. Radiat. Oncol. J. 2024, 42, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Muto, P.; Pastore, F. Radiotherapy in the Adjuvant and Advanced Setting of CSCC. Dermatol. Pract. Concept. 2021, 11, e2021168S. [Google Scholar] [CrossRef]
- Veness, M. Hypofractionated radiotherapy in older patients with non-melanoma skin cancer: Less is better. Australas. J. Dermatol. 2018, 59, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Deodato, F.; Macchia, G.; Gentileschi, S.; Cilla, S.; Torre, G.; Padula, G.D.A.; Nuzzo, M.; Massaccesi, M.; Valentini, V.; et al. Short-Course Radiotherapy in Elderly Patients with Early Stage Non-Melanoma Skin Cancer: A Phase II Study. Cancer Investig. 2015, 33, 34–38. [Google Scholar] [CrossRef]
- Katano, A.; Minamitani, M.; Tongyu, G.; Ohira, S.; Yamashita, H. Survival Following Palliative Radiotherapy for Head and Neck Squamous Cell Carcinoma: Examining Treatment Indications in Elderly Patients. Cancer Diagn. Progn. 2024, 4, 46–50. [Google Scholar] [CrossRef]
- Nguyen, N.T.A.; Doerwald-Munoz, L.; Wright, J.; Kim, D.H.; Sagar, S.M.; Hodson, D.I. Phase 2 Study of “0-7-21” Hypofractionated Palliative Radiation Therapy for Advanced Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, S128. [Google Scholar] [CrossRef]
- De Felice, F.; Serpone, M.; Cattaneo, C.G.; Di Giammarco, F.; Fallico, A.; Delle Donne, A.; Lanzilao, M.; Vitti, E.; Marampon, F.; Musio, D.; et al. Definitive weekly hypofractionated radiotherapy in surgery-ineligible older adults with cutaneous squamous cell carcinoma of the head and neck region. J. Geriatr. Oncol. 2024, 15, 101596. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, P.; Desideri, I.; Loi, M.; Lo Russo, M.; Olmetto, E.; Maragna, V.; Francolini, G.; Delli Paoli, C.; Grassi, R.; Pezzulla, D.; et al. Elderly patients affected by head and neck squamous cell carcinoma unfit for standard curative treatment: Is de-intensified, hypofractionated radiotherapy a feasible strategy? Oral Oncol. 2017, 74, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Thariat, J.; Gorobets, O.; Vinh-Hung, V.; Kim, L.; Blanco, S.C.; Vasileiou, M.; Arenas, M.; Mazibuko, T.; Giap, H.; et al. Immunotherapy and Hypofractionated Radiotherapy in Older Patients with Locally Advanced Cutaneous Squamous-Cell Carcinoma of the Head and Neck: A Proposed Paradigm by the International Geriatric Radiotherapy Group. Cancers 2023, 15, 4981. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, C.; Gennari, A.; Carriero, A.; Krengli, M.; Franco, P. An “Older Old” Woman with Large Squamous Cell Carcinoma of the Nasal Pyramid: Excellent Response to Ultra-Hypofractionated Radiation Therapy. Radiation 2024, 4, 232-241. https://doi.org/10.3390/radiation4030018
Pisani C, Gennari A, Carriero A, Krengli M, Franco P. An “Older Old” Woman with Large Squamous Cell Carcinoma of the Nasal Pyramid: Excellent Response to Ultra-Hypofractionated Radiation Therapy. Radiation. 2024; 4(3):232-241. https://doi.org/10.3390/radiation4030018
Chicago/Turabian StylePisani, Carla, Alessandra Gennari, Alessandro Carriero, Marco Krengli, and Pierfrancesco Franco. 2024. "An “Older Old” Woman with Large Squamous Cell Carcinoma of the Nasal Pyramid: Excellent Response to Ultra-Hypofractionated Radiation Therapy" Radiation 4, no. 3: 232-241. https://doi.org/10.3390/radiation4030018
APA StylePisani, C., Gennari, A., Carriero, A., Krengli, M., & Franco, P. (2024). An “Older Old” Woman with Large Squamous Cell Carcinoma of the Nasal Pyramid: Excellent Response to Ultra-Hypofractionated Radiation Therapy. Radiation, 4(3), 232-241. https://doi.org/10.3390/radiation4030018






