Magnetic Resonance Diffusion-Weighted Imaging for Detecting Fundal Intracholecystic Papillary Neoplasm inside Rokitansky-Aschoff Sinuses: A Comparison of Two Cases and a Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
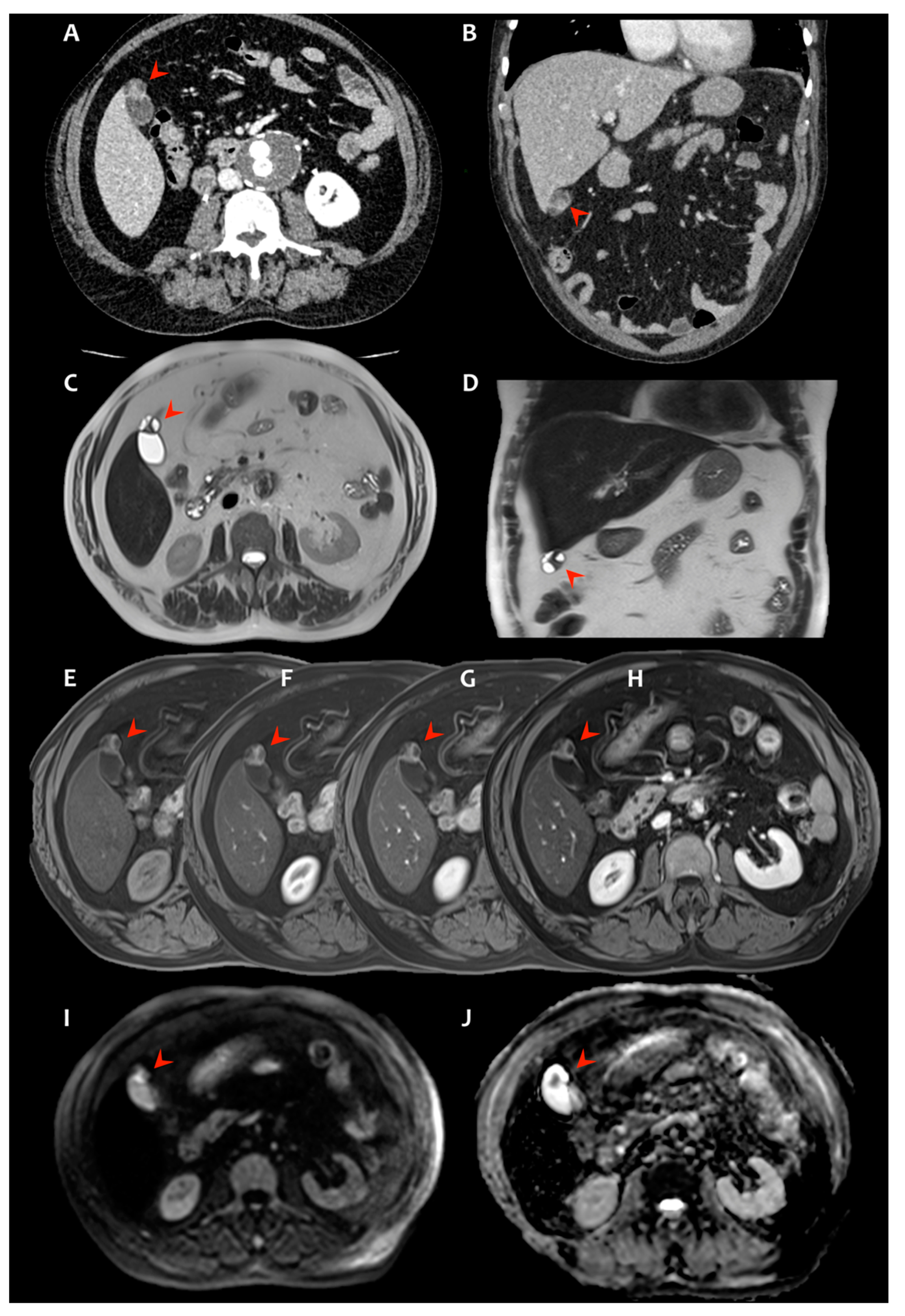
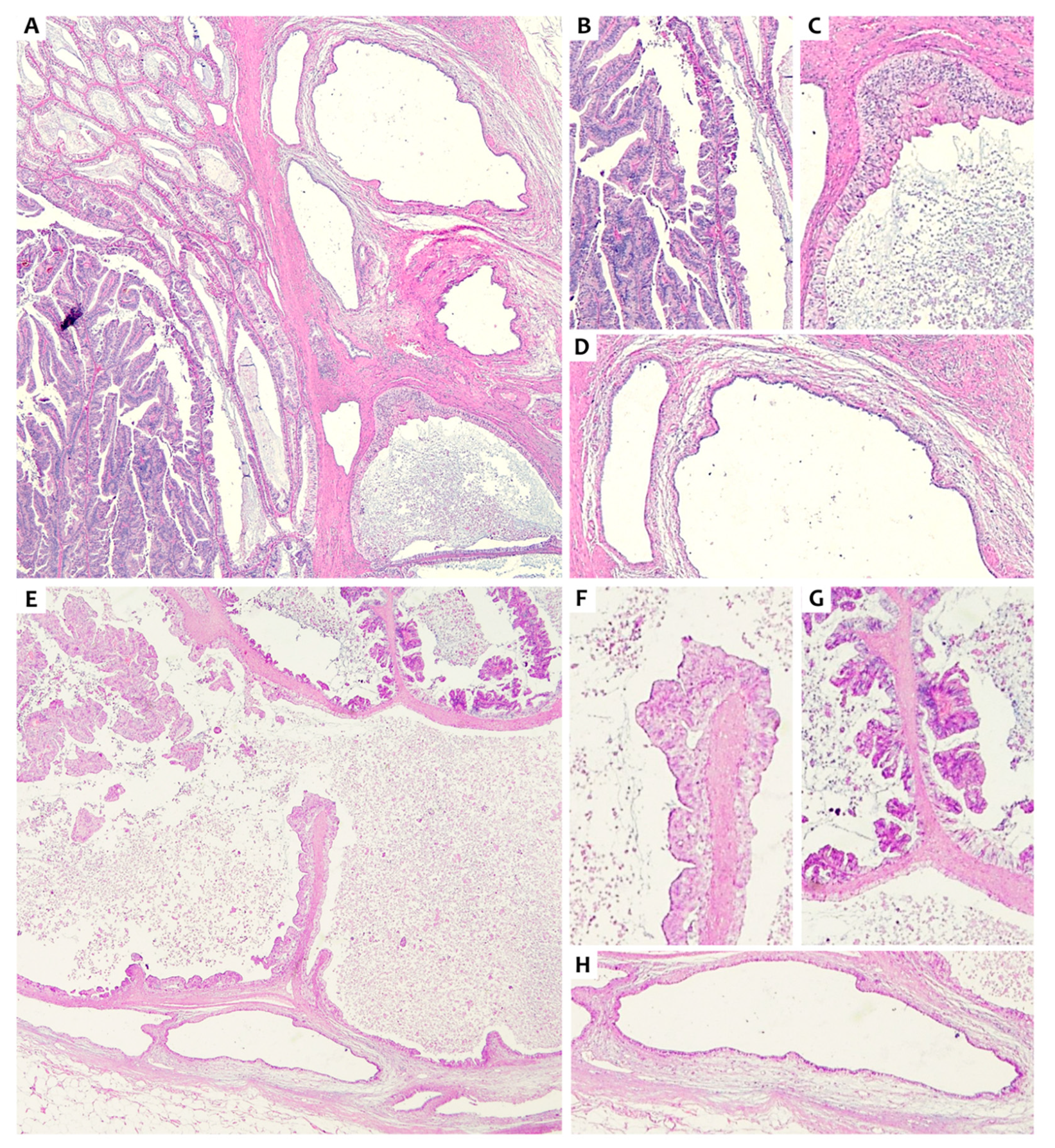
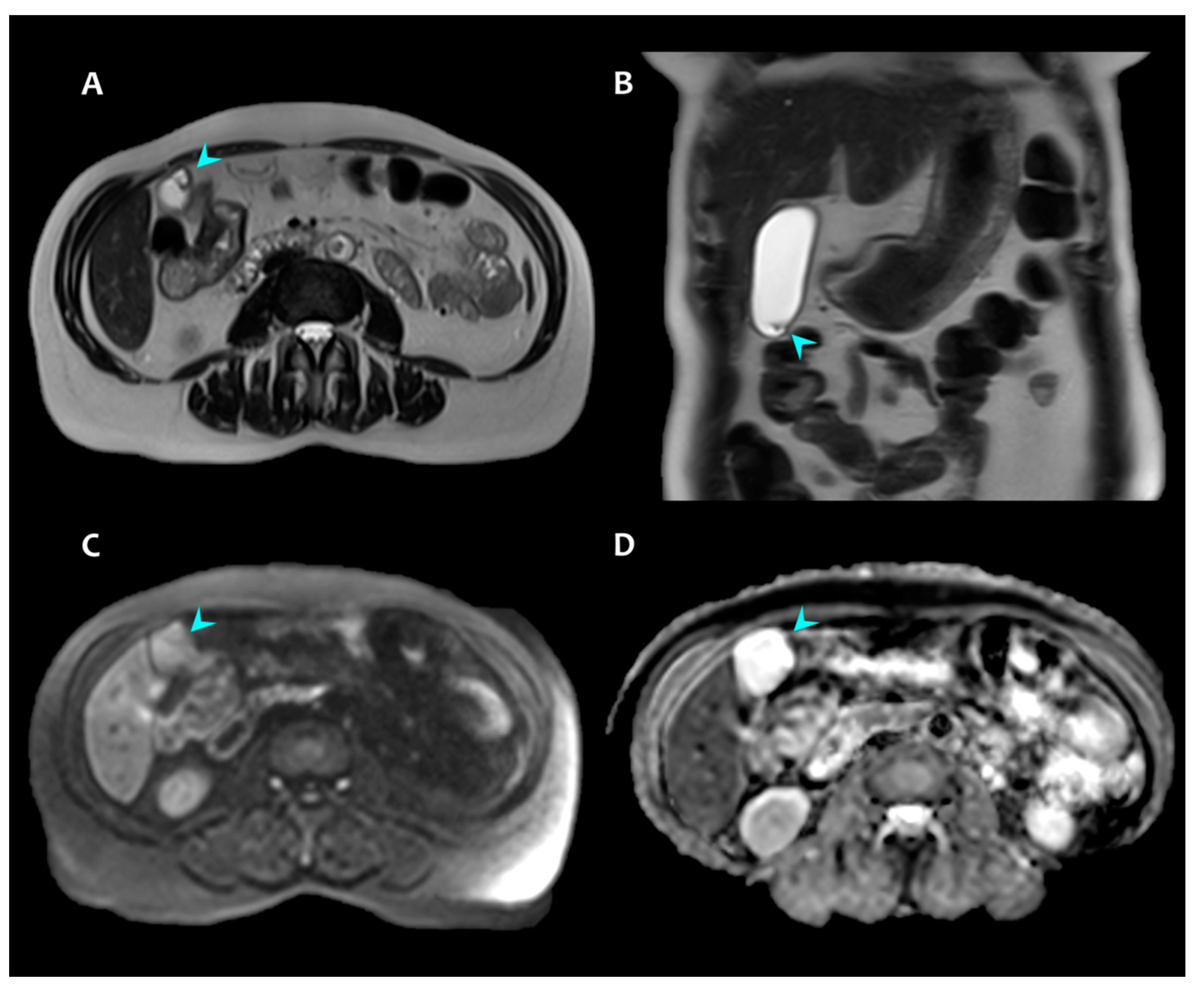
2. Presentation of Case #1
3. Presentation of Case #2
4. Discussion and Review of the Literature
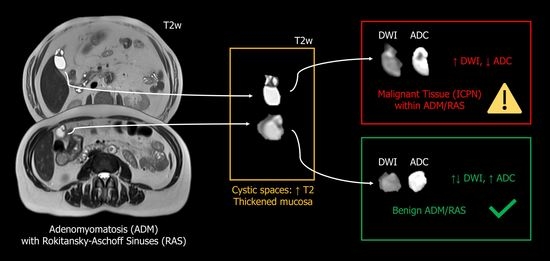
4.1. Intracholecystic Papillary Neoplasm (ICPN) and Rokitansky-Aschoff Sinuses (RAS)
4.2. Diffusion MRI to Discriminate Malignant and Benign Gallbladder Lesions
4.3. Diffusion MRI in ICPN/IPNB
4.4. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| AUC | area under the curve |
| ADM | adenomyomatosis |
| CECT | contrast-enhanced computed tomography |
| CE-MRI | contrast-enhanced MRI |
| ICPN | intracholecystic papillary neoplasm |
| IPMN | intraductal papillary-mucinous neoplasm |
| IPNB | intraductal papillary neoplasm of the bile duct |
| MR-DWI | Magnetic Resonance Diffusion-Weighted Imaging |
| MRI | Magnetic Resonance Imaging |
| RAS | Rokitansky-Aschoff sinuses |
| US | Ultrasound |
References
- Golse, N.; Lewin, M.; Rode, A.; Sebagh, M.; Mabrut, J.-Y. Gallbladder adenomyomatosis: Diagnosis and management. J. Visc. Surg. 2017, 154, 345–353. [Google Scholar] [CrossRef]
- Bonatti, M.; Vezzali, N.; Lombardo, F.; Ferro, F.; Zamboni, G.; Tauber, M.; Bonatti, G. Gallbladder adenomyomatosis: Imaging findings, tricks and pitfalls. Insights Imaging 2017, 8, 243–253. [Google Scholar] [CrossRef]
- Adsay, V.; Jang, K.-T.; Roa, J.C.; Dursun, N.; Ohike, N.; Bagci, P.; Basturk, O.; Bandyopadhyay, S.; Cheng, J.D.; Sarmiento, J.M.; et al. Intracholecystic Papillary-Tubular Neoplasms (ICPN) of the Gallbladder (Neoplastic Polyps, Adenomas, and Papillary Neoplasms That Are ≥1.0 cm): Clinicopathologic and immunohistochemical analysis of 123 cases. Am. J. Surg. Pathol. 2012, 36, 1279–1301. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Adsey, N.V. Carcinoma of gallbladder and extrahepatic bile ducts. In World Health Organization Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC: Lyon, France, 2010; pp. 266–272. [Google Scholar]
- Rocha, F.G.; Lee, H.; Katabi, N.; DeMatteo, R.P.; Fong, Y.; D’Angelica, M.I.; Allen, P.J.; Klimstra, D.S.; Jarnagin, W. Intraductal papillary neoplasm of the bile duct: A biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012, 56, 1352–1360. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Shukla, D.; Carrick, K.; Henson, D.E. In Situ and Invasive Adenocarcinomas of the Gallbladder Extending Into or Arising From Rokitansky-Aschoff Sinuses: A Clinicopathologic Study of 49 Cases. Am. J. Surg. Pathol. 2004, 28, 621–628. [Google Scholar] [CrossRef]
- Rowan, D.J.; Pehlivanoglu, B.; Memis, B.; Bagci, P.; Erbarut, I.; Dursun, N.; Jang, K.-T.; Sarmiento, J.; Mucientes, F.; Cheng, J.D.; et al. Mural Intracholecystic Neoplasms Arising in Adenomyomatous Nodules of the Gallbladder: An Analysis of 19 Examples of a Clinicopathologically Distinct Entity. Am. J. Surg. Pathol. 2020, 44, 1649–1657. [Google Scholar] [CrossRef]
- Le Bihan, D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003, 4, 469–480. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The Correlation between Apparent Diffusion Coefficient and Tumor Cellularity in Patients: A Meta-Analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef]
- Barral, M.; Jemal-Turki, A.; Beuvon, F.; Soyer, P.; Camparo, P.; Cornud, F. Cellular density of low-grade transition zone prostate cancer: A limiting factor to correlate restricted diffusion with tumor aggressiveness. Eur. J. Radiol. 2020, 131. [Google Scholar] [CrossRef]
- Driessen, J.P.; Caldas-Magalhaes, J.; Janssen, L.M.; Pameijer, F.A.; Kooij, N.; Terhaard, C.H.J.; Grolman, W.; Philippens, M.E.P. Diffusion-weighted MR Imaging in Laryngeal and Hypopharyngeal Carcinoma: Association between Apparent Diffusion Coefficient and Histologic Findings. Radiology 2014, 272, 456–463. [Google Scholar] [CrossRef]
- Sanvito, F.; Castellano, A.; Falini, A. Advancements in Neuroimaging to Unravel Biological and Molecular Features of Brain Tumors. Cancers 2021, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Muranushi, R.; Saito, H.; Matsumoto, A.; Kato, T.; Tanaka, N.; Nakazato, K.; Morinaga, N.; Shitara, Y.; Ishizaki, M.; Yoshida, T.; et al. A case report of intracholecystic papillary neoplasm of the gallbladder resembling a submucosal tumor. Surg. Case Rep. 2018, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Ando, T.; Tateno, H.; Rikiyama, T.; Furukawa, T.; Ebina, N. Intracystic papillary neoplasm with an associated mucinous adenocarcinoma arising in Rokitansky-Aschoff sinus of the gallbladder. Surg. Case Rep. 2016, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Kang, D.H.; Choi, B.H.; Kim, S.Y.; Lee, J.H. Intracystic Papillary Neoplasm of the Gallbladder Arising from a Localized Adenomyomatous Hyperplasia. Korean J. Pancreas Biliary Tract 2018, 23, 182–189. [Google Scholar] [CrossRef][Green Version]
- Roa, J.C.; Tapia, O.; Manterola, C.; Villaseca, M.; Guzman, P.; Araya, J.C.; Bagci, P.; Saka, B.; Adsay, V. Early gallbladder carcinoma has a favorable outcome but Rokitansky-Aschoff sinus involvement is an adverse prognostic factor. Virchows Arch. 2013, 463, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.Y.; Kim, Y.K.; Min, J.H.; Lee, J.; Cha, D.I.; Lee, S.J. Usefulness of noncontrast MRI in differentiation between gallbladder carcinoma and benign conditions manifesting as focal mild wall thickening. Clin. Imaging 2019, 54, 63–70. [Google Scholar] [CrossRef]
- Chatterjee, A.; Vendrami, C.L.; Nikolaidis, P.; Mittal, P.K.; Bandy, A.J.; Menias, C.O.; Hammond, N.A.; Yaghmai, V.; Yang, G.-Y.; Miller, F.H. Uncommon Intraluminal Tumors of the Gallbladder and Biliary Tract: Spectrum of Imaging Appearances. Radiographics 2019, 39, 388–412. [Google Scholar] [CrossRef]
- Kuipers, H.; Hoogwater, F.J.; Holtman, G.A.; van der Hoorn, A.; de Boer, M.T.; de Haas, R.J. Clinical value of diffusion-weighted MRI for differentiation between benign and malignant gallbladder disease: A systematic review and meta-analysis. Acta Radiol. 2021, 62, 987–996. [Google Scholar] [CrossRef]
- Yu, M.H.; Kim, Y.J.; Park, H.S.; Jung, S.I. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J. Gastroenterol. 2020, 26, 2967–2986. [Google Scholar] [CrossRef]
- Ogawa, T.; Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Koshita, S.; Kanno, Y.; Masu, K.; Sugita, R. High b-value diffusion-weighted magnetic resonance imaging for gallbladder lesions: Differentiation between benignity and malignancy. J. Gastroenterol. 2012, 47, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Shinozaki, F.; Fugo, K.; Sunaoshi, T.; Sugiyama, E.; Kano, D.; Shite, M.; Haga, R.; Fukamizu, Y.; Kagayama, S.; et al. Negative signals for adenomyomatosis of the gallbladder upon diffusion-weighted whole body imaging with background body signal suppression/T2-weighted image fusion analysis. Exp. Ther. Med. 2016, 11, 1777–1780. [Google Scholar] [CrossRef][Green Version]
- Sugita, R.; Yamazaki, T.; Furuta, A.; Itoh, K.; Fujita, N.; Takahashi, S. High b-value diffusion-weighted MRI for detecting gallbladder carcinoma: Preliminary study and results. Eur. Radiol. 2009, 19, 1794–1798. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, Y.K.; Jang, K.-T.; Lee, K.T.; Lee, J.K.; Choi, D.W.; Lim, J.H. Intraductal papillary neoplasm of the bile ducts: Description of MRI and added value of diffusion-weighted MRI. Abdom. Imaging 2013, 38, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.-P.; Rao, S.-X.; Sheng, R.-F.; Zeng, M.-S. Skewness of apparent diffusion coefficient (ADC) histogram helps predict the invasive potential of intraductal papillary neoplasms of the bile ducts (IPNBs). Abdom. Radiol. 2018, 44, 95–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanvito, F.; Gallotti, A.; Cobianchi, L.; Vanoli, A.; Cho, N.S.; Preda, L. Magnetic Resonance Diffusion-Weighted Imaging for Detecting Fundal Intracholecystic Papillary Neoplasm inside Rokitansky-Aschoff Sinuses: A Comparison of Two Cases and a Literature Review. Radiation 2022, 2, 52-61. https://doi.org/10.3390/radiation2010004
Sanvito F, Gallotti A, Cobianchi L, Vanoli A, Cho NS, Preda L. Magnetic Resonance Diffusion-Weighted Imaging for Detecting Fundal Intracholecystic Papillary Neoplasm inside Rokitansky-Aschoff Sinuses: A Comparison of Two Cases and a Literature Review. Radiation. 2022; 2(1):52-61. https://doi.org/10.3390/radiation2010004
Chicago/Turabian StyleSanvito, Francesco, Anna Gallotti, Lorenzo Cobianchi, Alessandro Vanoli, Nicholas S. Cho, and Lorenzo Preda. 2022. "Magnetic Resonance Diffusion-Weighted Imaging for Detecting Fundal Intracholecystic Papillary Neoplasm inside Rokitansky-Aschoff Sinuses: A Comparison of Two Cases and a Literature Review" Radiation 2, no. 1: 52-61. https://doi.org/10.3390/radiation2010004
APA StyleSanvito, F., Gallotti, A., Cobianchi, L., Vanoli, A., Cho, N. S., & Preda, L. (2022). Magnetic Resonance Diffusion-Weighted Imaging for Detecting Fundal Intracholecystic Papillary Neoplasm inside Rokitansky-Aschoff Sinuses: A Comparison of Two Cases and a Literature Review. Radiation, 2(1), 52-61. https://doi.org/10.3390/radiation2010004