The Role of NMDAR and BDNF in Cognitive Dysfunction Induced by Different Microwave Radiation Conditions in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Groups
2.2. Microwave Exposure
2.3. Morris Water Maze Test (MWM)
2.4. Transmission Electron Microscope (Tem)
2.5. Determination of Amino Acid Neurotransmitter Levels
2.6. Western Blot Analysis of the Expression of NMDAR Subunits, BDNF and Related Signaling Molecules
2.7. Statistical Analysis
3. Results
3.1. Spatial Learning and Memory Deficits after Microwave Exposure
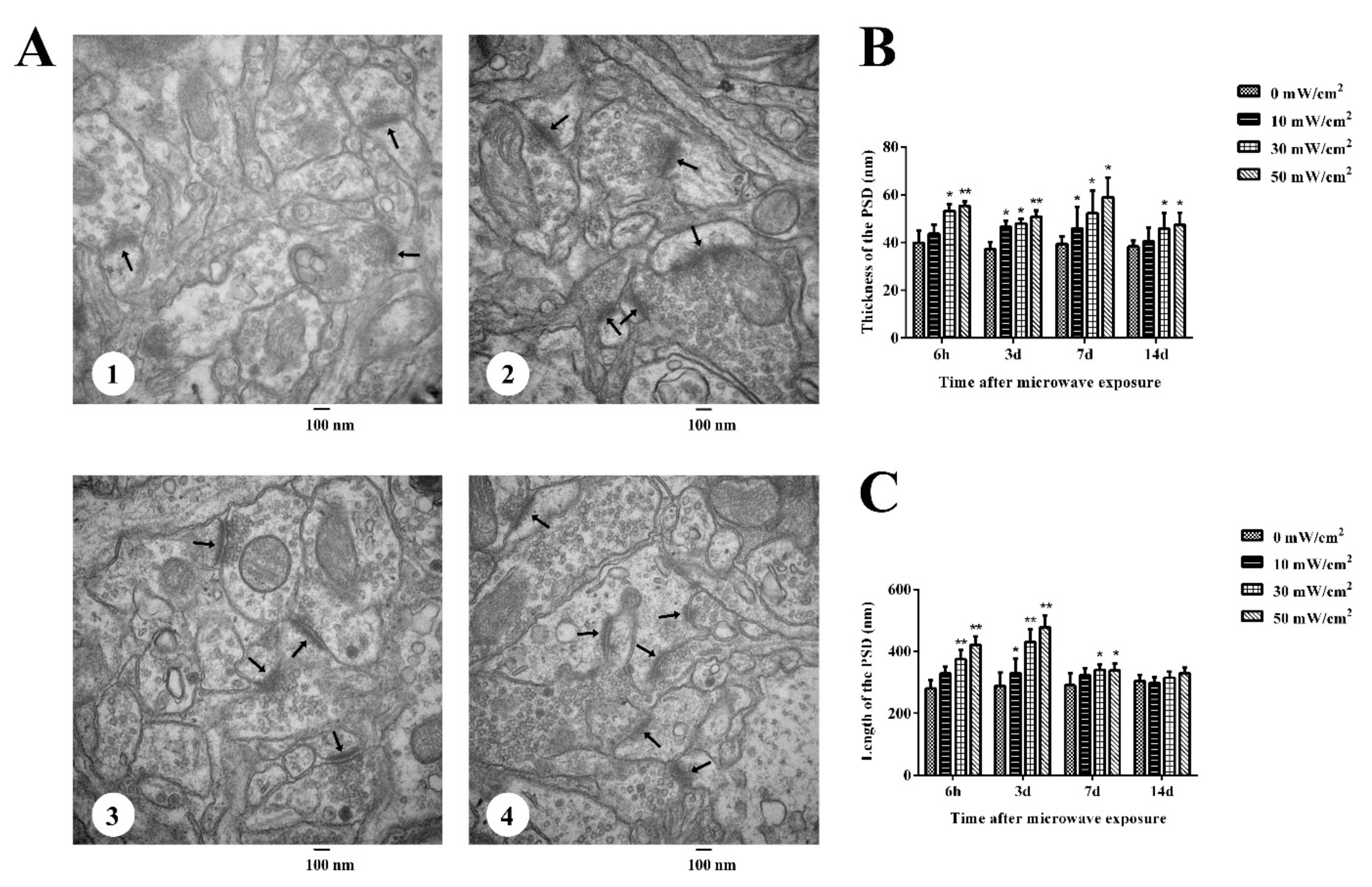
3.2. Ultrastructural Changes in the Rat Hippocampus after Microwave Radiation
3.3. Levels of Amino Acid Neurotransmitters in the Hippocampus
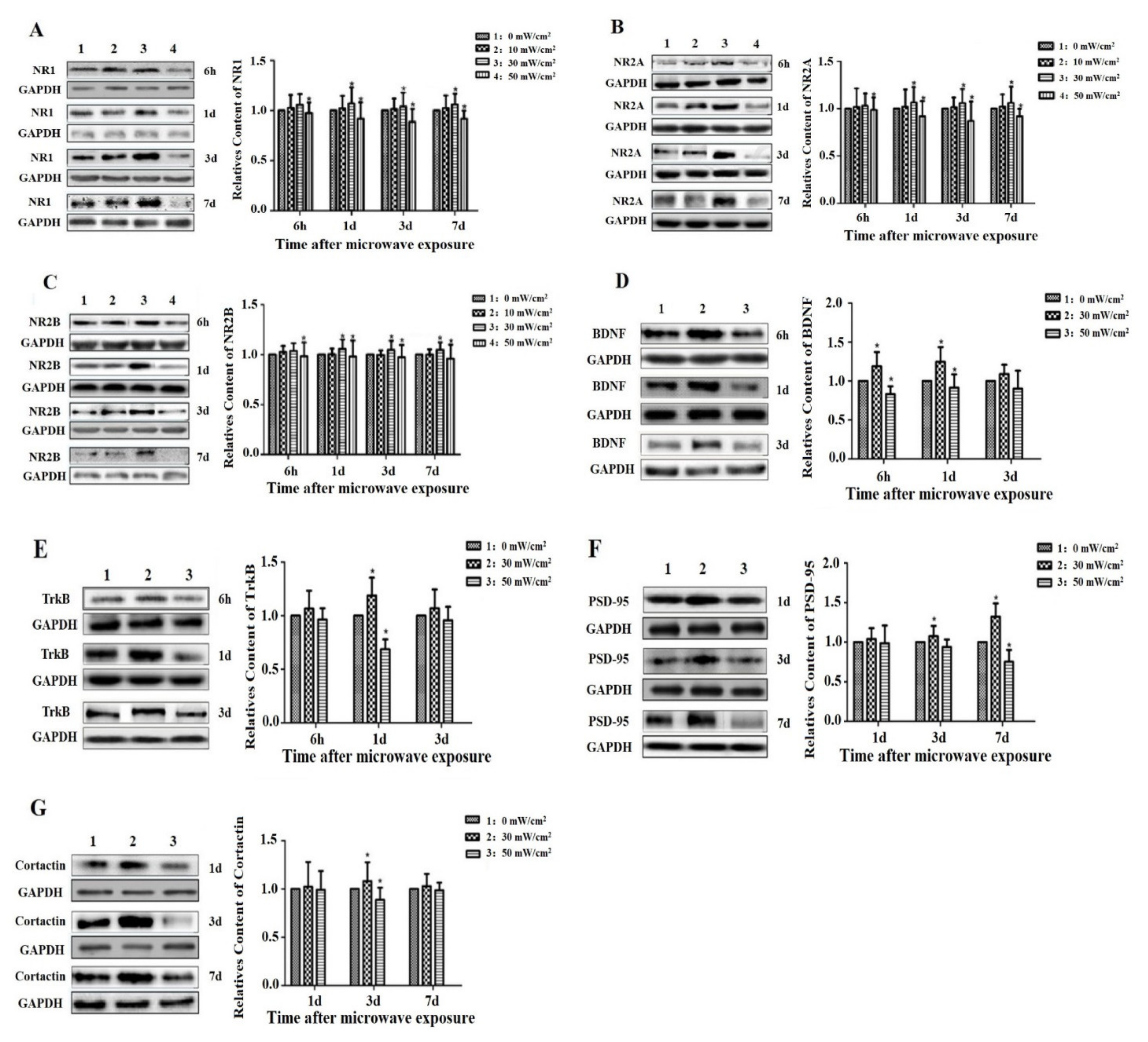
3.4. Effects of Microwave Exposure on the Levels of NMDAR, BDNF and Related Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foerster, M.; Thielens, A.; Joseph, W.; Eeftens, M.; Röösli, M. A Prospective Cohort Study of Adolescents’ Memory Performance and Individual Brain Dose of Microwave Radiation from Wireless Communication. Environ. Health Perspect. 2018, 126, 077007. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wan, P.; Wang, W.; Xiao, B.; Jin, H.; Jin, Q. Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res. Bull. 2019, 144, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.-J.; Peng, R.-Y.; Li, H.-J.; Zou, Y.; Yao, B.-W.; Wang, C.-Z.; Liu, Z.-H.; Gao, X.-H.; Xu, X.-P.; Dong, J.; et al. Microwave radiation leading to shrinkage of dendritic spines in hippocampal neurons mediated by SNK-SPAR pathway. Brain Res. 2018, 1679, 134–143. [Google Scholar] [CrossRef]
- Suárez-Pozos, E.; Thomason, E.J.; Fuss, B. Glutamate Transporters: Expression and Function in Oligodendrocytes. Neurochem. Res. 2020, 45, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, K. Behavioral consequences of co-administration of MTEP and the COX-2 inhibitor NS398 in mice. Part 1. Behav. Brain Res. 2019, 370, 111961. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, J. The C-terminus of NMDAR GluN1-1a Subunit Translocates to Nucleus and Regulates Synaptic Function. Front. Cell. Neurosci. 2018, 12, 334. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Hu, S.H.; Tan, S.Z.; Zhang, B.; Zhou, H.M.; Peng, R.Y. Real-time Microwave Exposure Induces Calcium Efflux in Primary Hippocampal Neurons and Primary Cardiomyocytes. Biomed. Environ. Sci. BES 2018, 31, 561–571. [Google Scholar] [CrossRef]
- Wang, H.; Tan, S.; Zhao, L.; Dong, J.; Yao, B.; Xu, X.; Zhang, B.; Zhang, J.; Zhou, H.; Peng, R. Protective Role of NMDAR for Microwave-Induced Synaptic Plasticity Injuries in Primary Hippocampal Neurons. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [Green Version]
- Magi, S.; Piccirillo, S.; Amoroso, S. The dual face of glutamate: From a neurotoxin to a potential survival factor—Metabolic implications in health and disease. Cell. Mol. Life Sci. CMLS 2019, 76, 1473–1488. [Google Scholar] [CrossRef]
- Lodge, D.; Watkins, J.C.; Bortolotto, Z.A.; Jane, D.E.; Volianskis, A. The 1980s: D-AP5, LTP and a Decade of NMDA Receptor Discoveries. Neurochem. Res. 2019, 44, 516–530. [Google Scholar] [CrossRef] [Green Version]
- Sadat-Shirazi, M.-S.; Ahmadian-Moghadam, H.; Khalifeh, S.; Zadeh-Tehrani, S.N.; Farahmandfar, M.; Zarrindast, M.-R. The role of calcium-calmodulin-dependent protein kinase II in modulation of spatial memory in morphine sensitized rats. Behav. Brain Res. 2019, 359, 298–303. [Google Scholar] [CrossRef]
- Kumar, A.; Foster, T.C. Alteration in NMDA Receptor Mediated Glutamatergic Neurotransmission in the Hippocampus During Senescence. Neurochem. Res. 2019, 44, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Coley, A.A.; Gao, W.-J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, G.; Yang, F.; Guan, Z.; Zhang, Z.; Zhao, J.; Liu, Y.; Chu, L.; Pei, L. Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed. Pharmacother. 2019, 116, 109054. [Google Scholar] [CrossRef] [PubMed]
- Mardones, M.D.; Jorquera, P.V.; Herrera-Soto, A.; Ampuero, E.; Bustos, F.J.; van Zundert, B.; Varela-Nallar, L. PSD95 regulates morphological development of adult-born granule neurons in the mouse hippocampus. J. Chem. Neuroanat. 2019, 98, 117–123. [Google Scholar] [CrossRef]
- Schnoor, M.; Stradal, T.E.; Rottner, K. Cortactin: Cell Functions of A Multifaceted Actin-Binding Protein. Trends Cell Biol. 2018, 28, 79–98. [Google Scholar] [CrossRef]
- Scherer, A.N.; Anand, N.S.; Koleske, A.J. Cortactin stabilization of actin requires actin-binding repeats and linker, is disrupted by specific substitutions, and is independent of nucleotide state. J. Biol. Chem. 2018, 293, 13022–13032. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-X.; Yan, D. Effects of dexmedetomidine on the growth and development of rat hippocampal neurons and its mechanism. Zhongguo Yingyong Shenglixue Zazhi Chin. J. Appl. Physiol. 2019, 35, 69–73. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.W.; Harward, S.C.; Huang, Y.Z.; McNamara, J.O. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology 2020, 167, 107734. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Singh, D. Dietary Flavonoids Interaction with CREB-BDNF Pathway: An Unconventional Approach for Comprehensive Management of Epilepsy. Curr. Neuropharmacol. 2019, 17, 1158–1175. [Google Scholar] [CrossRef]
- Wang, H.; Tan, S.; Xu, X.; Zhao, L.; Zhang, J.; Yao, B.; Gao, Y.; Zhou, H.; Peng, R. Long term impairment of cognitive functions and alterations of NMDAR subunits after continuous microwave exposure. Physiol. Behav. 2017, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Sun, C.F.; Zhang, J.; Gao, Y.B.; Wang, L.F.; Zuo, H.Y.; Wang, S.M.; Zhou, H.M.; Xu, X.P.; Dong, J.; et al. Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed. Environ. Sci. BES 2015, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bellmund, J.L.; Deuker, L.; Doeller, C.F. Mapping sequence structure in the human lateral entorhinal cortex. eLife 2019, 8. [Google Scholar] [CrossRef]
- Ramanathan, K.R.; Maren, S. Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav. Brain Res. 2019, 374, 112114. [Google Scholar] [CrossRef]
- Trinchero, M.F.; Herrero, M.; Monzón-Salinas, M.C.; Schinder, A.F. Experience-Dependent Structural Plasticity of Adult-Born Neurons in the Aging Hippocampus. Front. Neurosci. 2019, 13, 739. [Google Scholar] [CrossRef] [Green Version]
- Hassanshahi, A.; Shafeie, S.A.; Fatemi, I.; Hassanshahi, E.; Allahtavakoli, M.; Shabani, M.; Roohbakhsh, A.; Shamsizadeh, A. The effect of Wi-Fi electromagnetic waves in unimodal and multimodal object recognition tasks in male rats. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kesari, K.K.; Saxena, V.K.; Sisodia, R. Ten gigahertz microwave radiation impairs spatial memory, enzymes activity, and histopathology of developing mice brain. Mol. Cell. Biochem. 2017, 435, 1–13. [Google Scholar] [CrossRef]
- Barry, D.N.; Commins, S. A novel control condition for spatial learning in the Morris water maze. J. Neurosci. Methods 2019, 318, 1–5. [Google Scholar] [CrossRef]
- Levit, A.; Regis, A.M.; Gibson, A.; Hough, O.H.; Maheshwari, S.; Agca, Y.; Agca, C.; Hachinski, V.; Allman, B.L.; Whitehead, S.N. Impaired behavioural flexibility related to white matter microgliosis in the TgAPP21 rat model of Alzheimer disease. Brain Behav. Immun. 2019, 80, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.C.; Zhong, J.Y.; Clendinen, C.A.; Moffat, S.D.; Magnusson, K.R. Age-related differences in brain activations during spatial memory formation in a well-learned virtual Morris water maze (vMWM) task. NeuroImage 2019, 202, 116069. [Google Scholar] [CrossRef] [PubMed]
- Vouros, A.; Gehring, T.V.; Szydlowska, K.; Janusz, A.; Tu, Z.; Croucher, M.; Lukasiuk, K.; Konopka, W.; Sandi, C.; Vasilaki, E. A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Sci. Rep. 2018, 8, 15089. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Zhou, H.; Zhou, N.; Dong, D.; Chu, Y.; Shen, J.; Han, Y.; Chu, X.P.; Zhu, K. Dynamic Evaluation Indices in Spatial Learning and Memory of Rat Vascular Dementia in the Morris Water Maze. Sci. Rep. 2019, 9, 7224. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Peng, R.Y.; Wang, S.M.; Wang, L.F.; Gao, Y.B.; Dong, J.; Li, X.; Su, Z.T. Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed. Environ. Sci. BES 2012, 25, 182–188. [Google Scholar] [CrossRef]
- Deshmukh, P.S.; Megha, K.; Nasare, N.; Banerjee, B.D.; Ahmed, R.S.; Abegaonkar, M.P.; Tripathi, A.K.; Mediratta, P.K. Effect of Low Level Subchronic Microwave Radiation on Rat Brain. Biomed. Environ. Sci. BES 2016, 29, 858–867. [Google Scholar] [CrossRef]
- Olivares-Bañuelos, T.N.; Chi-Castañeda, D.; Ortega, A. Glutamate transporters: Gene expression regulation and signaling properties. Neuropharmacology 2019, 161, 107550. [Google Scholar] [CrossRef]
- Di, G.; Kim, H.; Xu, Y.; Kim, J.; Gu, X. A comparative study on influences of static electric field and power frequency electric field on cognition in mice. Environ. Toxicol. Pharmacol. 2019, 66, 91–95. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, X.; Qu, S. Glutamate, Glutamate Transporters, and Circadian Rhythm Sleep Disorders in Neurodegenerative Diseases. ACS Chem. Neurosci. 2019, 10, 175–181. [Google Scholar] [CrossRef]
- Avoli, M. Inhibition, oscillations and focal seizures: An overview inspired by some historical notes. Neurobiol. Dis. 2019, 130, 104478. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Parkin, G.M.; Udawela, M.; Gibbons, A.; Dean, B. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J. Psychiatry 2018, 8, 51–63. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Zhao, L.; Wang, S.; Gao, Y.; Wang, L.; Zuo, H.; Dong, J.; Xu, X.; Zhou, H.; et al. The relationship between NMDA receptors and microwave-induced learning and memory impairment: A long-term observation on Wistar rats. Int. J. Radiat. Biol. 2015, 91, 262–269. [Google Scholar] [CrossRef]
- Wang, L.-F.; Wei, L.; Qiao, S.-M.; Gao, X.-N.; Gao, Y.-B.; Wang, S.-M.; Zhao, L.; Dong, J.; Xu, X.-P.; Zhou, H.-M.; et al. Microwave-Induced Structural and Functional Injury of Hippocampal and PC12 Cells Is Accompanied by Abnormal Changes in the NMDAR-PSD95-CaMKII Pathway. Pathobiology 2015, 82, 181–194. [Google Scholar] [CrossRef]
- Wang, H.; Tan, S.; Dong, J.; Zhang, J.; Yao, B.; Xu, X.; Hao, Y.; Yu, C.; Zhou, H.; Zhao, L.; et al. iTRAQ quantitatively proteomic analysis of the hippocampus in a rat model of accumulative microwave-induced cognitive impairment. Environ. Sci. Pollut. Res. Int. 2019, 26, 17248–17260. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, X.; Wang, D.; Li, Y.; Xu, X.; Peng, R.; Song, T. RKIP-Mediated NF-κB Signaling is involved in ELF-MF-mediated improvement in AD rat. Int. J. Med. Sci. 2018, 15, 1658–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Li, W.; Wang, H.; Zhang, J.; Yu, C.; Tan, S.; Wang, H.; Xu, X.; Dong, J.; Yao, B.; et al. Autophagy mediates the degradation of synaptic vesicles: A potential mechanism of synaptic plasticity injury induced by microwave exposure in rats. Physiol. Behav. 2018, 188, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, S.P.; Chaturvedi, C.M. Chronic Nonmodulated Microwave Radiations in Mice Produce Anxiety-like and Depression-like Behaviours and Calcium- and NO-related Biochemical Changes in the Brain. Exp. Neurobiol. 2016, 25, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Wang, H.; Xu, X.; Zhao, L.; Zhang, J.; Dong, J.; Yao, B.; Wang, H.; Zhou, H.; Gao, Y.; et al. Study on dose-dependent, frequency-dependent, and accumulative effects of 1.5 GHz and 2.856 GHz microwave on cognitive functions in Wistar rats. Sci. Rep. 2017, 7, 10781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, N.; Bayat, M.; Haghani, M.; Saadi, H.F.; Ghazipour, G.R. 2.45 GHz microwave radiation impairs learning, memory, and hippocampal synaptic plasticity in the rat. Toxicol. Ind. Health 2018, 34, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Sabet, M.; Mofidi, H.; Attarian-Khosroshahi, M.-S. NMDA receptors in the dorsal hippocampal area are involved in tramadol state-dependent memory of passive avoidance learning in mice. Can. J. Physiol. Pharmacol. 2018, 96, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ethell, I.M.; Pasquale, E.B. Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 2005, 75, 161–205. [Google Scholar] [CrossRef]
- Ueda, S.; Negishi, M.; Katoh, H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol. Biol. Cell 2013, 24, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Nandini, H.S.; Paudel, Y.N.; Krishna, K.L. Envisioning the neuroprotective effect of Metformin in experimental epilepsy: A portrait of molecular crosstalk. Life Sci. 2019, 233, 116686. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Liu, Z.; Zhi, W.; Ma, L.; Zhou, H.; Peng, R.; Hu, X.; Zou, Y.; Wang, L. The Role of NMDAR and BDNF in Cognitive Dysfunction Induced by Different Microwave Radiation Conditions in Rats. Radiation 2021, 1, 277-289. https://doi.org/10.3390/radiation1040023
Liao S, Liu Z, Zhi W, Ma L, Zhou H, Peng R, Hu X, Zou Y, Wang L. The Role of NMDAR and BDNF in Cognitive Dysfunction Induced by Different Microwave Radiation Conditions in Rats. Radiation. 2021; 1(4):277-289. https://doi.org/10.3390/radiation1040023
Chicago/Turabian StyleLiao, Shiyao, Zonghuan Liu, Weijia Zhi, Lizhen Ma, Hongmei Zhou, Ruiyun Peng, Xiangjun Hu, Yong Zou, and Lifeng Wang. 2021. "The Role of NMDAR and BDNF in Cognitive Dysfunction Induced by Different Microwave Radiation Conditions in Rats" Radiation 1, no. 4: 277-289. https://doi.org/10.3390/radiation1040023
APA StyleLiao, S., Liu, Z., Zhi, W., Ma, L., Zhou, H., Peng, R., Hu, X., Zou, Y., & Wang, L. (2021). The Role of NMDAR and BDNF in Cognitive Dysfunction Induced by Different Microwave Radiation Conditions in Rats. Radiation, 1(4), 277-289. https://doi.org/10.3390/radiation1040023





