The German Uranium Miners’ Biobank—A Biobank for OMICs Radiation Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
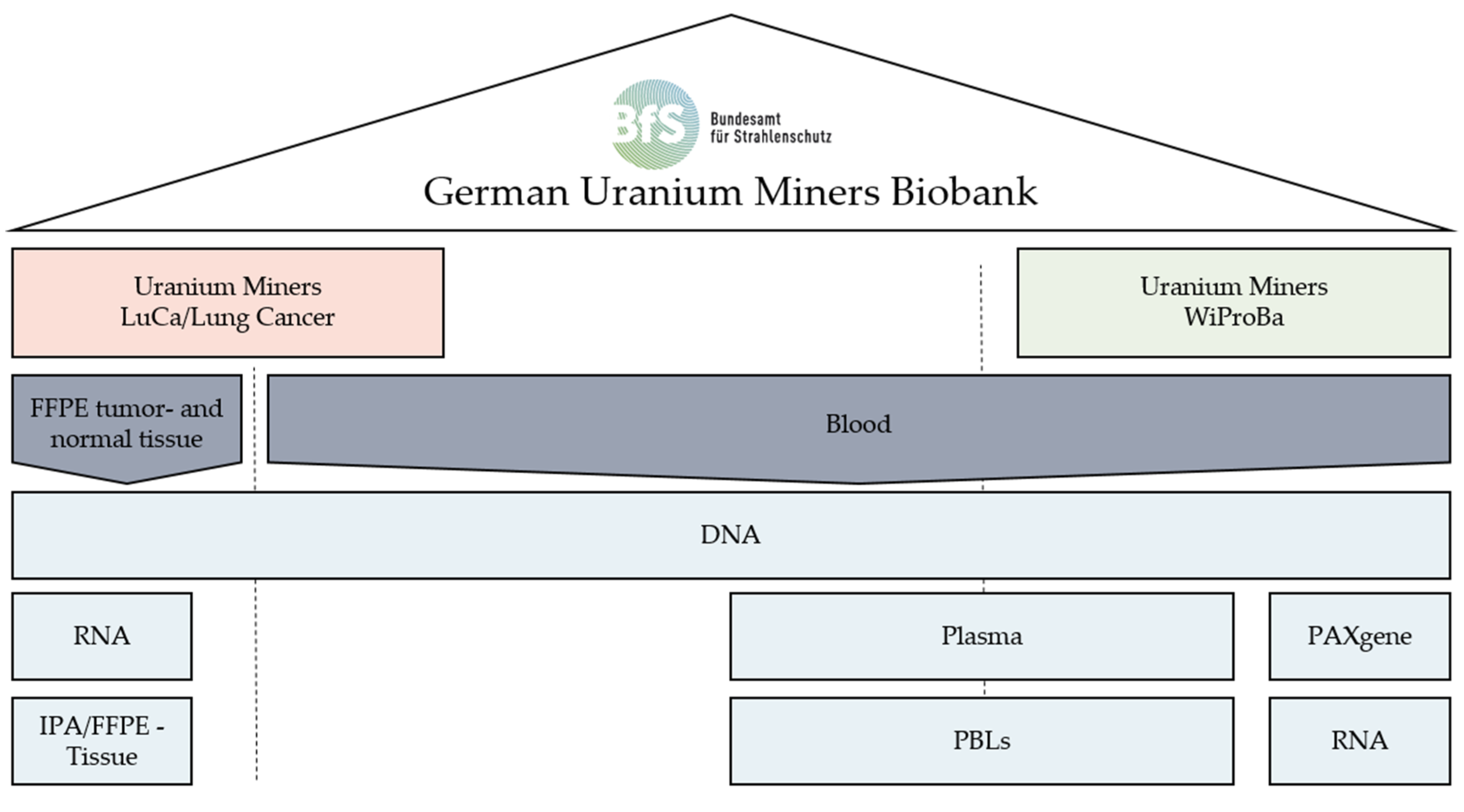
2.1. Wismut Biosample Bank from Blood: WiProBa
2.1.1. Biological Samples
2.1.2. DNA Extraction
2.1.3. PAXgene Tube Sampling for RNA Extraction
2.1.4. Plasma Biobanking
2.1.5. Isolation of Peripheral Blood Leukocytes
2.1.6. Individual Data
2.1.7. Protection of Personal Data
2.2. Sampling of DNA and RNA from the Tissue of Lung Cancer Cases in the Pathological Archive: LuCa
2.2.1. DNA Isolation from FFPE Tissue
2.2.2. RNA Isolation from FFPE Tissue
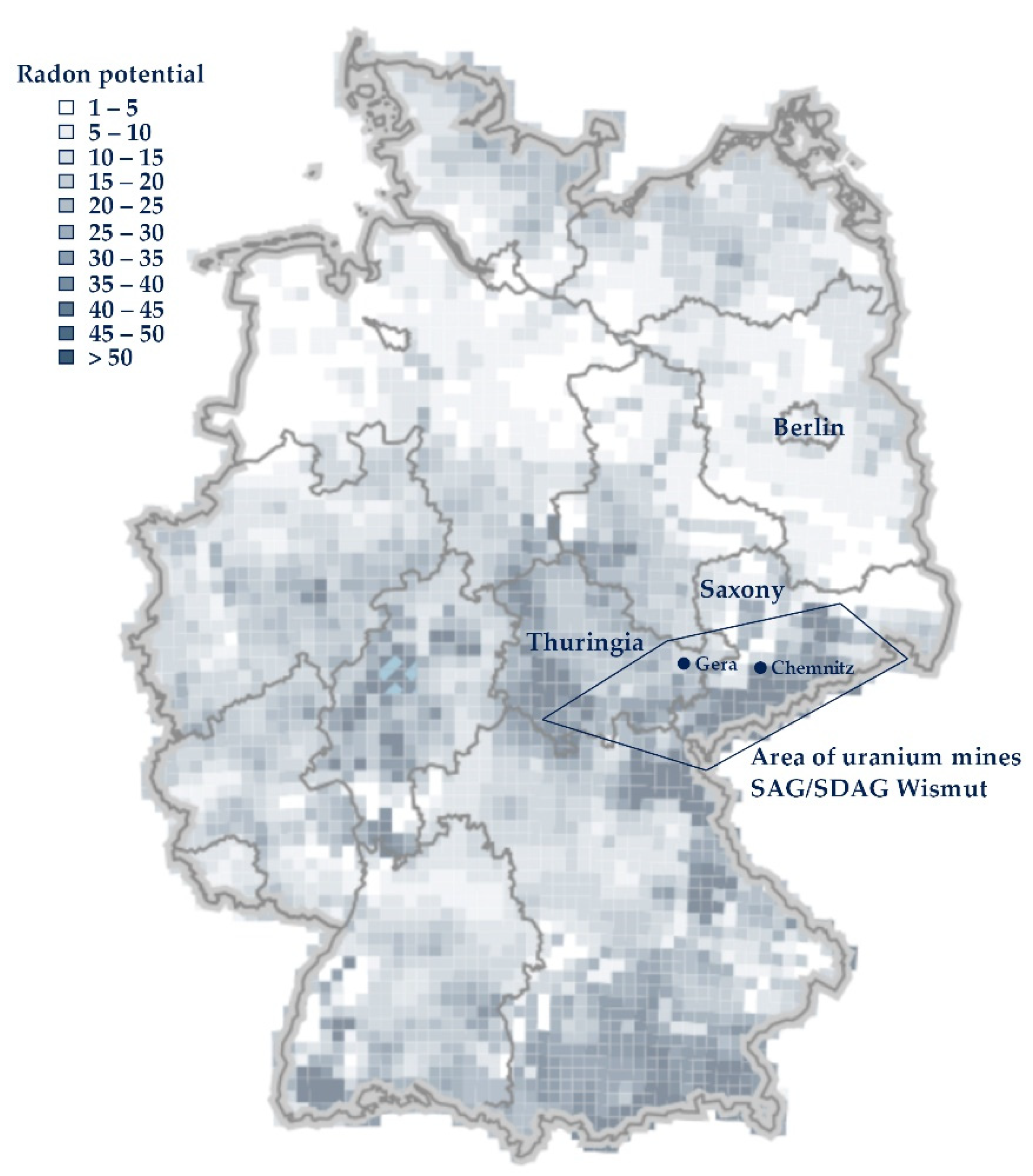
2.3. Indoor Radon (Wismut Cases): Lung Cancer
3. Results
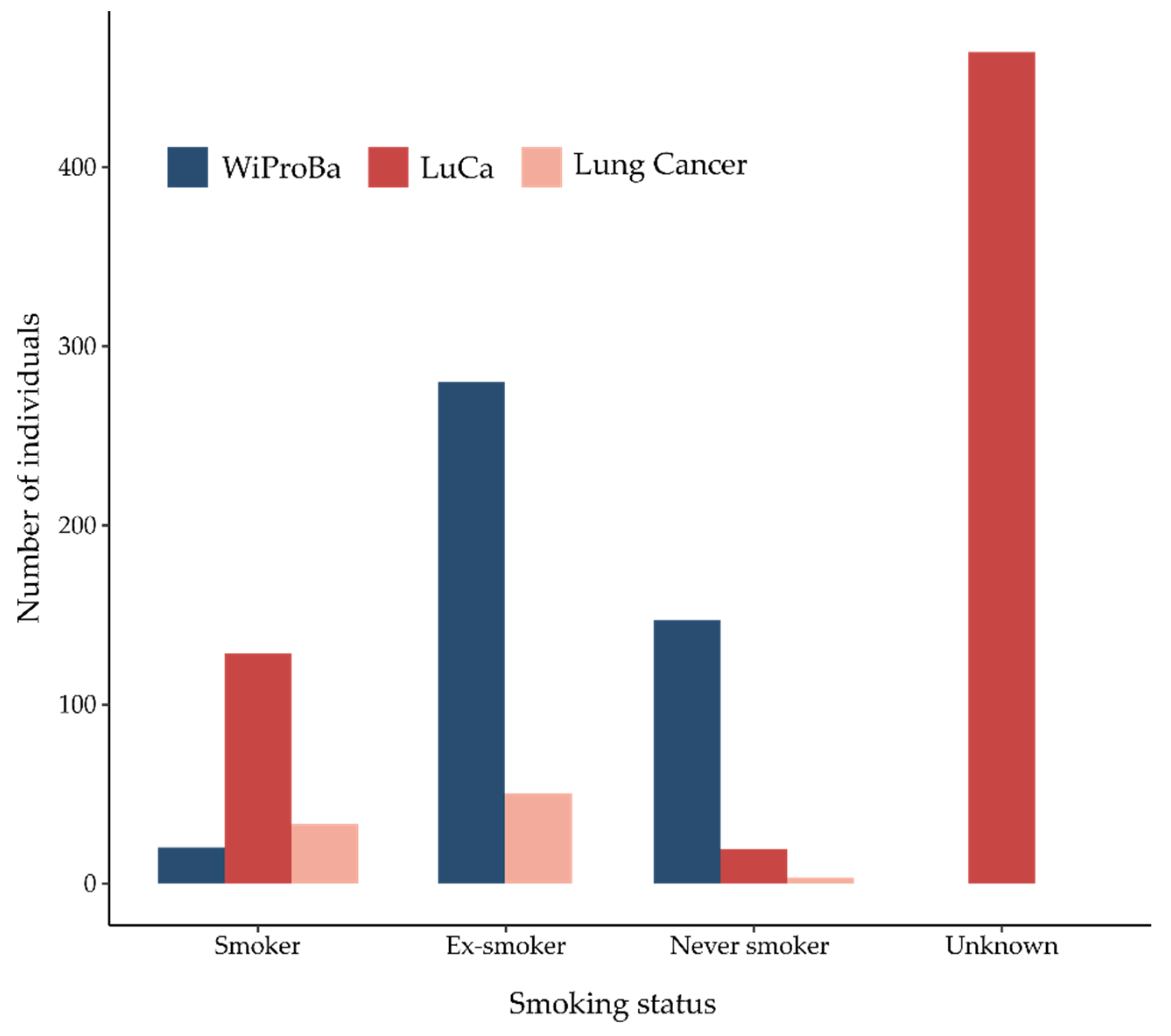
3.1. WiProBa
3.2. Lung Cancer Cases
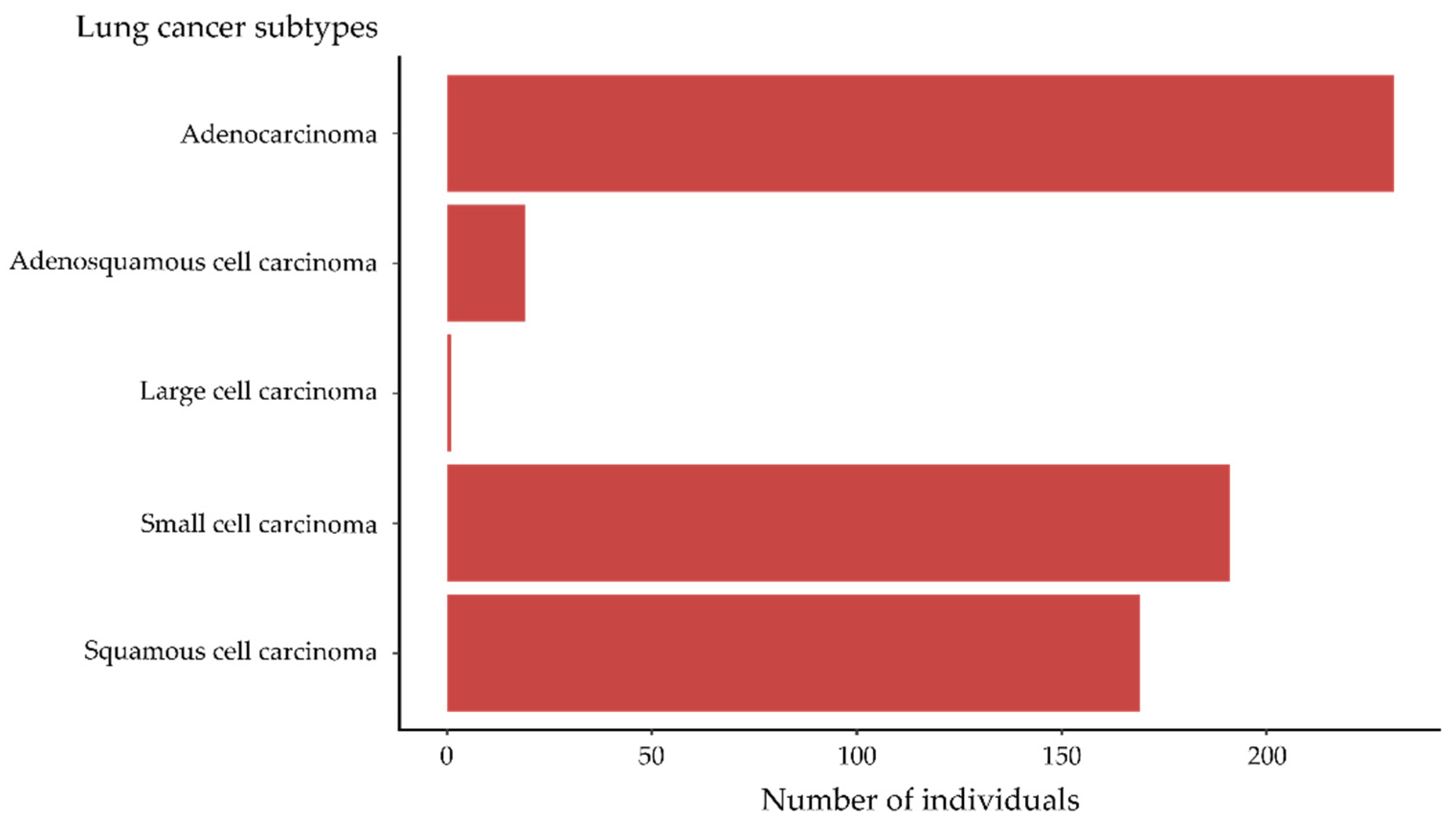
3.2.1. LuCa
3.2.2. Lung Cancer
3.3. Available Samples and Data
3.4. Access to GUMB Material and Data
4. Discussion
- the risk of low doses for organs other than lungs,
- individual genetic susceptibility to radon-induced lung cancer,
- long-term radiation-induced biological effects and their associated adverse health effects other than lung cancer,
- the combined effects of co-carcinogenic lung exposure such as radiation and arsenic dust,
- potential radon-specific fingerprints in lung cancer tissue depending on tumor subtype.
4.1. High-Dose and Low-Dose-Associated Health Effects
4.2. Radon Risk-Modifying Factors
4.3. Signatures in Radon-Induced Lung Tumor Tissue
4.4. Radiation-Induced Long-Term Effects
4.5. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreuzer, M.; Grosche, B.; Brachner, A.; Martignoni, K.; Schnelzer, M.; Schopka, H.J.; Brüske-Hohlfeld, I.; Wichmann, H.E.; Burkart, W. The German Uranium Miners Cohort Study: Feasibility and First Results. Radiat. Res. 1999, 152, S56–S58. [Google Scholar] [CrossRef] [PubMed]
- Wesch, H.; Wiethege, T.; Spiethoff, A.; Wegener, K.; Müller, K.M.; Mehlhorn, J. German Uranium Miner Study—Historical Background and Available Histopathological Material. Radiat. Res. 1999, 152, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Petermann, E.; Meyer, H.; Nussbaum, M.; Bossew, P. Mapping the Geogenic Radon Potential for Germany by Machine Learning. Sci. Total Environ. 2021, 754, 142291. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Schnelzer, M.; Tschense, A.; Walsh, L.; Grosche, B. Cohort Profile: The German Uranium Miners Cohort Study (Wismut Cohort), 1946–2003. Int. J. Epidemiol. 2010, 39, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuzer, M.; Deffner, V.; Schnelzer, M.; Fenske, N. Mortality in Underground Miners in a Former Uranium Ore Mine. Dtsch. Ärzteblatt Int. 2021, 118, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dahmann, D.; Bauer, H.D.; Stoyke, G. Retrospective Exposure Assessment for Respirable and Inhalable Dust, Crystalline Silica and Arsenic in the Former German Uranium Mines of Sag/Sdag Wismut. Int. Arch. Occup. Environ. Health 2008, 81, 949–958. [Google Scholar] [CrossRef]
- HVBG; BBG. Belastung Durch Ionisierende Strahlung, Staub und Arsen im Uranerzbergbau der Ehemaligen DDR (Version 08/2005); Bergbau BG (BBG): Gera, Germany; Hauptverband der Gewerblichen Berufsgenossenschaften (HVBG) (CD-ROM): Sankt Augustin, Germany, 1998. [Google Scholar]
- Lehmann, F.; Hambeck, L.; Linhart, K.K.; Lutze, H.; Meyer, H.; Reiber, H.; Renner, H.K.; Reinisch, A.; Seifert, T.; Wolf, F. Belastung Durch Ionisierende Strahlung im Uranerzbergbau der Ehemaligen DDR; Hauptverband der Gewerblichen Berufgenossenschaften: Sankt Augustin, Germany, 1998. [Google Scholar]
- Kreuzer, M.; Grosche, B.; Schnelzer, M.; Tschense, A.; Walsh, L. The German Uranium Miners Cohort Study (Wismut Cohort), 1946–2003: Technical Report; Bundesamt für Strahlenschutz (BfS): Salzgitter, Germany, 2011; Available online: http://nbn-resolving.de/urn:nbn:de:0221-201102185211 (accessed on 5 November 2021).
- Marsh, J.W.; Bessa, Y.; Birchall, A.; Blanchardon, E.; Hofmann, W.; Nosske, D.; Tomasek, L. Dosimetric Models Used in the Alpha-Risk Project to Quantify Exposure of Uranium Miners to Radon Gas and Its Progeny. Radiat. Prot. Dosim. 2008, 130, 101–106. [Google Scholar] [CrossRef]
- Marsh, J.W.; Blanchardon, E.; Gregoratto, D.; Hofmann, W.; Karcher, K.; Nosske, D.; Tomásek, L. Dosimetric Calculations for Uranium Miners for Epidemiological Studies. Radiat. Prot. Dosim. 2012, 149, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Brüske-Hohlfeld, I.; Rosario, A.S.; Wölke, G.; Heinrich, J.; Kreuzer, M.; Kreienbrock, L.; Wichmann, H.E. Lung Cancer Risk among Former Uranium Miners of the Wismut Company in Germany. Health Phys. 2006, 90, 208–216. [Google Scholar] [CrossRef]
- Rosenberger, A.; Hung, R.J.; Christiani, D.C.; Caporaso, N.E.; Liu, G.; Bojesen, S.E.; le Marchand, L.; Haiman, C.A.; Albanes, D.; Aldrich, M.C.; et al. Genetic Modifiers of Radon-Induced Lung Cancer Risk: A Genome-Wide Interaction Study in Former Uranium Miners. Int. Arch. Occup. Environ. Health 2018, 91, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Pesch, B.; Johnen, G.; Lehnert, M. Aufbau Einer Bioproben-Bank von Eeemaligen Beschäftigten der SAG/SDAG Wismut—Pilotstudie Vorhaben 3608S04532. In Ressortforschungsberichte zur Kerntechnischen Sicherheit und Zum Strahlenschutz; Bundesamt für Strahlenschutz (BfS): Salzgitter, Germany, 2015; Available online: http://nbn-resolving.de/urn:nbn:de:0221-2015102213745 (accessed on 29 November 2021).
- Guéguen, Y.; Roy, L.; Hornhardt, S.; Badie, C.; Hall, J.; Baatout, S.; Pernot, E.; Tomasek, L.; Laurent, O.; Ebrahimian, T.; et al. Biomarkers for Uranium Risk Assessment for the Development of the Cure (Concerted Uranium Research in Europe) Molecular Epidemiological Protocol. Radiat. Res. 2017, 187, 107–127. [Google Scholar] [CrossRef]
- Walsh, L.; Grosche, B.; Schnelzer, M.; Tschense, A.; Sogl, M.; Kreuzer, M. A Review of the Results from the German Wismut Uranium Miners Cohort. Radiat. Prot. Dosim. 2015, 164, 147–153. [Google Scholar] [CrossRef]
- Storedatabase. Available online: https://www.storedb.org/store_v3/ (accessed on 29 November 2021).
- Wiethege, T.; Wesch, H.; Wegener, K.; Müller, K.M.; Mehlhorn, J.; Spiethoff, A.; Schömig, D.; Hollstein, M.; Bartsch, H. German Uranium Miner Study-Pathological and Molecular Genetic Findings. German Uranium Miner Study, Research Group Pathology. Radiat. Res. 1999, 152, S52–S55. [Google Scholar] [CrossRef]
- Kreuzer, M.; Sogl, M.; Brüske, I.; Möhner, M.; Nowak, D.; Schnelzer, M.; Walsh, L. Silica Dust, Radon and Death from Non-Malignant Respiratory Diseases in German Uranium Miners. Occup. Environ. Med. 2013, 70, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sogl, M.; Taeger, D.; Pallapies, D.; Brüning, T.; Dufey, F.; Schnelzer, M.; Straif, K.; Walsh, L.; Kreuzer, M. Quantitative Relationship between Silica Exposure and Lung Cancer Mortality in German Uranium Miners, 1946–2003. Br. J. Cancer 2012, 107, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.G.; Casjens, S.; Rozynek, P.; Lehnert, M.; Zilch-Schöneweis, S.; Bryk, O.; Taeger, D.; Gomolka, M.; Kreuzer, M.; Otten, H.; et al. Assessment of mRNA and microRNA Stabilization in Peripheral Human Blood for Multicenter Studies and Biobanks. Biomark Insights 2010, 5, 95–102. [Google Scholar] [CrossRef]
- Bonin, M. Expression Changes as Cause of Chronical Radiation Exposition to Uranium (Wismut)-Miners 3611s10010. In Strahlenschutzforschung Programmreport; Bundesamt für Strahlenschutz (BfS): Salzgitter, Germany, 2015; pp. 2–5. [Google Scholar]
- Johnen, G.; Brüning, T.; Weber, D.G. Analyse Epigenetischer Effekte (Mikro Rnas) in Ehemaligen Wismutbeschäftigten—Vorhaben 3610s10001. In Ressortforschungsberichte zur Kerntechnischen Sicherheit und Zum Strahlenschutz; Bundesamt für Strahlenschutz (BfS): Salzgitter, Germany, 2014; Available online: http://nbn-resolving.de/urn:nbn:de:0221-2014051311415 (accessed on 29 November 2021).
- Salomaa, S.; Jourdain, J.R.; Kreuzer, M.; Jung, T.; Repussard, J. Multidisciplinary European Low Dose Initiative: An Update of the Melodi Program. Int. J. Radiat. Biol. 2017, 93, 1035–1039. [Google Scholar] [CrossRef]
- Kreuzer, M.; Auvinen, A.; Cardis, E.; Durante, M.; Harms-Ringdahl, M.; Jourdain, J.R.; Madas, B.G.; Ottolenghi, A.; Pazzaglia, S.; Prise, K.M.; et al. Multidisciplinary European Low Dose Initiative (Melodi): Strategic Research Agenda for Low Dose Radiation Risk Research. Radiat. Environ. Biophys. 2018, 57, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurier, D.; Marsh, J.W.; Rage, E.; Tomasek, L. Miner Studies and Radiological Protection against Radon. Ann. ICRP 2020, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Rage, E.; Richardson, D.B.; Demers, P.A.; Do, M.; Fenske, N.; Kreuzer, M.; Samet, J.; Wiggins, C.; Schubauer-Berigan, M.K.; Kelly-Reif, K.; et al. Puma—Pooled Uranium Miners Analysis: Cohort Profile. Occup. Environ. Med. 2020, 77, 194–200. [Google Scholar] [CrossRef]
- Kreuzer, M.; Sobotzki, C.; Fenske, N.; Marsh, J.W.; Schnelzer, M. Leukaemia Mortality and Low-Dose Ionising Radiation in the Wismut Uranium Miner Cohort (1946–2013). Occup. Environ. Med. 2017, 74, 252–258. [Google Scholar] [CrossRef]
- Präleukämische Genetische Veränderungen Und Klonale Hämatopoese Bei Deutschen Uranbergarbeitern Der Wismut Biobank. Available online: https://gepris.dfg.de/gepris/projekt/433083317?context=projekt&task=showDetail&id=433083317& (accessed on 5 November 2021).
- Steensma, D.P.; Ebert, B.L. Clonal Hematopoiesis as a Model for Premalignant Changes During Aging. Exp. Hematol. 2020, 83, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [Green Version]
- Möhner, M.; Lindtner, M.; Otten, H.; Gille, H.G. Leukemia and Exposure to Ionizing Radiation among German Uranium Miners. Am. J. Ind. Med. 2006, 49, 238–248. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; la Vecchia, C.; Boffetta, P. Risk Factors for Lung Cancer Worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Kreuzer, M.; Sobotzki, C.; Schnelzer, M.; Fenske, N. Factors Modifying the Radon-Related Lung Cancer Risk at Low Exposures and Exposure Rates among German Uranium Miners. Radiat. Res. 2018, 189, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, A.; Rössler, U.; Hornhardt, S.; Sauter, W.; Bickeböller, H.; Wichmann, H.E.; Gomolka, M. Heritability of Radiation Response in Lung Cancer Families. Genes 2012, 3, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarnke, A.M.; Tharmalingam, S.; Boreham, D.R.; Brooks, A.L. Beir Vi Radon: The Rest of the Story. Chem. Biol. Interact. 2019, 301, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Choi, J.W.; Hong, M.H.; Jung, D.; Lee, C.Y.; Park, S.Y.; Shim, H.S.; Sheen, S.; Kwak, K.I.; Kang, D.R.; et al. Indoor Radon Exposure Increases Tumor Mutation Burden in Never-Smoker Patients with Lung Adenocarcinoma. Lung Cancer 2019, 131, 139–146. [Google Scholar] [CrossRef]
- Azimzadeh, O.; Gomolka, M.; Birschwilks, M.; Saigusa, S.; Grosche, B.; Moertl, S. Advanced Omics and Radiobiological Tissue Archives: The Future in the Past. Appl. Sci. 2021, 11, 11108. [Google Scholar] [CrossRef]
- Wang, T.; Shao, W.; Huang, Z.; Tang, H.; Zhang, J.; Ding, Z.; Huang, K. Mogonet Integrates Multi-Omics Data Using Graph Convolutional Networks Allowing Patient Classification and Biomarker Identification. Nat. Commun. 2021, 12, 3445. [Google Scholar] [CrossRef] [PubMed]
- Daga, S.; Fallerini, C.; Baldassarri, M.; Fava, F.; Valentino, F.; Doddato, G.; Benetti, E.; Furini, S.; Giliberti, A.; Tita, R.; et al. Employing a Systematic Approach to Biobanking and Analyzing Clinical and Genetic Data for Advancing COVID-19 Research. Eur J. Hum. Genet. 2021, 29, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Thapa, I.; Ali, H. A Multiomics Graph Database System for Biological Data Integration and Cancer Informatics. J. Comput. Biol. 2021, 28, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.J. Can Radiation Research Impact the Estimation of Risk? Int. J. Radiat. Biol. 2017, 93, 1009–1014. [Google Scholar] [CrossRef]



| Cohort | Number of Individuals | Origin of Material | DNA | RNA | Cells | Plasma | PAXgene |
|---|---|---|---|---|---|---|---|
| WiProBa | 419 | Blood | 419 | 418 | 399 | 369 | 418 |
| LuCa | 617 | FFPE tissue | |||||
| Tumor | 617 | 612 | |||||
| Non-Tumor | 612 | 607 |
| Experimental Data | Number of Samples | Literature | Technique |
|---|---|---|---|
| DNA methylation: | |||
| ATM methylation | 441 | Pesch et al. 2015 [14] | Bisulfite/PCR |
| LINE1 methylation | 441 | Pesch et al. 2015 [14] | Bisulfite/PCR |
| SNP typing | 413 | Rosenberger et al. 2018 [13] | Infinium OncoArray-500K chip |
| RNA expression: | |||
| ATM expression | 438 | Pesch et al. 2015 [14] | qRT PCR |
| GAPDH expression | 438 | Pesch et al. 2015 [14] | qRT PCR |
| Whole genome RNA expression | 196 | Bonin 2015 [22] | Affymetrix (Human Genome U219 GeneChip) |
| RNAseq analyses | 22 | Bonin 2015 [22] | NGS |
| miRNA expression | 61 | Johnen et al. 2014 [23] | Custom-based Microarray |
| Chromosomal aberrations | 120 | Unpublished | mFISH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomolka, M.; Bucher, M.; Duchrow, L.; Hochstrat, B.; Taeger, D.; Johnen, G.; Moertl, S. The German Uranium Miners’ Biobank—A Biobank for OMICs Radiation Research. Radiation 2022, 2, 62-77. https://doi.org/10.3390/radiation2010005
Gomolka M, Bucher M, Duchrow L, Hochstrat B, Taeger D, Johnen G, Moertl S. The German Uranium Miners’ Biobank—A Biobank for OMICs Radiation Research. Radiation. 2022; 2(1):62-77. https://doi.org/10.3390/radiation2010005
Chicago/Turabian StyleGomolka, Maria, Martin Bucher, Lukas Duchrow, Beate Hochstrat, Dirk Taeger, Georg Johnen, and Simone Moertl. 2022. "The German Uranium Miners’ Biobank—A Biobank for OMICs Radiation Research" Radiation 2, no. 1: 62-77. https://doi.org/10.3390/radiation2010005
APA StyleGomolka, M., Bucher, M., Duchrow, L., Hochstrat, B., Taeger, D., Johnen, G., & Moertl, S. (2022). The German Uranium Miners’ Biobank—A Biobank for OMICs Radiation Research. Radiation, 2(1), 62-77. https://doi.org/10.3390/radiation2010005






