2. Case Presentation
A 22-year-old lactating female bottlenose dolphin (
Tursiops truncatus), weighing 220 kg, housed with her 1½ year old calf and 14 other dolphins in an outdoor natural sea water habitat (32 parts per thousand (ppt), 20 °C, approximately 2.31 × 10
6 liters (L)) at a public oceanarium presented with lethargy and acute anorexia [
1]. On 22 January 2010, (Day 0), she displayed periodic, non-specific signs of behavioral avoidance to her caretakers but consumed seventy percent of her normal diet, consisting of 16 kg of herring
(Clupea harengus), capelin (
Mallotus villosus), and mackerel (
Scomber japonicus). The following morning (Day 1 of clinical presentation), the dolphin showed complete anorexia but co-operated for an unrestrained blood sample from the ventral fluke vein or periarterial venous rete (PAVR) using a 21-gauge × 1.9 cm (¾ inch) butterfly needle (Nipro Medical Corporation, Doral, FL) and syringe. The blood analysis demonstrated leukocytosis [white blood cell count (WBC) 11.3 × 10
3/uL, normal range 5.0–9.0 × 10
3 cells/uL] and markedly abnormal serum chemistry changes; azotemia [blood urea nitrogen (BUN) = 53.8 mmol/L, range 14.0–21.0 mmol/L, creatinine, (Cr) = 495.0 µmol/L, range 88.0–186.0 µmol/L)], hyperkalemia [potassium (K) = 6.7 mmol/L, range 3.2–4.1 mmol/L)], and hyperphosphatemia [phosphorus (P) = 2.62 mmol/L, range 1.16–1.84 mmol/L)] suggestive of AKI (
Table 1) [
1,
2].
Differential diagnoses considered for this clinical presentation in this species included bacterial infection, metabolic kidney insufficiency, or failure secondary to inflammation, obstructive nephrolithiasis, urinary tract infection, gastrointestinal inflammation, colic, or foreign body ingestion. The dolphin was transported from her habitat to a hospital medical pool with lifting bottom for additional treatment and monitoring that afternoon. Initial medical treatment consisted of oral fluids (2 L fresh water) and dexamethasone (0.1 mg/kg) via orogastric tube, plus intramuscular (IM) ceftriaxone (20 mg/kg) [
1].
The next morning (Day 2), the dolphin was placed in a stretcher for movement to a foam-padded deck to recheck blood analysis and administer oral fluids. Frank hematuria was observed during placement for the exam, but caretakers were unable to collect a urine sample. The dolphin received oral fluids (2 L fresh water) and oral dexamethasone (0.05 mg/kg) by orogastric tube and ate 1.5 kg of fish when returned to the medical pool. Blood was collected and analysis demonstrated a worsening azotemia (BUN = 76.0 mmol/L, Cr = 839.8 µmol/L), hyperkalemia (7.4 mmol/L), hypernatremia (165 mmol/L, 150–157 mmol/L), and hyperphosphatemia (3.71 mmol/L) (
Table 1) [
1,
2].
That same day, the dolphin was again stretchered and craned out of the medical pool for more diagnostics. She was placed in right lateral recumbency on a foam-padded table for additional oral fluid therapy, diagnostic ultrasound, urinary catheterization, and cardiac telemetry. Ultrasonography examination of her abdomen demonstrated bilateral nephrolithiasis, bilateral hydroureter, and mild left hydronephrosis suggestive of bilateral post-kidney obstruction. A grossly hematuric urine sample was collected via urethral catheterization and evaluated with a colorimetric reagent Multistix
® strip (Siemens Health, Malvern, PA, USA) to confirm marked hematuria (4+), proteinuria (2+), pH = 6.0, 10–15 WBC/high power field (hpf), and urine specific gravity (USG) of 1.020 [
1]. An electrocardiogram (ECG) monitor demonstrated a heart rate of 84 beats per minute (normal range, 80–100 bpm) with tented T waves, secondary to hyperkalemia [
1]. The dolphin received 1 L lactated Ringers solution (LRS) IV and 3 L LRS subcutaneously (SC) and was then returned to the medical pool filled with brackish seawater, approximately 10 ppt (local natural sea water is 32 ppt) to augment oral hydration [
1]. Blood and urine samples submitted for aerobic and anaerobic bacterial culture to the on-site clinical diagnostic lab were subsequently negative for microbial growth with standard laboratory incubation measures.
The next morning (Day 3), a follow-up exam and blood collection were performed. The blood analysis showed persistent worsening azotemia (BUN = 91.5 mmol/L, Cr = 1069.6 µmol/L), hyperkalemia (8.3 mmol/L), hyperphosphatemia (4.72 mmol/L), and signs of metabolic acidosis (CO
2 = 18 mmol/L, range 23–30 mmol/L) despite prior fluid therapy (
Table 1) [
2]. Recheck abdominal ultrasound showed persistent dilated ureters and bi-lateral hydronephrosis. Fluid therapy boluses were repeated with 1 L LRS IV and 3 L LRS SC, as a more intensive treatment plan was devised. The dolphin’s behavior became more lethargic and less responsive over the ensuing hours; therefore, a recheck blood analysis was conducted later that day. The blood analysis showed progressive marked azotemia (BUN = 92.2 mmol/L, Cr = 1069.6 µmol/L), critically elevated hyperkalemia (9.1 mmol/L), hyperphosphatemia (4.84 mmol/L), acidosis (CO
2 = 16 mmol/L), and hyperglycemia (11.65 mmol/L, 4.11–7.94 mmol/L) (
Table 1) [
1,
2]. Within an hour of obtaining finalized results, the dolphin received emergency treatment for hyperkalemia with 10% calcium gluconate (0.05 mL/kg), sodium bicarbonate (1 mEq/kg), 25% dextrose solution (0.25 mL/kg), and regular insulin SC (0.1 unit/kg) [
1]. Due to the critical condition of the dolphin and worsening non-responsive azotemia, a procedure to coordinate aggressive IV fluid therapy and placement of a peritoneal catheter for dialysis was planned.
At the end of Day 3, the dolphin was stretchered and moved to a foam-padded hydraulic-lift table inside a hospital treatment room for fluid therapy and peritoneal catheter placement. With the dolphin in sternal recumbency, the caudoventral peduncle was aseptically prepped for venipuncture of the caudoventral spinal vein or PAVR with a 19-gauge × 3.8 cm (1.5 inch) venous needle catheter for fluid therapy administration with LRS at an approximate rate of 10 mls/kg/h or 2 L/h drip rate. A repeat blood analysis demonstrated mild improvement in potassium (8.6 mmol/L), but azotemia (BUN = 87.1 mmol/L, Cr = 1069.4 µmol/L) and acidosis (CO
2 = 15 mmol/L) persisted. Initial ECG monitoring displayed a heart rate of 65–75 bpm, wide QRS complexes, flat p waves, tented T waves, and periodic premature ventricular complexes (PVCs) secondary to hyperkalemia from primary ureteral obstruction and a normal respiration rate of 1–2 respirations/minute. Medical treatment for hyperkalemia was repeated with slow IV boluses of sodium bicarbonate (1 mEq/kg), 10% calcium gluconate (0.05 mL/kg), and regular insulin (Humulin R) SC (0.2 units/kg). Repeat ultrasound examination revealed moderate abdominal ascites, pleural effusion, bilateral nephrolithiasis, mild bilateral hydronephrosis, bilateral hydroureter, and the presence of distally obstructing ureteroliths, (1.0 cm × 0.5 cm) at the right ureteral orifice (UO) and (0.87 cm × 0.87 cm) at the left UO [
1]. Spontaneous urination occurred during fluid therapy and a urolith measuring (1.0 cm × 0.5 cm) was recovered [
1]. The urolith was later analyzed by the Urolith Center at the University of Minnesota, College of Veterinary Medicine (St. Paul, MN, USA) and found to consist of 100% ammonium urate [
1]. Following spontaneous passage of the urolith and fluid therapy, the ECG showed improved QRS complexes and shortened, normalized T waves. Subsequent abdominal ultrasound found the left ureterolith still present at the left UO, confirming that the right ureterolith passed with fluid therapy.
Peritoneal dialysis (PD) was still warranted to further stabilize the dolphin with AKI and refractory hyperkalemia until the remaining obstructing ureterolith could be ad-dressed. With the dolphin restrained in left lateral recumbency, a local anesthetic (2% lidocaine solution) was administered in a reverse “L” pattern cranial to the planned entry site in the caudoventral right flank, extending subcutaneously and deep to the external oblique muscle layer. The catheterization site was surgically prepped using Betadine
® and chlorhexidine. A stab incision with a #11 scalpel blade was made for introducing an 18-gauge introducer needle followed by guidewire insertion. A 15 French × 15 cm dilator and introducer sheath was advanced over the guidewire using a modified-Seldinger technique into the abdominal cavity. The wire and dilator were removed, and a 62 cm (24 in) curled human peritoneal dialysis (PD) catheter (Quinton
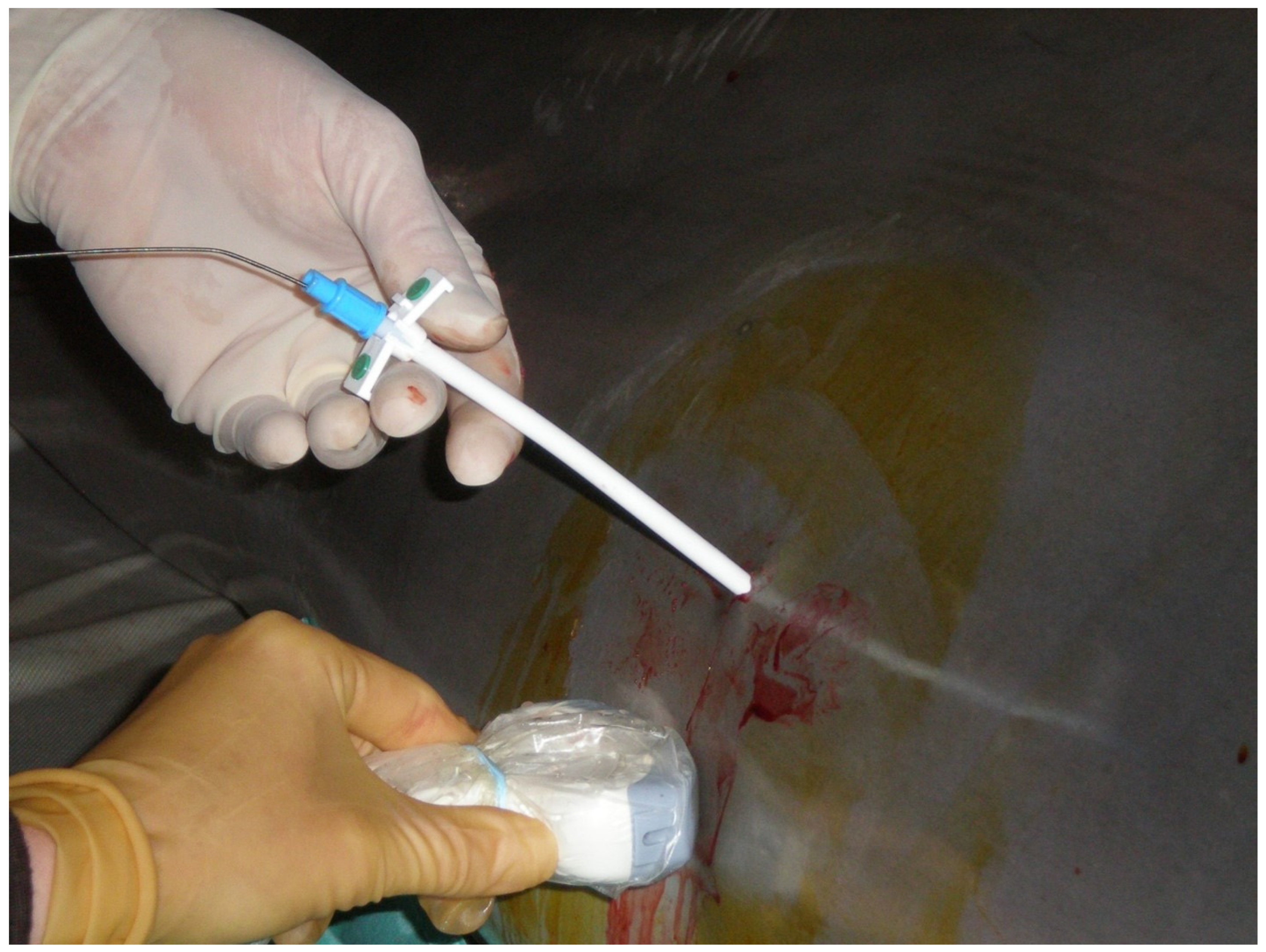
®, Tyco Healthcare, now Covidien, Mansfield, MA, USA) was successfully inserted through the sheath percutaneously into the right retroperitoneal space with ultrasound guidance (
Figure 1).
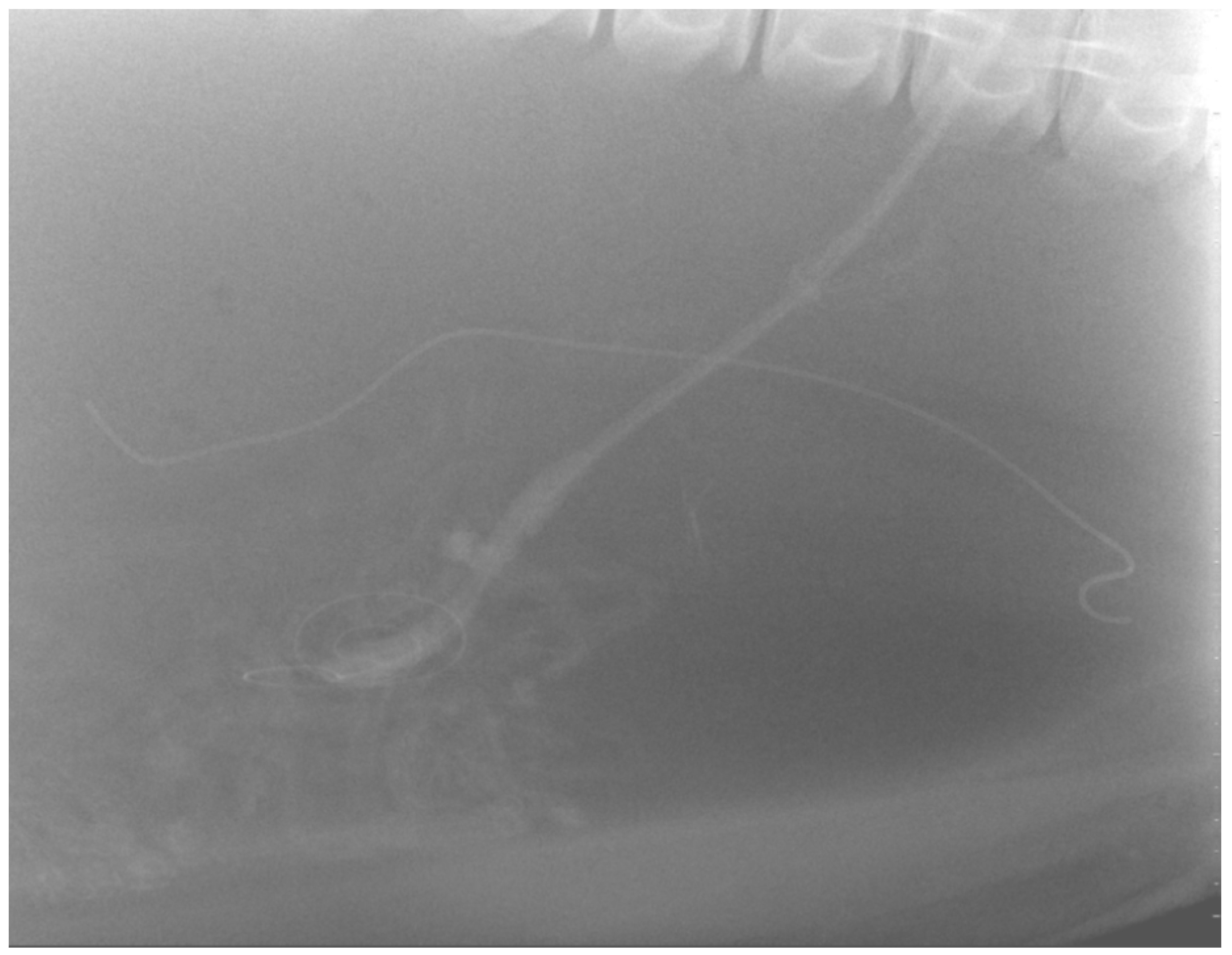
The peel-away-sheath was removed, and the PD catheter was sutured in place to the skin of the right flank, using 2-0 Maxon™ (Tyco Healthcare). A subcutaneous catheter tunnel was not performed to minimize injury to the dense blubber layer. A lateral abdominal radiograph was taken to assess the position of the PD catheter (
Figure 2).
Six liters of warmed dialysis fluid (Dianeal
® low calcium (2 mEq/L) peritoneal dialysis solution with 1.5% dextrose, Baxter Healthcare Corp., Irvine, CA, USA) was infused into the abdominal cavity by gravity feed and allowed to dwell for approximately one hour, then 5 L dialysate fluid was drained into a closed sterile collection bag. The dialysate fluid (#1) sample was analyzed and found to have a K concentration of 4.4 mmol/L (
Table 2). A post-procedural blood analysis showed improvement in hyperkalemia (7.2 mmol/L) and azotemia (BUN = 84.9 mmol/L, Cr = 892.8 µmol/L) (
Table 1). Antibiotic coverage consisted of 20 mg/kg ceftazidime IM BID. The dolphin appeared clinically more stable; therefore, the PD catheter was capped, and the dolphin was returned to the medical pool for observation, where she appeared more responsive and consumed a few fish after the procedure. A 0.7 mg/kg tramadol dose was administered with the fish. A night watch was instituted to monitor the overall behavior of the dolphin.
Later that morning, on Day 4, the dolphin showed no interest in food, and was therefore stretchered and craned to a foam-padded table to perform blood collection, dialysate ex-change, and recheck ultrasound examination. The blood analysis indicated mild improvement in kidney function parameters (BUN = 77.5 mmol/L, Cr = 751.4 µmol/L), hyperkalemia (7.4 mmol/L), hyperglycemia (24.53 mmol/L), and mild acidosis (CO
2 = 21 mmol/L) (
Table 1). The overnight dwell peritoneal-dialysate fluid #2 (~1 L) was collected into a collection bag by gravity feed, and 6 L of warmed dialysate with 1.5% dextrose was infused back in to dwell. The collected dialysate fluid (#2) was found to contain 14.3 mmol/L of K (
Table 2).
Post-infusion, the dolphin became bradycardic (40 bpm) with shallow respirations within twenty minutes after receiving the new dialysate infusion. One liter of dialysate fluid was immediately eluted off and an additional 2 L of 0.45% saline and 2.5% dextrose were administered SC. The dolphin’s heart rate and respirations gradually returned to normal within 10–15 min. Low dose tamsulosin (0.005 mg/kg) was administered per os that morning to aid with ureteral dilation and possible spontaneous urolith passage. Ultrasound of the left abdomen showed persistent left hydroureter with a round hyperechoic foci, suggestive of a ureterolith, still obstructing the left UO. The dolphin was returned to the medical pool for observation. Later on Day 4, a dialysate exchange and cystoscopy procedure was attempted to visualize the obstruction. The dolphin was stretchered and placed in left lateral recumbency on a foam-padded table. Dialysate fluid (#3) was collected and K = 10.5 mmol/L; an exchange of 4 L × 1.5% glucose dialysate was conducted (
Table 2). The genital slit was aseptically prepped with dilute 2% chlorhexidine. A flexible 4 mm endoscope was inserted into the urethra and passed approximately 7 cm; the dolphin became acutely bradycardic (40 bpm) and the scope was quickly removed. Emergency treatment with sodium bicarbonate (0.5 mEq/kg), calcium gluconate (0.05 mL/kg), insulin (0.4 units/kg), furosemide (150 mg), and 0.9% saline (500 mls) boluses was administered slowly IV in the caudoventral spinal PAVR. The dolphin’s vital signs normalized, and she was moved back to the medical pool for overnight observation.
The following morning, on Day 5, the dolphin appeared more active and attentive to staff trainers. A recheck blood analysis was performed prior to the dialysate exchange (4 L × 1.5% dextrose dialysate) and showed mild improvement in azotemia (BUN = 72.9 mmol/L, Cr = 751.4 µmol/L) and hyperkalemia (K = 6.9 mmol/L); however, muscle and hepatic transaminase enzymes [creatine kinase (CK) = 9598 IU/L, normal 64–191 IU/L, lactate dehydrogenase (LDH) = 2993 IU/L, 328–565 IU/L, alanine aminotransferase (ALT) = 181 IU/L, range 19–67 IU/L, and aspartate aminotransferase (AST) = 1006 U/L, normal range 153–386 IU/L] were significantly elevated, as well as, cholesterol (Chol) (10.4 mmol/L, range 3.7–6.9 mmol/L) and triglycerides (Trig) (9.18 mmol/L, range 0.36–1.89 mmol/L) indicating potential impacts to muscle and liver function (
Table 1) [
2]. Dialysate fluid (#4) was analyzed and found to have 9.1 mmol/L K (
Table 2). After 8 h, another blood collection and dialysate exchange (#5) were performed on the lifted medical floor with 4 L of gravity-fed 1.5% dextrose dialysate; the collected dialysate was found to have 8.1 mmol/L K, respectively. (
Table 2). Repeat blood analysis demonstrated improving azotemia (BUN = 69.9 mmol/L, Cr = 707.36 µmol/L), hyperkalemia (7.1 mmol/L), hyperphosphatemia (4.46 mmol/L), inflammatory leukogram (WBC = 8.89 × 10
3/μL with 9% band neutrophils); however, muscle and liver transaminase enzymes were further increased (CK = 13,275 IU/L, AST = 1117 IU/L, LDH = 3971 IU/L). Prophylactic antibiotics (10 mg/kg, 2 g ceftazidime) and antifungals (1 mg/kg, 200 mg fluconazole) were administered intraperitoneally (IP) as an additive treatment for possible local peritonitis. A follow-up abdominal ultrasound showed mild ascites, focal hyperechoic peritoneal membrane, and intestinal serosal borders near the right-side insertion site of the PD catheter location. With the dolphin stabilized clinically, a second procedure utilizing cystoscopy enabled subsequent ureteral stent placement in the left ureter [
1]. Atropine (0.02 mg/kg) IM was administered prior to the procedure to potentially mitigate a vagal response during the cystoscopy. Approximately 1 L of dialysate (#6) was collected and analyzed and found to have 5.3 mmol/L K (
Table 2). An additional 2 L × 1.5% dextrose dialysate was infused through the PD catheter into the abdomen and allowed to dwell overnight. Oral voriconazole (2 mg/kg) once daily was added for antifungal prophylaxis.
On the morning of Day 6, a recheck blood analysis was performed, and approximately 1 L of dialysate (#7, K = 6.2 mmol/L) was removed and 3 L of fresh 1.5% dextrose dialysate infused back in to dwell (
Table 2). A second out-of-water procedure was performed later using laser lithotripsy to obliterate the obstructing left ureterolith and replace the ureteral stent [
1]. After the lithotripsy procedure, a final dialysate (#8, K = 6.3 mmol/L) sample was collected, and additional doses of Ceftazidime (20 mg/kg) and Fluconazole (2 mg/kg) were instilled IP and flushed through the PD catheter. The PD catheter was then removed and fluid from catheter cultured, while the remaining dialysate solution drained from the catheter site. The PD catheter entry site of subcutaneous blubber was closed with a deep horizontal mattress suture and skin closed with a simple interrupted suture pattern using 0-0 Maxon™. The left ureteral stent was left in place, exiting the genital slit, and was sutured to the right lateral flank with 2-0 Maxon™. A post-procedural blood analysis indicated an active inflammatory leukogram with a WBC of 9.3 × 10
3/μL with 19% band neutrophils, and persistent moderate azotemia (BUN 52.1 mmol/L, Cr 468.63 μmol/L), mild hyperkalemia (5.1 mmol/L), hypernatremia (169 mmol/L), hyperglycemia (13.15 mmol/dL), hypercholesterolemia (8.2 mmol/L), hypertriglyceridemia (5.92 mmol/L), erythrocyte sedimentation rate (ESR) (30 mm/h, 4–17 mm/h), and elevated fibrinogen (14.76 µmol/L, 4.70–8.85 µmol/L) (
Table 1) [
2]. Over the course of 72 h, PD proved effective in clearance of urea, creatinine, and potassium, with five exchanges of dialysate fluid resulting in a reduction of BUN (92.2 to 52.1 mmol/L), Cr (1069.6 to 468.5 μmol/L) and, most importantly, K (9.1 to 5.1 mmol/L) (
Table 1). A total of eight dialysate fluid samples were collected over the course of 72 h, showing adequate range of major ion absorption from one to twelve-hour dwell periods, notably BUN (130.3 to 320 mmol/L), creatinine (521.68 to 2113.24 μmol/L), and potassium (4.4 to 14.3 mmol/L) (
Table 2).
Following lithotripsy of the obstructing left ureterolith and the removal of the PD catheter, the dolphin had several episodes of anorexia, dehydration, local peritonitis, systemic infection and phlebitis. However, she made a full recovery after several months of supportive therapy. For approximately 7 days following the procedure, the dolphin was supported with maintenance oral and subcutaneous fluid therapy due to anorexia. She was treated with antibiotics [amikacin (10 mg/kg SID), ceftazidime (20 mg/kg IM BID), vancomycin (1.5 mg/kg TID], antifungals [voriconazole 2 mg/kg SID then itraconazole, 2.5 mg/kg BID], and low-dose steroids [prednisolone, (0.05 mg/kg (SID)]. On Day 5 post-PD catheter removal, her blood analysis showed significant inflammation with marked leukocytosis, WBC 30.9 × 10
3 cells/µL with 3% band neutrophils, elevated fibrinogen (18.70 µmol/L) and ESR (70 mm/h) (
Table 1), and signs of persistent mild azotemia (BUN = 40.7 mmol/L, Cr = 168.0 μmol/L), mild hyperkalemia (5.3 mmol/L), hypernatremia (177 mmol/L), elevated liver transaminases (LDH = 4155 IU/L, ALT = 313 IU/L, and GGT = 147 IU/L, range = 30–50 IU/L), and persistent elevation of muscle enzymes (CK = 4678 IU/L, AST = 1579 IU/L) (
Table 1) [
2]. Voriconazole was discontinued (Day 5 post-procedure) due to possible hepatoxicity. The dolphin’s appetite returned nine days after the procedure, and her disposition gradually improved. On Day 10 post-PD catheter removal, the dolphin’s CBC showed persistent inflammation (WBC 29.3 × 10
3 cells/µL) with 3% band neutrophils and ESR of 91 mm/h, however serum chemistries showed mild azotemia (BUN = 42.0 mmol/L, Cr = 159.1 μmol/L), hypercholesterolemia (Chol = 7.6 mmol/L) and hypertriglyceridemia (6.16 mmol/L), with elevated fibrinogen (23.7 µmol/L), normokalemia (4.0 mmol/L), hypernatremia (171 mmol/L), elevated liver transaminases (LDH 1647 IU/L, ALT 125 IU/L, GGT 90 IU/L), and normalizing muscle enzymes (CK 360 IU/L) (
Table 1) [
2]. Additional medications were prescribed in the form of s-adenosylmethionine (15 mg/kg) SID, silymarin (1.5 mg/kg) TID (three times daily), and alpha lipoic acid (4 mg/kg) BID to aid liver metabolism. Abdominal ultrasound showed persistent ascites, bilateral nephrolithiasis, and left hydronephrosis. Although her metabolic condition was improving, there was physical and thermographic evidence of significant bilateral phlebitis of the ventral fluke veins incurred by repeated IV fluid treatments and blood collections. The ventral surface of both fluke blades showed swelling and heat of the epithelium along the vascular path of the ventral fluke PAVR. The epithelium of the distal fluke tips began to slough exposing the white dermis below. Topical treatment for both fluke tips required superficial debridement of necrotic epithelium, cold-laser therapy, and aggressive intravenous antibiotic treatment (vancomycin 1.5 mg/kg) IV TID. A multi-resistant Staphylococcus epidermidis was identified on culture of blood and peritoneal fluid aspirate, so the treatment was changed to address sepsis and phlebitis with cefuroxime (20 mg/kg) PO BID, while levofloxacin, and amoxicillin–clavulanate were discontinued. Additional low-dose analgesic therapy, tramadol (0.75 mg/kg PO BID), was added to address possible pain. Treatment of bilateral fluke tip necrosis continued with daily topical application of Betadine and weekly cold laser therapy of the affected fluke tip regions.
Approximately two weeks after the procedure, the dolphin resumed consuming her entire mixed fish diet that was increased to 8.2 kg. However, because of the fluke tip necrosis and chronic inflammation, her appetite waxed and waned daily, so low-dose (0.1 mg/kg) diazepam (20 mg PO once daily) and (0.75 mg/kg) tramadol (150 mg PO BID), were given to aid appetite stimulation and analgesia. The dolphin was assist-fed when she showed no interest, to mitigate dehydration and weight loss. Her appetite was stable one month following the procedure. Concurrent dipstick urinalysis and urine chemistry demonstrated a specific gravity (SG) of 1.042, pH = 6.5, blood = 4+, protein = 1210 g/L, rare WBC’s, glucose 0.22 mmol/L, GGT 59 U/L, and negative for bilirubin, ketone, and nitrite. However, a multi-resistant Escherichia coli was repeatedly cultured from free-catch urine samples, sensitive only to amikacin, imipenem, and nitrofurantoin. Due to the recent AKI with ureterolith obstruction, nitrofurantoin (2.5 mg/kg) PO TID was chosen instead of aminoglycosides to treat the chronic urinary tract infection for 3 days. Side-effects of inappetence were noted during the nitrofurantoin treatment, as her appetite waned after each nitrofurantoin dose. The nitrofurantoin dose was reduced to 1.5 mg/kg PO TID, and the treatment extended 4 more days to alleviate effects on appetite. Approximately 2.5 months after the procedure, a recheck blood analysis demonstrated a normalized WBC (8.6 × 103 cells/µL), glucose (6.72 mmol/dL), but chronically elevated liver transaminases (LDH 603 IU/L, AST 502 IU/L, ALP 335 IU/L, ALT 95 IU/L, GGT 115 IU/L), and decreased fibrinogen (10.82 µmol/L). Urinalysis and urine culture showed mild proteinuria (1008 g/L), normal USG 1.038, and a negative result for the growth of aerobic and anaerobic bacteria. Due to fluke tip necrosis and phlebitis, she remained on antibiotic therapy and oral nystatin (7500 IU/kg PO TID) for another 8 weeks.
4. Discussion
This case demonstrates that intermittent peritoneal dialysis (PD) can be a life-saving procedure in zoo or aquaria-based animals that may not tolerate conventional fluid therapy or be refractory to it. Common techniques for intravenous (IV) fluid administration in veterinary medicine are extremely challenging in marine mammals and are limited by the aqueous environment, patient accessibility, movement, duration of treatment, technical catheter insertion, and achieving fluid maintenance requirements. More often, marine mammal patients are managed with bolus IV fluid therapy, subcutaneous fluids, or periodic oral hydration via an oral gastric tube. As marine mammals, dolphins are unique patients that normally receive all of their maintenance fluid requirements from the fish and invertebrate diet they consume [
3]. Therefore, anorexia can severely complicate health with the progression of dehydration and metabolic dysfunction. They also evolved with a dependency for living in water and an anatomical structure that is uniquely adapted to the weightless aqueous medium; hence, they cannot remain out of water for extended periods of time without suffering respiratory and circulatory compromise and dehydration of their skin [
4,
5]. Dolphin patients are most often temporarily managed in smaller medical pools with or without a lifting-floor or placed in a stretcher suspended in a container of water for easier access to monitor clinical changes. In the face of rapidly progressing life-threatening AKI with hyperkalemia, hypernatremia, and metabolic acidosis secondary to post-renal obstruction, conventional fluid therapy was quickly recognized as inadequate in this case. Consequently, interventional patient management and PD were emergent procedures that temporized the patient’s life-threatening metabolic derangements until the underlying ureterolith obstruction could be addressed.
While not common in veterinary practice due to technical complications, PD may be a life-saving procedure with proper patient selection [
6,
7,
8]. The method has been used to treat acute kidney injury, chronic kidney disease, manage post-kidney obstruction, or remove other dialyzable toxins (ethylene glycol, ethanol, and barbiturates) and uroabdomen [
9,
10]. In addition, PD can also be used to treat hypo/hyperthermia, pancreatitis, congestive heart failure, peritonitis, and other metabolic conditions (hypercalcemia, hyperkalemia, hepatic encephalopathy) [
10]. PD is more commonly performed on small domestic animals (cats and dogs), as studies have indicated when animals are larger than 13.6 kg in size, the management techniques of PD are increasingly difficult to perform [
9]. Due to the dolphin’s size and progressive clinical presentation, it seemed reasonable to adapt the methodology used for human PD treatment with this case.
PD has been effectively used to treat human AKI since 1946; however, after the introduction of continuous renal replacement therapy (CRRT) for AKI in critically ill human patients, there was a rapid decline in the use of PD in acute settings, except in pediatric populations as well as some lower-income countries [
11]. Acute PD for AKI has some recognized benefits in human medicine over CRRT and hemodialysis in that it requires less training for nursing staff, has more cardiovascular stability in hypotensive patients, does not require anticoagulation, reduces the risk of bacteremia, enables a more rapid recovery of renal function, incurs lower overall cost, and does not require water and electricity when performed manually. In aquatic animals, the risk associated with PD catheter insertion may be favorable to the repeated vascular access requirement of hemodialysis. Disadvantages of PD can include lack of trained physicians to place catheters, mechanical catheter complications, unreliable ultrafiltration, high glucose exposure, risk of peritonitis, raised intra-abdominal pressure, and reduced enteral tolerance [
11]. The use of prophylactic antibiotics prior to PD catheter implantation is recommended to decrease the incidence of peritonitis. Cycle times (length of dwell of dialysate) should be dictated by the clinical circumstances: short cycle times (1–2 h) are likely to more rapidly correct uremia, hyperkalemia, fluid overload and/or metabolic acidosis, while longer cycles facilitate the clearance of larger sized solutes. When fluid overload is present, the concentration of dextrose is increased and cycle time reduced to 2 h. There remains controversy and a lack of robust data regarding the most appropriate dose of PD in patients with AKI [
12].
With large patient size and decreased accessibility, the prospect for successful management of a PD in a zoologic or aquarium facility has unique challenges. In fact, there are no reports of successful non-domestic animal PD treatment in any other zoological facility. In 1992, an attempt to perform PD in a pilot whale, with acute kidney injury, was complicated by technical insertion of the peritoneal catheter into the peritoneal cavity and dialysate was rather deposited into the subcutaneous space and unable to absorb uremic toxins [
13,
14]. More recently, intestinal dialysis with oral mannitol was attempted in a beluga, achieving the dialytic goals of reducing blood urea nitrogen (BUN), potassium, and phosphorous, but creatinine continued to rise, and the whale expired on Day 15 of treatment [
15]. These two cases highlight the challenge of patient-size limiting access or complicating placement of the peritoneal catheter and the need for exploring other means of dialysis. For dolphin patients, their average size of 200–300 kg requires equipment necessary for movement (i.e., crane, transport vehicle, stretcher, rigging, etc.), specialized housing (medical or hospital pool with or without lifting-floor) for intensive care management, and trained personnel. The successful outcome of this case was facilitated by isolating this dolphin to a medical pool that enabled moving her in and out of water as needed for interventional medical care and monitoring her clinical status with an on-site diagnostic laboratory.
The management of acute kidney injury in other marine mammals, mainly pinnipeds, is complicated by vascular access and underlying disease. Pinnipeds are mobile with flexible fore-flippers and hind-flippers on land, and it is difficult to maintain intravenous catheters unless severely debilitated. Therefore, most cases of acute or chronic kidney injury in seals, sea lions, or walrus are treated with subcutaneous fluids or bolused IV fluids. Transient (e.g., 2–3 days) intravenous fluid therapy has been maintained by the author to successfully correct azotemia in moribund juvenile seals and sea lion pups. There are no other reports of PD in marine mammals.
For dialysis, the peritoneal catheter is a vital conduit to instill dialysate to dwell in the abdomen and then exchange the dialysate laden with toxic solutes and concentrated electrolytes. However, the indwelling PD catheter has also been reported to cause complications in human and domestic animal patients [
7,
16,
17]. PD-associated peritonitis is a serious complication of PD and prevention, and treatment is important in reducing patient morbidity and mortality [
18,
19]. Sterile placement of the PD catheter in the dolphin was achieved during an out-of-water procedure, and the catheter remained patent throughout the three-day treatment period. However, because the peritoneal catheter cannot stay dry and hygienic in a fully aquatic mammal, as it would in domestic animals or humans, there is a high risk for bacterial translocation and secondary peritonitis; therefore, empiric antibiotic therapy was warranted. Additionally, collecting gravity-fed effluent fluid and maintaining sterility while changing catheter tips and collection bags with movement of the patient in and out of the water also posed minor risk of contamination.
Aseptic technique was used for injections, venipuncture, and replacing dialysate fluids. It is plausible that increased injections and venipunctures performed on this dolphin during emergency treatments caused secondary phlebitis of flukes and myositis of dorsal musculature from SC fluids and IM injections. Due to aquatic management, installed injection ports did not seem feasible at the time for remaining in place and probably posed additional risk of infection. However, from experience gained with this case, the authors advise being judicious with repeated venipuncture of the fluke PAVR in cetaceans when collecting repeated blood samples and to dilute IV medications to decrease the risk of phlebitis and secondary infection. In the last ten years, improved catheterization techniques of the lateral caudal dorsal or ventral subcutaneous vein, used with dolphin anesthesia, may provide better sustainable treatment for emergency triage [
20].
The dialysis fluid, Dianeal® low calcium (2 mEq/L) peritoneal dialysis solution with 1.5% dextrose, was a commercial product chosen with the lowest osmolarity and sugar content. Standard commercial dialysates for human use are available with higher levels of glucose when rapid osmotic fluid removal is desired, but it was not needed in this case. This Dianeal® solution had electrolyte concentrations of Na (132 mmol/L), K (0 mmol/L), Ca (1.0 mmol/L = 2 mEq/L), Mg (0.25 mmol/L = 0.5 mEq/L), and Cl 96 (mmol/L), with lactate 40 mmol/L and pH 5.2. Although serum sodium and chloride concentrations typically run about 15 mmol/L higher in cetaceans than in humans (healthy baseline in this dolphin was Na 156 and Cl 119; normal ranges for humans are Na 136–145 and Cl 98–107), this patient’s serum Na and Cl both rose during PD treatment, so dialysate levels did not need to be augmented. The recommended dose for humans is based on clinical condition, body size, and electrolyte needs. Generally, treatment volume of dialysate solution instilled into an adult human abdomen ranges between 2.0 and 2.5 L, with the goal of exchanging dialysate 2–3 times daily for maintenance dialysis, and more frequently in acute intensive care situations (often every 4 h), depending on the patient needs. An extrapolated dose for this 220 kg animal was estimated to be between 5.0 and 6.0 L, and the goal was to maximize exchanges in the early critical phase and then try to perform at least two dialysate exchanges per day, with a minimum of 8 h dwell time.
Logistically, we were monitoring the dolphin’s condition and conducting procedures such as cystoscopy and stent placement, which may have decreased some of the desired dwell times. However, the dialysis achieved goals of lowering urea, creatinine, and electrolytes. Most notably, the reduction in the initial severe hyperkalemia (K 9.1 mq/L) was paralleled by progressive improvement in the critical ECG abnormalities and was clearly life-saving. It is important to note that the intraperitoneal infusion of any nonphysiologic solution has been shown to incite an inflammatory reaction of the peritoneum in animal models [
21]. The hypertonic dialysate with low pH and high glucose concentration can inflame the peritoneum, suppress the function of peritoneal cells, and cause aseptic peritonitis and/or lead to degrees of peritoneal fibrosis with chronic treatment [
17,
20]. Additives such as heparin and other osmotic solutes (i.e., N-acetylglucosamine (NAG)) have been shown to decrease the fibrinogenic response of the peritoneum, prevent catheter obstruction, and improve dialysis efficiency in animal models. These were not used in this case [
21]. In addition, antibiotics have been shown to decrease the incidence of intraperitoneal infection; therefore, intraperitoneal boluses of prophylactic antibiotics and antifungals, ceftazidime and fluconazole, respectively, were administered to the dolphin [
21].
PD-associated peritonitis can be diagnosed in humans when at least two of the following are present: (1) clinical evidence of peritonitis (abdominal pain and/or cloudy dialysis effluent); (2) a dialysis effluent WBC > 100 um/L with >50% polymorphonuclear leukocytes (PMN); and (3) a positive dialysis effluent culture [
18]. Focal peritonitis was indeed a significant complication in this dolphin, likely secondary to the progressive external contamination of the PD catheter translocating to the peritoneal space. Abdominal ultrasound displayed persistent abdominal ascites and hyperintense peritoneal serosa that lasted for approximately two weeks after the PD catheter was removed. Aggressive antibiotic therapy was initiated with amikacin but was replaced with less-nephrotoxic antibiotics: amoxicillin/clavulanic acid, ceftazidime, cefuroxime, and vancomycin. Chronic peritonitis and associated hepatopathy secondary to illness and possible drug toxicity (antifungal) contributed to hyporexia and weight loss, so additional liver supportive medications were added. Over the course of a month, the dolphin reached her lowest weight of 179 kg, having lost approximately 50 kg of weight from the start of her presenting illness. She soon began consuming 8–9 kg fish daily, approximately 50% of her normal 16 kg diet, but still had periods of decreased activity. Clinical resolution of peritonitis, as demonstrated by normal hematology and serum chemistry analysis, took approximately 75 days following the removal of the PD catheter.
From a welfare perspective, it was important to have a behavioral baseline from the animal care specialists familiar with this individual animal to follow her status from being moved from a social community pool to being a solo patient in a medical pool. The AKI-related signs (e.g., lethargy, anorexia, avoidance behavior) progressed rapidly to a critical uremic condition that required life-saving intensive care, frequent monitoring, fluid therapy and PD catheter placement. She was treated with steroid anti-inflammatories or local anesthetics to manage pain or discomfort; more potent analgesics were considered a risk to her acutely weakened condition. The dolphin was monitored twenty-four hours a day to evaluate changes in respiratory rate, activity, appetite, and behavioral responsiveness until her condition was stable after ten days. She was tolerant of being stretchered and physically moved from the medical pool to hospital table for dialysis procedures, as she was accustomed to being placed in a stretcher and moved to different locations in the past. Her appetite was a key display of positive operant conditioning, and it was remarkable following return to the holding pool that she would eat and respond to trainers after dialysis. A week following the critical phase of PD and recovery from the lithotripsy procedure, a companion animal was moved to the holding pool to remain with her for the duration of the treatments. Unfortunately, complications from the interventional PD catheterization and IV treatments were significant and required several weeks to recover from. At no point did the veterinarians and animal care specialists consider her quality of life to be poor, as all understood the temporary nature of the recovery and focused on the resources needed to address discomfort, socialization, wound healing, secondary infection, and caloric deficits.
Other medical complications observed such as bradycardia, metabolic syndrome, and vasculitis in this dolphin appeared secondary to volume overload, iatrogenic metabolic disorders, venipuncture, and intravenous fluid therapy. The dialysate volume administered was estimated from human dosage recommendations, 5–6 L, and approximated for the animal’s weight. A 1 L portion of the initial 6 L dialysate was eluted off when the dolphin subsequently displayed bradycardia (40 bpm) and shallow respirations suggestive of possible vagal response from increased abdominal pressure. Following dialysate volume infusion totals were moderated (4–5 L), but found to achieve dialytic goals of reducing creatinine, potassium, and BUN. Iatrogenic metabolic syndrome, secondary to systemic glucose absorption from the hyperosmolar glucose-based dialysate contributed to hyperglycemia, metabolic hyperlipidemia, and mild dehydration. A secondary fasting hyperglycemia (9.9 mmol/L) was chronically persistent during the dialysis treatment, suggesting possible insulin resistance, steroid induction or iatrogenic hyperglycemia. Insulin levels were measured to progressively elevate post-dialysis with insulin levels measuring 9.0 µIU/mL (54 days post-procedure), then 40.4 µIU/mL (64 days post-procedure) (insulin in managed dolphins, range 3–13 µIU/mL) [
22]. Serum glucose levels rose to a level of 24.65 mmol/L (
= 5.82 mmol/L), cholesterol to 10.4 mmol/L (
= 5.2 mmol/L), and triglycerides to 9.18 mmol/L (
= 0.73 mmol/L), by the end of the dialysis period [
2]. While the hyperglycemia resolved within 24 h after the removal of the peritoneal catheter, cholesterol and triglyceride levels remained elevated for 20 and 48 days, respectively.
Recurrent IV bolus treatments administered for fluid diuresis, peritonitis, and secondary septicemia likely contributed to bilateral vasculitis of the ventral fluke PAVR, distal to the venipuncture sites. The dolphin exhibited chronic leukocytosis, with WBC persistently in the mid-to-high 20s (×103/µL) for approximately 60 days, and fibrinogen levels exceeding 340 mg/dL for 80 days. Full resolution of the fluke tip wounds required approximately eight months. These complications were likely associated with IV therapy and peritonitis secondary to contamination of the peritoneal dialysis (PD) catheter, an inherent risk when managing such devices in an aquatic environment. Fortunately, the underlying ureterolith obstruction was identified and addressed within days of the initial presentation, allowing PD to serve as a short-term intervention. Nonetheless, the potential for complications is likely heightened in fully aquatic mammals and should be carefully considered during treatment planning.
Following peritoneal catheter placement and initial dialysis collection, elevations in AST, CK, and LDH were noted beginning on Day 3, suggesting possible hepatic or muscle injury. Contributing factors likely included the invasive nature of peritoneal catheter placement, dialysate infusion, and repeated patient handling. However, myocardial injury could not be excluded. ECG monitoring and echocardiography were intermittently utilized during out-of-water procedures to assess cardiac rhythm and function. During active ureteral obstruction, ECG abnormalities—including widened QRS complexes, flattened P waves, tented T waves, and intermittent premature ventricular complexes (PVCs)—were observed. These findings resolved with the passage of a unilateral urolith and medical management of hyperkalemia. Subsequent ECG monitoring during out-of-water procedures remained within normal limits for this individual, with only mild, transient changes (e.g., bradycardia) and no arrhythmia recorded.
Chronic elevation of liver enzymes later indicated hepatopathy, likely secondary to antifungal toxicity. Voriconazole was administered at an extrapolated dose of 2 mg/kg SID, which has since been recognized as excessive for dolphins. Retrospective drug level analysis revealed serum concentrations ranging from 4.5 to 9.9 µg/mL within 1–7 days post-treatment, exceeding the commonly accepted therapeutic range of 1.0–5.0 µg/mL for susceptible fungal pathogens [
23]. Hepatic dysfunction was therefore likely multifactorial, compounded by voriconazole toxicity and underlying metabolic disturbances, including insulin resistance, transient hyperglycemia, persistent hyperlipidemia, and hypercholesterolemia.
With dialysis, there is continuous exchange of solutes between the blood and dialysate, as water, small solutes, and ions move by diffusion, convection, or ultrafiltration across the peritoneal membrane [
21,
24]. In this case, the dwell period ranged from 1 h to 12 h, after which the dialysate containing uremic toxins, solutes, and excess fluid was collected into a sterile collection bag. Optimally, 4–5 fluid exchanges per day are desirable in humans; however, logistically 1–2 exchanges were performed per day over 3 days in the case of this dolphin. Kidney function was evaluated by monitoring BUN, Cr, P, K, and USG. AKI peaked on the evening of Day 3 with severe life-threatening elevations in BUN, Cr, P, and K (
Table 1). USG did not go lower than 1.020 ppt. With fluid therapy aiding the expulsion of one urolith combined with PD, kidney function drastically improved within 4–6 h and then through Day 6. There was steady improvement in kidney function through Day 11; however, BUN was observed to lag and did not return to normal for over 2.5 months (
Table 1). The lag in BUN normalization may correspond with other extra-renal factors that this dolphin was contending with, such as chronic inflammation, chronic wound healing, and recovering from septicemia—all requiring increased metabolic breakdown and the use of protein filtered by the kidneys. With AKI, there may also be residual kidney dysfunction, evident in electrolyte disregulation through Day 16 (
Table 1). Six months after the AKI (Day186), the kidney function indicators and USG were within normal limits (
Table 1).
This dolphin presented with AKI secondary to bilateral ureteral obstruction and recovered with the intervention treatment of PD and laser lithotripsy. She recovered full kidney function and, to our knowledge, is the first reported case of successful peritoneal dialysis in a marine mammal. This case also highlights the cooperation between veterinary and human medical teams who worked together with a One Health philosophy to apply common human medical techniques to a critically ill marine mammal to facilitate clinical stabilization and treatment of bilateral ureteral obstruction [
1].