The Effects of Artificial UV-B Provision on Positional Sleeping Behaviour and Vitamin D3 Metabolites of Captive Aye-Ayes (Daubentonia madagascariensis)
Abstract
1. Introduction
- Effect of UV-B Exposure on Vitamin D3 Levels
- 2.
- Effect of UV-B Exposure on Positional Sleeping Behaviour
2. Materials and Methods
2.1. Location and Date
2.2. Test Subjects
2.3. UV and Thermal Variation
2.4. Vitamin D3 Blood Serum Collection and Measurement Protocol
2.5. Positional Sleep Behaviour Data Collection
2.6. Statistical Analysis
2.6.1. Vitamin D3 Blood Serum Levels
2.6.2. Positional Sleep Behaviour
3. Results
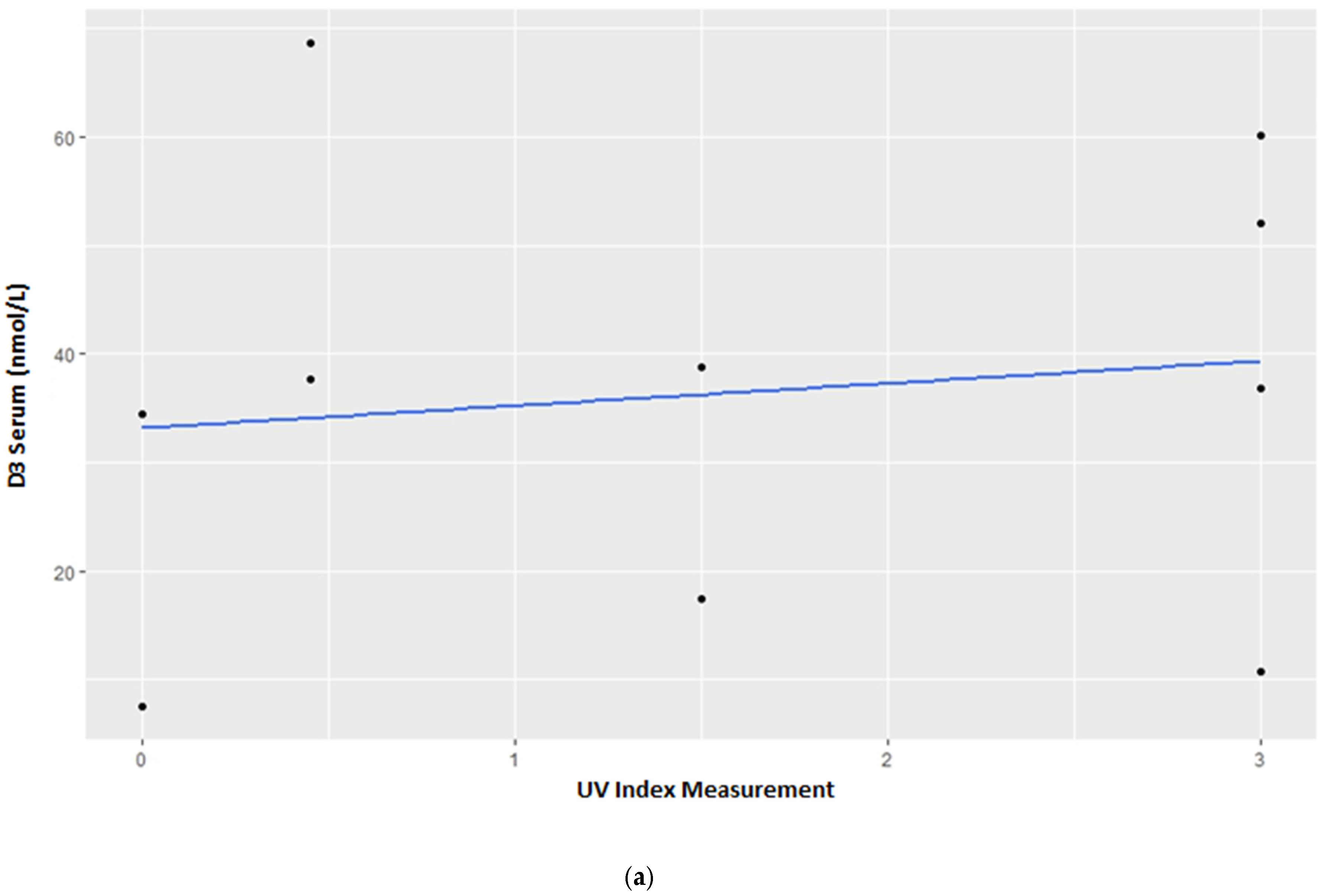
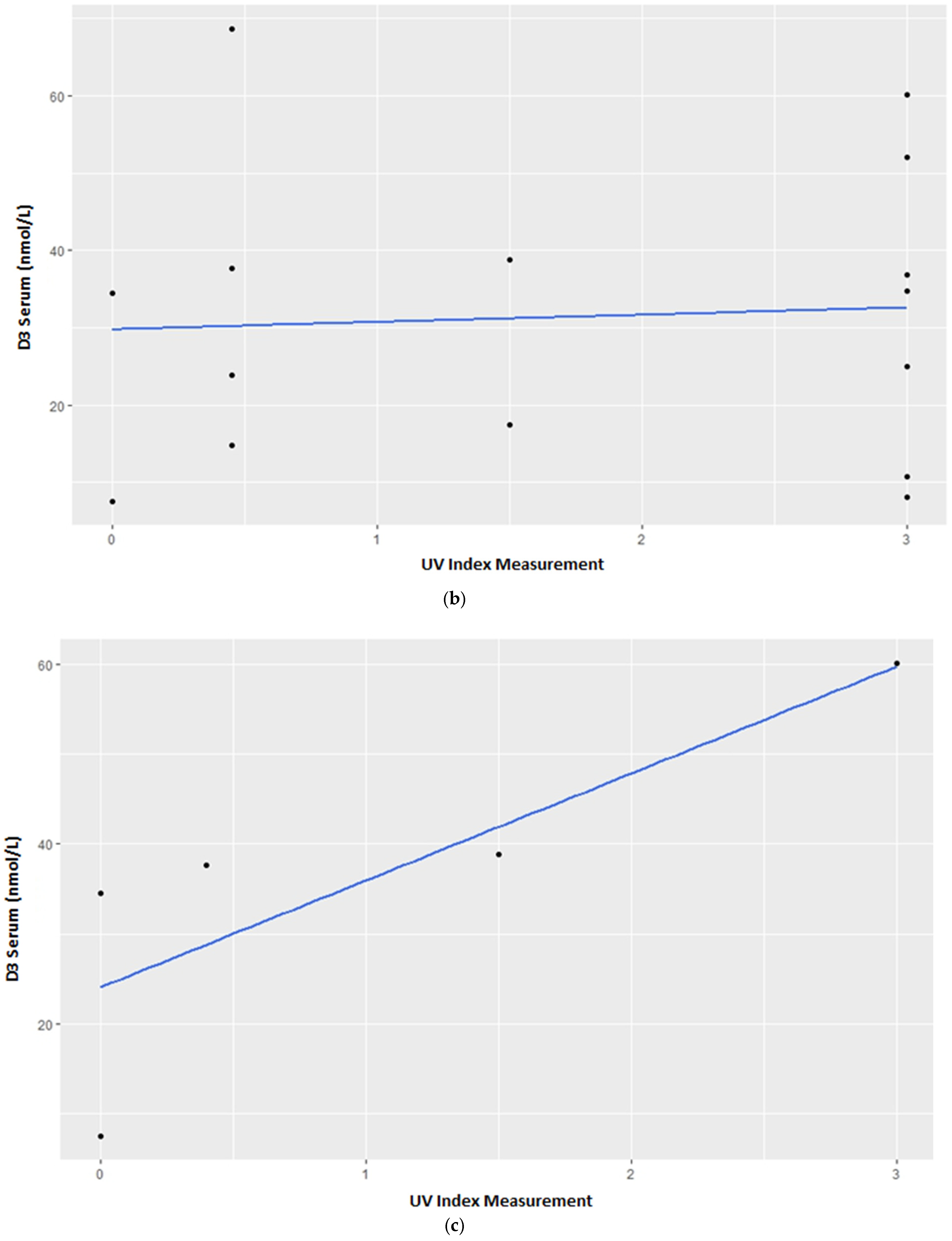
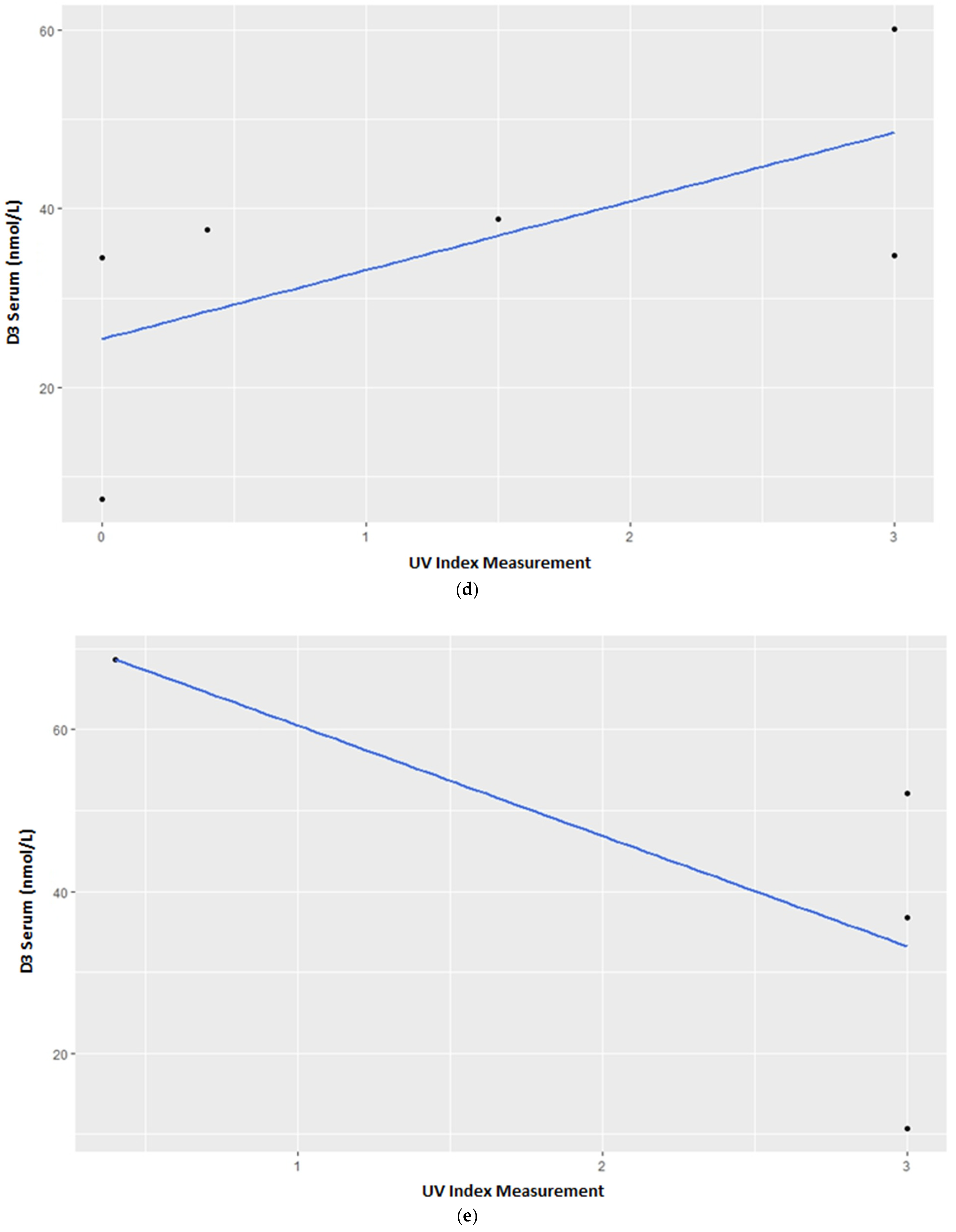
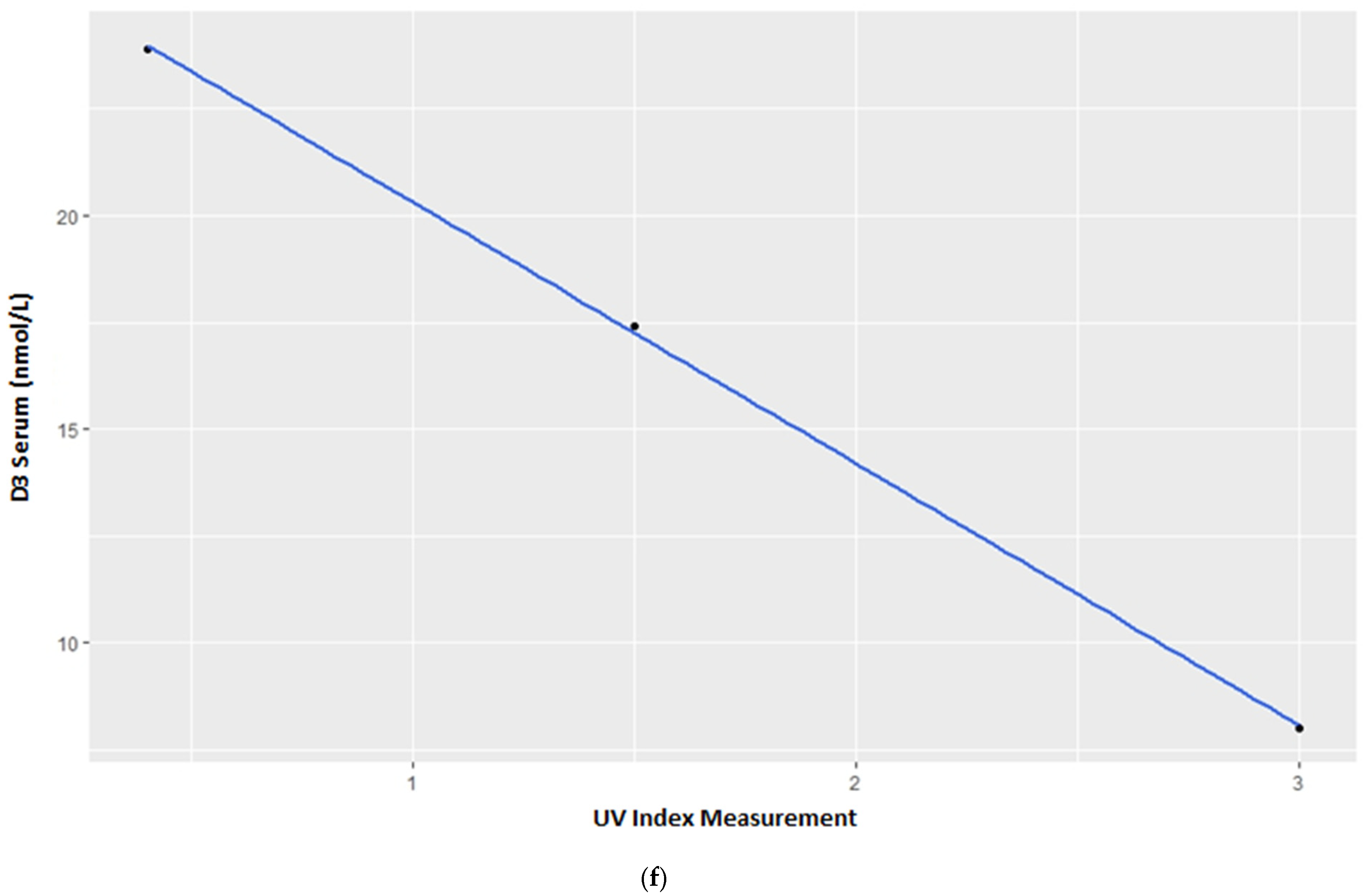
3.1. Vitamin D3 Blood Serum Levels
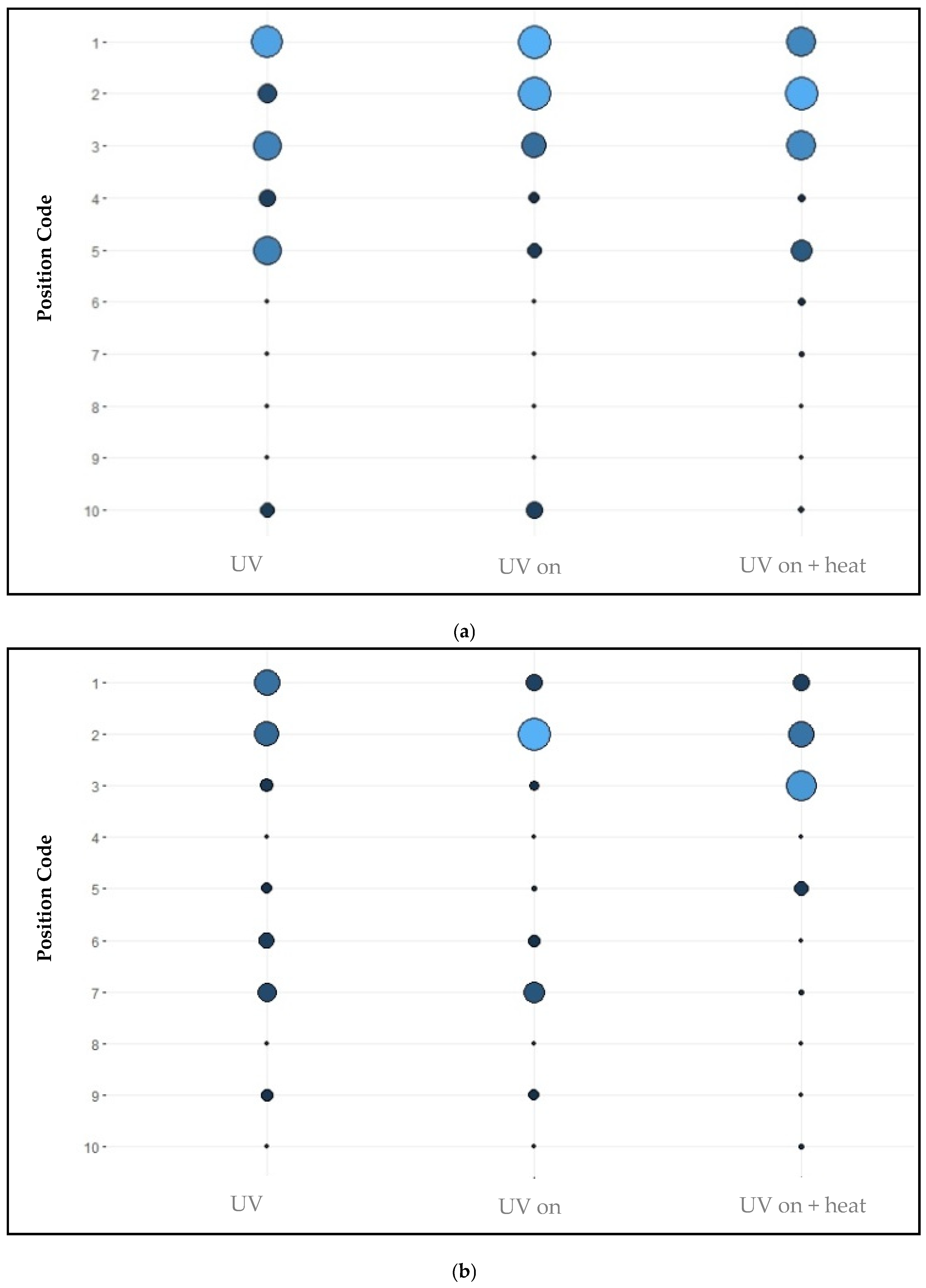
3.2. Positional Sleep Behaviour
4. Discussion
4.1. Vitamin D3 Blood Serum Levels—Group Census
4.2. Vitamin D3 Blood Serum Levels—Individual Variations
4.3. Positional Sleep Behaviour
4.4. Limitations
4.5. Study Applications, Benefits, and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV-B | Ultraviolet-B |
| UVI | Ultraviolet index |
Appendix A. Ferguson Zones
| Zone | Characteristics | Zone Range UVI | Maximum UVI |
|---|---|---|---|
| Zone 1 | Crepuscular or shade dweller, thermal conformer | 0–0.7 | 0.6–1.4 |
| Zone 2 | Partial sun/occasional basker, Thermoregulator | 0.7–1 | 1.1–3.0 |
| Zone 3 | Open or partial sun basker, Thermoregulator | 1–2.6 | 2.9–7.4 |
| Zone 4 | Mid-day sun basker, Thermoregulator | 2.6–3.5 | 4.5–9.5 |
Appendix B. Pairwise Fisher’s Exact Test Results
Appendix B.1
| UV-Off and UV-On | UV-Off and UV-On | N | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 130.070936 | 1.31 × 10−5 | 5.9 × 10−4 | *** |
| position 1 | position 3 | 52.780741 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 1 | position 4 | 43.249530 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 1 | position 5 | 47.431244 | 5.60 × 10−1 | 1.00000 × 10 | ns |
| position 1 | position 6 | 56.551378 | 7.30 × 10−1 | 1.00000 × 10 | ns |
| position 1 | position 7 | 77.724120 | 5.75 × 10−3 | 1.2900 × 10−1 | ns |
| position 1 | position 8 | 43.249530 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 1 | position 9 | 51.689233 | 4.19 × 10−1 | 1.00000 × 10 | ns |
| position 1 | position 10 | 43.249530 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 2 | position 3 | 96.352616 | 3.59 × 10−2 | 3.2300 × 10−1 | ns |
| position 2 | position 4 | 86.821405 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 2 | position 5 | 91.003119 | 1.53 × 10−2 | 2.2900 × 10−1 | ns |
| position 2 | position 6 | 100.123253 | 2.84 × 10−2 | 3.2000 × 10−1 | ns |
| position 2 | position 7 | 121.295995 | 4.05 × 10−1 | 1.00000 × 10 | ns |
| position 2 | position 8 | 86.821405 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 2 | position 9 | 95.261108 | 2.72 × 10−1 | 1.00000 × 10 | ns |
| position 2 | position 10 | 86.821405 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 3 | position 4 | 9.531210 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 3 | position 5 | 13.712924 | 5.05 × 10−1 | 1.00000 × 10 | ns |
| position 3 | position 6 | 22.833058 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 3 | position 7 | 44.005800 | 1.65 × 10−1 | 1.00000 × 10 | ns |
| position 3 | position 8 | 9.531210 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 3 | position 9 | 17.970913 | 6.50 × 10−1 | 1.00000 × 10 | ns |
| position 3 | position 10 | 9.531210 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 5 | 4.181714 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 6 | 13.301847 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 7 | 34.474590 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 8 | 0.000000 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 9 | 8.439703 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 4 | position 10 | 0.000000 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 5 | position 6 | 17.483561 | 5.19 × 10−1 | 1.00000 × 10 | ns |
| position 5 | position 7 | 38.656304 | 4.71 × 10−2 | 3.5300 × 10−1 | ns |
| position 5 | position 8 | 4.181714 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 5 | position 9 | 12.621417 | 2.28 × 10−1 | 1.00000 × 10 | ns |
| position 5 | position 10 | 4.181714 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 6 | position 7 | 47.776437 | 1.93 × 10−1 | 1.00000 × 10 | ns |
| position 6 | position 8 | 13.301847 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 6 | position 9 | 21.741550 | 6.62 × 10−1 | 1.00000 × 10 | ns |
| position 6 | position 10 | 13.301847 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 7 | position 8 | 34.474590 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 7 | position 9 | 42.914293 | 7.10 × 10−1 | 1.00000 × 10 | ns |
| position 7 | position 10 | 34.474590 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 8 | position 9 | 8.439703 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 8 | position 10 | 0.000000 | 1.00 × 10 | 1.00000 × 10 | ns |
| position 9 | position 10 | 8.439703 | 1.00 × 10 | 1.00000 × 10 | ns |
Appendix B.2
| UV-On and UV-On with Heat | UV-On and UV-On with Heat | n | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 112.72657952 | 4.56 × 10−1 | 1.00 × 10 | ns |
| position 1 | position 3 | 72.94662309 | 2.22 × 10−5 | 2.50 × 10−4 | *** |
| position 1 | position 4 | 21.49346405 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 5 | 29.26361656 | 1.16 × 10−2 | 4.35 × 10−2 | * |
| position 1 | position 6 | 25.77015251 | 1.25 × 10−1 | 4.02 × 10−1 | ns |
| position 1 | position 7 | 41.40522876 | 4.79 × 10−4 | 3.08 × 10−3 | ** |
| position 1 | position 8 | 21.45642702 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 9 | 24.96623094 | 1.25 × 10−1 | 4.02 × 10−1 | ns |
| position 1 | position 10 | 21.62309368 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 3 | 142.76034858 | 1.06 × 10−12 | 2.38 × 10−1 | **** |
| position 2 | position 4 | 91.30718954 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 5 | 99.07734205 | 5.58 × 10−4 | 3.14 × 10−3 | ** |
| position 2 | position 6 | 95.58387800 | 2.94 × 10−1 | 8.27 × 10−1 | ns |
| position 2 | position 7 | 111.21895425 | 6.96 × 10−4 | 3.48 × 10−3 | ** |
| position 2 | position 8 | 91.27015251 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 9 | 94.77995643 | 2.94 × 10−1 | 8.27 × 10−1 | ns |
| position 2 | position 10 | 91.43681917 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 4 | 51.52723312 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 5 | 59.29738562 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 6 | 55.80392157 | 9.53 × 10−5 | 7.15 × 10−4 | *** |
| position 3 | position 7 | 71.43899782 | 5.68 × 10−15 | 2.56 × 10−13 | **** |
| position 3 | position 8 | 51.49019608 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 9 | 55.00000000 | 9.53 × 10−5 | 7.15 × 10−4 | *** |
| position 3 | position 10 | 51.65686275 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 5 | 7.84422658 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 6 | 4.35076253 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 7 | 19.98583878 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 8 | 0.03703704 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 9 | 3.54684096 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 10 | 0.20370370 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 6 | 12.12091503 | 2.02 × 10−3 | 8.26 × 10−3 | ** |
| position 5 | position 7 | 27.75599129 | 3.22 × 10−7 | 4.83 × 10−6 | **** |
| position 5 | position 8 | 7.80718954 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 9 | 11.31699346 | 2.02 × 10−3 | 8.26 × 10−3 | ** |
| position 5 | position 10 | 7.97385621 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 7 | 24.26252723 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 8 | 4.31372549 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 9 | 7.82352941 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 10 | 4.48039216 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 8 | 19.94880174 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 9 | 23.45860566 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 10 | 20.11546841 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 9 | 3.50980392 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 10 | 0.16666667 | 1.00 × 10 | 1.00 × 10 | ns |
| position 9 | position 10 | 3.67647059 | 1.00 × 10 | 1.00 × 10 | ns |
Appendix B.3
| UV-Off and UV-On with Heat | UV-Off and UV-On with Heat | n | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 103.97398563 | 4.24 × 10−3 | 2.07 × 10−2 | * |
| position 1 | position 3 | 97.64893232 | 1.10 × 10−10 | 4.18 × 10−9 | **** |
| position 1 | position 4 | 42.31162175 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 5 | 53.87133148 | 1.23 × 10−2 | 5.03 × 10−2 | ns |
| position 1 | position 6 | 51.26270655 | 1.76 × 10−1 | 4.95 × 10−1 | ns |
| position 1 | position 7 | 57.20778052 | 4.89 × 10−2 | 1.69 × 10−1 | ns |
| position 1 | position 8 | 42.27458472 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 9 | 47.20448338 | 5.69 × 10−1 | 1.00 × 10 | ns |
| position 1 | position 10 | 42.44125138 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 3 | 117.07374852 | 5.35 × 10−5 | 6.02 × 10−4 | *** |
| position 2 | position 4 | 61.73643795 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 5 | 73.29614768 | 5.30 × 10−1 | 1.00 × 10 | ns |
| position 2 | position 6 | 70.68752275 | 2.71 × 10−3 | 1.52 × 10−2 | * |
| position 2 | position 7 | 76.63259671 | 7.57 × 10−5 | 6.81 × 10−4 | *** |
| position 2 | position 8 | 61.69940091 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 9 | 66.62929957 | 5.34 × 10−2 | 1.72 × 10−1 | ns |
| position 2 | position 10 | 61.86606758 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 4 | 55.41138464 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 5 | 66.97109437 | 9.42 × 10−2 | 2.83 × 10−1 | ns |
| position 3 | position 6 | 64.36246944 | 3.58 × 10−7 | 5.37 × 10−6 | **** |
| position 3 | position 7 | 70.30754341 | 1.86 × 10−10 | 4.18 × 10−9 | **** |
| position 3 | position 8 | 55.37434761 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 9 | 60.30424627 | 1.33 × 10−4 | 9.98 × 10−4 | *** |
| position 3 | position 10 | 55.54101427 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 5 | 11.63378380 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 6 | 9.02515887 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 7 | 14.97023284 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 8 | 0.03703704 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 9 | 4.96693570 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 10 | 0.20370370 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 6 | 20.58486860 | 4.60 × 10−3 | 2.07 × 10−2 | * |
| position 5 | position 7 | 26.52994257 | 2.23 × 10−4 | 1.43 × 10−3 | ** |
| position 5 | position 8 | 11.59674677 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 9 | 16.52664543 | 2.94 × 10−2 | 1.10 × 10−1 | ns |
| position 5 | position 10 | 11.76341343 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 7 | 23.92131764 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 8 | 8.98812184 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 9 | 13.91802050 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 10 | 9.15478850 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 8 | 14.93319580 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 9 | 19.86309446 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 10 | 15.09986247 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 9 | 4.92989866 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 10 | 0.16666667 | 1.00 × 10 | 1.00 × 10 | ns |
| position 9 | position 10 | 5.09656533 | 1.00 × 10 | 1.00 × 10 | ns |
Appendix B.4
| UV-Off and UV-On | UV-Off and UV-On | n | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 109.561584 | 7.96 × 10−3 | 7.16 × 10−2 | ns |
| position 1 | position 3 | 109.383293 | 4.31 × 10−1 | 1.00 × 10 | ns |
| position 1 | position 4 | 75.546192 | 2.62 × 10−1 | 1.00 × 10 | ns |
| position 1 | position 5 | 95.236908 | 2.71 × 10−3 | 4.07 × 10−2 | * |
| position 1 | position 6 | 66.955979 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 7 | 66.955979 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 8 | 66.955979 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 9 | 66.955979 | 1.00 × 10 | 1.00 × 10 | ns |
| position 1 | position 10 | 78.095938 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 3 | 85.032920 | 1.57 × 10−3 | 3.53 × 10−2 | * |
| position 2 | position 4 | 51.195818 | 5.98 × 10−3 | 6.73 × 10−2 | ns |
| position 2 | position 5 | 70.886534 | 5.82 × 10−7 | 2.62 × 10−5 | **** |
| position 2 | position 6 | 42.605605 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 7 | 42.605605 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 8 | 42.605605 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 9 | 42.605605 | 1.00 × 10 | 1.00 × 10 | ns |
| position 2 | position 10 | 53.745565 | 1.34 × 10−1 | 7.54 × 10−1 | ns |
| position 3 | position 4 | 51.017528 | 4.50 × 10−1 | 1.00 × 10 | ns |
| position 3 | position 5 | 70.708244 | 3.85 × 10−2 | 2.85 × 10−1 | ns |
| position 3 | position 6 | 42.427315 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 7 | 42.427315 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 8 | 42.427315 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 9 | 42.427315 | 1.00 × 10 | 1.00 × 10 | ns |
| position 3 | position 10 | 53.567274 | 5.18 × 10−1 | 1.00 × 10 | ns |
| position 4 | position 5 | 36.871142 | 6.39 × 10−1 | 1.00 × 10 | ns |
| position 4 | position 6 | 8.590213 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 7 | 8.590213 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 8 | 8.590213 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 9 | 8.590213 | 1.00 × 10 | 1.00 × 10 | ns |
| position 4 | position 10 | 19.730172 | 3.52 × 10−1 | 1.00 × 10 | ns |
| position 5 | position 6 | 28.280929 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 7 | 28.280929 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 8 | 28.280929 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 9 | 28.280929 | 1.00 × 10 | 1.00 × 10 | ns |
| position 5 | position 10 | 39.420888 | 4.43 × 10−2 | 2.85 × 10−1 | ns |
| position 6 | position 7 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 8 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 9 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 6 | position 10 | 11.139959 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 8 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 9 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 7 | position 10 | 11.139959 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 9 | 0.000000 | 1.00 × 10 | 1.00 × 10 | ns |
| position 8 | position 10 | 11.139959 | 1.00 × 10 | 1.00 × 10 | ns |
| position 9 | position 10 | 11.139959 | 1.00 × 10 | 1.00 × 10 | ns |
Appendix B.5
| UV-On and UV-On with Heat | UV-On and UV-On with Heat | n | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 127.47167756 | 0.37400 | 1.000 × 10 | ns |
| position 1 | position 3 | 104.71350763 | 0.11200 | 7.59 × 10−1 | ns |
| position 1 | position 4 | 63.27995643 | 1.00000 | 1.000 × 10 | ns |
| position 1 | position 5 | 77.95315904 | 0.03160 | 3.56 × 10−1 | ns |
| position 1 | position 6 | 60.87254902 | 0.42600 | 1.000 × 10 | ns |
| position 1 | position 7 | 60.26143791 | 1.00000 | 1.000 × 10 | ns |
| position 1 | position 8 | 60.22440087 | 1.00000 | 1.000 × 10 | ns |
| position 1 | position 9 | 60.22440087 | 1.00000 | 1.000 × 10 | ns |
| position 1 | position 10 | 66.79411765 | 0.07540 | 6.79 × 10−1 | ns |
| position 2 | position 3 | 111.73638344 | 0.43900 | 1.000 × 10 | ns |
| position 2 | position 4 | 70.30283224 | 1.00000 | 1.000 × 10 | ns |
| position 2 | position 5 | 84.97603486 | 0.11800 | 7.59 × 10−1 | ns |
| position 2 | position 6 | 67.89542484 | 1.00000 | 1.000 × 10 | ns |
| position 2 | position 7 | 67.28431373 | 1.00000 | 1.000 × 10 | ns |
| position 2 | position 8 | 67.24727669 | 1.00000 | 1.000 × 10 | ns |
| position 2 | position 9 | 67.24727669 | 1.00000 | 1.000 × 10 | ns |
| position 2 | position 10 | 73.81699346 | 0.02710 | 3.56 × 10−1 | ns |
| position 3 | position 4 | 47.54466231 | 0.56700 | 1.000 × 10 | ns |
| position 3 | position 5 | 62.21786492 | 0.39600 | 1.000 × 10 | ns |
| position 3 | position 6 | 45.13725490 | 1.00000 | 1.000 × 10 | ns |
| position 3 | position 7 | 44.52614379 | 1.00000 | 1.000 × 10 | ns |
| position 3 | position 8 | 44.48910675 | 1.00000 | 1.000 × 10 | ns |
| position 3 | position 9 | 44.48910675 | 1.00000 | 1.000 × 10 | ns |
| position 3 | position 10 | 51.05882353 | 0.00847 | 1.91 × 10−1 | ns |
| position 4 | position 5 | 20.78431373 | 0.24700 | 1.000 × 10 | ns |
| position 4 | position 6 | 3.70370370 | 1.00000 | 1.000 × 10 | ns |
| position 4 | position 7 | 3.09259259 | 1.00000 | 1.000 × 10 | ns |
| position 4 | position 8 | 3.05555556 | 1.00000 | 1.000 × 10 | ns |
| position 4 | position 9 | 3.05555556 | 1.00000 | 1.000 × 10 | ns |
| position 4 | position 10 | 9.62527233 | 0.33300 | 1.000 × 10 | ns |
| position 5 | position 6 | 18.37690632 | 1.00000 | 1.000 × 10 | ns |
| position 5 | position 7 | 17.76579521 | 1.00000 | 1.000 × 10 | ns |
| position 5 | position 8 | 17.72875817 | 1.00000 | 1.000 × 10 | ns |
| position 5 | position 9 | 17.72875817 | 1.00000 | 1.000 × 10 | ns |
| position 5 | position 10 | 24.29847495 | 0.00343 | 1.54 × 10−1 | ns |
| position 6 | position 7 | 0.68518519 | 1.00000 | 1.000 × 10 | ns |
| position 6 | position 8 | 0.64814815 | 1.00000 | 1.000 × 10 | ns |
| position 6 | position 9 | 0.64814815 | 1.00000 | 1.000 × 10 | ns |
| position 6 | position 10 | 7.21786492 | 0.14300 | 8.04 × 10−1 | ns |
| position 7 | position 8 | 0.03703704 | 1.00000 | 1.000 × 10 | ns |
| position 7 | position 9 | 0.03703704 | 1.00000 | 1.000 × 10 | ns |
| position 7 | position 10 | 6.60675381 | 1.00000 | 1.000 × 10 | ns |
| position 8 | position 9 | 0.00000000 | 1.00000 | 1.000 × 10 | ns |
| position 8 | position 10 | 6.56971678 | 1.00000 | 1.000 × 10 | ns |
| position 9 | position 10 | 6.56971678 | 1.00000 | 1.000 × 10 | ns |
Appendix B.6
| UV-Off and UV-On with Heat | UV-Off and UV-On with Heat | n | p | P. Adj. | P. Adj. Signif. |
|---|---|---|---|---|---|
| position 1 | position 2 | 100.71953587 | 0.000465 | 1.050 × 10−2 | * |
| position 1 | position 3 | 107.07719317 | 0.442000 | 1.00000 × 10 | ns |
| position 1 | position 4 | 64.08105011 | 0.225000 | 8.4400 × 10−1 | ns |
| position 1 | position 5 | 93.54300788 | 0.520000 | 1.00000 × 10 | ns |
| position 1 | position 6 | 57.75009632 | 0.448000 | 1.00000 × 10 | ns |
| position 1 | position 7 | 57.13898521 | 1.000000 | 1.00000 × 10 | ns |
| position 1 | position 8 | 57.10194817 | 1.000000 | 1.00000 × 10 | ns |
| position 1 | position 9 | 57.10194817 | 1.000000 | 1.00000 × 10 | ns |
| position 1 | position 10 | 62.30182045 | 0.075600 | 4.8600 × 10−1 | ns |
| position 2 | position 3 | 93.59283270 | 0.008940 | 8.050 × 10−2 | ns |
| position 2 | position 4 | 50.59668965 | 0.001820 | 2.050 × 10−2 | * |
| position 2 | position 5 | 80.05864741 | 0.000185 | 8.32 × 10−3 | ** |
| position 2 | position 6 | 44.26573585 | 1.000000 | 1.00000 × 10 | ns |
| position 2 | position 7 | 43.65462474 | 1.000000 | 1.00000 × 10 | ns |
| position 2 | position 8 | 43.61758770 | 1.000000 | 1.00000 × 10 | ns |
| position 2 | position 9 | 43.61758770 | 1.000000 | 1.00000 × 10 | ns |
| position 2 | position 10 | 48.81745999 | 0.001170 | 1.760 × 10−2 | * |
| position 3 | position 4 | 56.95434695 | 0.105000 | 5.9100 × 10−1 | ns |
| position 3 | position 5 | 86.41630471 | 0.189000 | 7.7300 × 10−1 | ns |
| position 3 | position 6 | 50.62339315 | 1.000000 | 1.00000 × 10 | ns |
| position 3 | position 7 | 50.01228204 | 1.000000 | 1.00000 × 10 | ns |
| position 3 | position 8 | 49.97524500 | 1.000000 | 1.00000 × 10 | ns |
| position 3 | position 9 | 49.97524500 | 1.000000 | 1.00000 × 10 | ns |
| position 3 | position 10 | 55.17511729 | 0.053000 | 3.9700 × 10−1 | ns |
| position 4 | position 5 | 43.42016166 | 0.396000 | 1.00000 × 10 | ns |
| position 4 | position 6 | 7.62725009 | 0.250000 | 8.6500 × 10−1 | ns |
| position 4 | position 7 | 7.01613898 | 1.000000 | 1.00000 × 10 | ns |
| position 4 | position 8 | 6.97910195 | 1.000000 | 1.00000 × 10 | ns |
| position 4 | position 9 | 6.97910195 | 1.000000 | 1.00000 × 10 | ns |
| position 4 | position 10 | 12.17897423 | 1.000000 | 1.00000 × 10 | ns |
| position 5 | position 6 | 37.08920786 | 0.378000 | 1.00000 × 10 | ns |
| position 5 | position 7 | 36.47809675 | 1.000000 | 1.00000 × 10 | ns |
| position 5 | position 8 | 36.44105972 | 1.000000 | 1.00000 × 10 | ns |
| position 5 | position 9 | 36.44105972 | 1.000000 | 1.00000 × 10 | ns |
| position 5 | position 10 | 41.64093200 | 0.160000 | 7.5200 × 10−1 | ns |
| position 6 | position 7 | 0.68518519 | 1.000000 | 1.00000 × 10 | ns |
| position 6 | position 8 | 0.64814815 | 1.000000 | 1.00000 × 10 | ns |
| position 6 | position 9 | 0.64814815 | 1.000000 | 1.00000 × 10 | ns |
| position 6 | position 10 | 5.84802043 | 0.167000 | 7.5200 × 10−1 | ns |
| position 7 | position 8 | 0.03703704 | 1.000000 | 1.00000 × 10 | ns |
| position 7 | position 9 | 0.03703704 | 1.000000 | 1.00000 × 10 | ns |
| position 7 | position 10 | 5.23690932 | 1.000000 | 1.00000 × 10 | ns |
| position 8 | position 9 | 0.00000000 | 1.000000 | 1.00000 × 10 | ns |
| position 8 | position 10 | 5.19987229 | 1.000000 | 1.00000 × 10 | ns |
| position 9 | position 10 | 5.19987229 | 1.000000 | 1.00000 × 10 | ns |
Appendix C. Ethogram of Aye-Aye Positional Sleep Behaviour
Appendix C.1
| Position | Code | Description |
|---|---|---|
| Exposed ball and ears hidden | 1 | The aye-aye is in a fully closed ball-like position, lying laterally on their side. Their tail either follows their curved body on the side or is placed on top of their body. The arms, legs, and face are fully tucked within the body. The ears are curled over and appear flattered to the body. Less than 20% of the body is covered by nesting or woodwool material. |
| Exposed ball and ears out | 2 | The aye-aye is in a fully closed ball-like position, lying laterally on their side. The tail either follows their curved body on the side or is placed on top of their body. The arms, legs, and face are fully tucked within the body. The face may be fully or partially tucked within the body. The ears are protruding and appear fully erect. Less than 20% of the body is covered by nesting or woodwool material. |
| Exposed stretched out | 3 | The aye-aye is lying laterally on their side. The arms, legs, head, and ears are free from the torso and can be easily identified. The tail is also free from the torso but may be covering some of the legs. Less than 20% of the body is covered by nesting or woodwool material. |
| Exposed tail over torso laid on back | 4 | The aye-aye is lying posteriorly, and the underbelly can be clearly seen. The tail comes up between the back legs covering the torso. The arms are lying either at the side of or perpendicular to the torso. The legs are splayed out apart. The head is lying recumbently or laterally. Less than 20% of the body is covered by nesting or woodwool material. |
| Exposed laid on back | 5 | The aye-aye is lying posteriorly, and the underbelly can be clearly seen. The tail lies away from the body. The arms are lying either at the side of or perpendicular to the torso. The legs are splayed out apart. The head is lying recumbently or laterally. Less than 20% of the body is covered by nesting or woodwool material. |
| Partial—face exposed | 6 | From 20% to 70% of the body is covered in nesting or woodwool material. The face and top of the torso are exposed. |
| Partial—body exposed | 7 | From 20% to 70% of the body is covered in nesting or woodwool material. The tail, legs, and hips are exposed. |
| Under nesting material | 8 | Approximately 80% of the body is covered by nesting material, and the position cannot be determined. |
| Under woodwool | 9 | Approximately 80% of the body is covered by woodwool, and the position cannot be determined. |
| Exposed stretched out tail over torso | 10 | The aye-aye is lying laterally on their side. The arms, legs, head, and ears are free from the torso and can be easily identified. The tail is either fully or partially covering the torso. Less than 20% of the body is covered by nesting or woodwool material. |
Appendix D. Raw Vitamin D3 Serum Blood Results
Appendix D.1
| Test Subject ID | UVI Measurement | nmol/L | Control Box Access |
|---|---|---|---|
| A | 0 | 7.5 | N/A |
| 0 | 34.5 | N/A | |
| 0.4 | 37.6 | N | |
| 1.5 | 38.8 | N | |
| 3 | 60.1 | N | |
| 3 | 34.8 | Y | |
| B | 0.4 | 23.9 | Y |
| 1.5 | 17.4 | N | |
| 3 | 8 | Y | |
| C | 0.4 | 14.8 | Y |
| D | 0.4 | 68.7 | N |
| 3 | 52.1 | N | |
| 3 | 36.8 | N | |
| 3 | 10.7 | N | |
| E | 3 | 25.02 | Y |
Appendix D.2
| UVI Measurement | Control Box Access | n | Mean (nmol/L) | Range (nmol/L) |
|---|---|---|---|---|
| 0 | N/A | 2 | 24.75 | 7.5–34.5 |
| 0.4 | Yes | 2 | 19.35 | 14.8–23.9 |
| 0.4 | No | 2 | 53.15 | 37.6–68.7 |
| 1.5 | Yes | - | - | - |
| 1.5 | No | 2 | 28.6 | 17.4–38.8 |
| 3 | Yes | 3 | 22.61 | 8–34.8 |
| 3 | No | 4 | 39.93 | 10.7–60.1 |
References
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Xie, Z.; Komuves, L.; Yu, Q.C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Bikle, D.D. Lack of the Vitamin D Receptor is Associated with Reduced Epidermal Differentiation and Hair Follicle Growth. J. Investig. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Arvold, D.S.; Odean, M.J.; Dornfeld, M.P.; Regal, R.R.; Arvold, J.G.; Karwoski, G.C.; Mast, D.J.; Sanford, P.B.; Sjoberg, R.J. Correlation of Symptoms with Vitamin D Deficiency and Symptom Response to Cholecalciferol Treatment: A Randomized Controlled Trial. Endocre. Pract. 2009, 15, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D. Production of Ergocalciferol (Vitamin D2) and Related Sterols in Mushrooms with Exposure to Pulsed Ultraviolet Light. Ph.D. Thesis, The Pennsylvania State University, University Park, PA, USA, January 2012. Available online: https://etda.libraries.psu.edu/catalog/13864 (accessed on 12 July 2025).
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for it’s Treatment. Metabolites 2021, 11, 225. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in Foods and as Supplements. Prog. Biophys. Mol. Bio. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Anderson, J.J.B.; Toverud, S.U. Diet and Vitamin D: A Review with an Emphasis on Human Function. J. Nutr. Biochem. 1994, 5, 58–65. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Brenner, M.; and Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. J. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Norman, A.W. Sunlight, Season, Skin Pigmentation, Vitamin D, and 25-hydroxyvitamin D: Integral Components of the Vitamin D Endocrine System. Am. J. Clin. Nutr. 1998, 67, 1108–1110. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D2: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D2 Synthesis in Humans. J. Clin. Endocrinol. Metab. 1998, 67, 373–378. [Google Scholar] [CrossRef]
- Moan, J.; Lagunova, Z.; Cicarma, E.; Aksnes, L.; Dalhback, A.; Grant, W.B.; Porojnicu, A.C. Sunbeds as Vitamin D Sources. J Photochem. Photobiol. 2009, 85, 1474–1479. [Google Scholar] [CrossRef]
- Morais, P. Artificial tanning devices (Sunbeds): Where do we stand? Cutan. Ocul. Toxicol. 2002, 41, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M. Contributions of Sunlight and Diet to Vitamin D Status. Calcif. Tissue Int. 2012, 92, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D Supplementation, 25-Hydroxyvitamin D Concentrations, and Safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Veith, R.; et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “Sunshine” Vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Dittmer, K.E.; Thompson, K.G. Vitamin D Metabolism and Rickets in Domestic Animals: A Review. Vet. Pathol. 2011, 48, 389–407. [Google Scholar] [CrossRef]
- Uhl, E.W. The Pathology of Vitamin D Deficiency in Domestic Animals: An Evolutionary and Comparative Overview. Int. J. Paleopathol. 2018, 23, 100–109. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Williams, C.J.; Proulx, J.G. Plasma Vitamin D3 Response in Cattle and Sheep Exposed to Ultraviolet Radiation. Int. J. Vitam. Nutr. Res. 1985, 55, 41–46. [Google Scholar]
- Junge, R.E.; Gannon, F.H.; Porton, I.; McAlister, W.H.; Whyte, M.P. Management and Prevention of Vitamin D Deficiency Rickets in Captive-Born Juvenile Chimpanzees. J. Zoo Wildl. Med. 2000, 31, 361–369. [Google Scholar] [CrossRef]
- Hunt, R.D.; Garcia, F.G.; Hegsted, D.M.; Kaplinsky, N. Vitamins D2 and D3 in New World Primates: Influence on Calcium Absorption. Science 1967, 157, 943–945. [Google Scholar] [CrossRef]
- Power, M.L.; Oftedal, O.T.; Savage, A.; Blumer, E.S.; Soto, L.H.; Chen, C.T.; Hollck, M.F. Assessing Vitamin D Status of Callitrichids: Baseline Data from Wild Cotton-top Tamarins (Saguinus oesipus) in Colombia. Zoo Biol. 1997, 16, 39–46. [Google Scholar] [CrossRef]
- Gacad, M.A.; Deseran, M.W.; Adams, J.S. Influence of Ultraviolet B Radiation on Vitamin D3 Metabolism in Vitamin D3-Resistant New World Primates. Am. J. Primatol. 1992, 28, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.L.; Chen, T.C.; Murphy, H.; Holick, M.F.; Tlusty, M.; Baitchman, E. Assessment of Serum 25-Hydrooxyvitamin D Concentrations in Two Collections of Captive Gorillas (Gorilla gorilla gorilla). J. Zoo Wildl. Med. 2017, 48, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 973–1088. [Google Scholar] [CrossRef] [PubMed]
- Killick, R.; Saunders, R.; Redrobe, S.P. Summer and Winter Vitamin D3 Levels in Four Lemur Species Housed at a British Zoo, with Reference to UVB Levels. J. Zoo Wildl. Med. 2015, 46, 498–505. [Google Scholar] [CrossRef]
- Abelló, T.; Rietkerk, F.; Bemment, N. EAZA Best Practice Guidelines for Gorilla (Gorilla gorilla gorilla), 2nd ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Byczyk, K.; Dunn, N.; Příbrský, F.; Tang, C. EAZA Best Practice Guidelines for the Slow Loris (Nycticebus) Species, 1st ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Van den Heuvel, S.; Hoevens, S.; Daalhuisen, F.; Cabana, F.; de Boer, A.; Persaud, S. EAZA Best Practice Guidelines for the White-Faced Saki Monkey (Pithecia pithecia), 1st ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Lόpez, J.; Wormell, D.; Rodríguez, A. Preliminary Evaluation of the Efficacy and Safety of a UVB Lamp Used to Prevent Metabolic Bone Disease in Pied Tamarins Saguinus bicolor at Jersey Zoo. Dodo 2001, 37, 41–49. [Google Scholar]
- Herczeg, G.; Gonda, A.; Saarikivi, J.; Merilä, J. Experimental Support for the Cost-Benefit Model of Lizard Thermoregulation. Behav. Ecol. Sociobiol. 2006, 60, 405–414. [Google Scholar] [CrossRef]
- Stannard, H.J.; Fabian, M.; Old, J.M. To Bask or Not to Bask: Behavioural Thermoregulation in Two Species of Dasyruid, Phascogale calura and Antechinomys laniger. J. Therm. Bio. 2015, 53, 66–71. [Google Scholar] [CrossRef]
- Chaplin, G.; Jablonski, N.G.; Sussman, R.W.; Kelley, E.A. The Role of Piloerection in Primate Thermoregulation. Folia Primatol. 2014, 85, 1–17. [Google Scholar] [CrossRef]
- Gestich, C.C.; Caselli, C.B.; Setz, E.Z.F. Behavioural Thermoregulation in a Small Neotropical Primate. Ethology 2014, 120, 331–339. [Google Scholar] [CrossRef]
- Junge, R.E.; Williams, C.V.; Rakotondrainibe, H.; Mahefarisoa, K.L.; Rajaonarivelo, T.; Faulkner, C.; Mass, V. Baseline Health and Nutrition Evaluation of Two Sympatric Nocturnal Lemur Species (Avahi langier and Lepilemur mustelinus) Residing Near an Active Mine Site at Ambatovy, Madagascar. J. Zoo Wildl. Med. 2017, 48, 794–803. [Google Scholar] [CrossRef]
- Bethge, J.; Razafimampiandra, J.C.; Wulff, A.; Dausmann, K.H. Sportive Lemurs Elevate Their Metabolic Rate During Challenging Seasons and Do Not Enter Regular Heterothermy. Conserv. Physiol. 2021, 9, coab075. [Google Scholar] [CrossRef]
- Fernandez-Duque, E. An Owl Monkey (Aotus azarai azarai) Social Group Sunbasking During a Cold Morning in the Ar-gentinean Chaco [medium]. In Primates in Perspective; Campbell, C., Fuentes, A., MacKinnon, K., Bearder, S., Stumpf, R., Eds.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Geiser, F.; Goodship, N.; Pavey, C.R. Was Basking Important in the Evolution of Mammalian Endothermy? Naturwissenschaften 2002, 89, 412–414. [Google Scholar] [CrossRef]
- McLain, A.T.; Meyer, T.J.; Faulk, C.; Herke, S.W.; Oldenburg, J.M.; Bourgeois, M.G.; Abshire, C.F.; Roos, C.; Batzer, M.A. An Alu-Based Phylogeny of Lemurs (Infraorder: Lemuriformes). PLoS ONE 2012, 7, E0044035. [Google Scholar] [CrossRef]
- Duke Lemur Centre; Aye-Aye Studbook Keeper. Aye-Aye (Daubentonia madagascariensis) Housing and Husbandry Guidelines, 2nd ed.; Duke Lemur Centre: Durham, NC, USA, 2009. [Google Scholar]
- Carroll, J.B.; Haring, D.M. Maintenance and Breeding of Aye-Ayes (Daubentonia madagascariensis) in Captivity: A Review. Folia Primatol. 1994, 62, 54–62. [Google Scholar] [CrossRef]
- Sterling, E.J.; Dierenfeld, E.S.; Ashbourne, C.J.; Feistner, A.T.C. Dietary Intake, Food Composition and Nutrient Intake in Wild and Captive Populations of Daubentonia madagascariensis. Folia Primatol. 1994, 62, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Crissey, S.; Feeser, T.; Glander, K. Evaluation and Reformulation of Diets of Captive Aye-Aye (Daubentonia madagascariensis). In Proceedings of the First Annual Conference of the Nutrition Advisory Group of the American Association of Zoos and Aquariums, Scarborough, ME, USA; 1995. [Google Scholar]
- Simons, E.L.; Meyers, D.M. Folklore and Beliefs About the Aye-aye (Daubentonia madagascariensis). Group. Lemur News 2001, 6, 11–16. [Google Scholar]
- Randimbiharinirina, D.R.; Raharivololona, B.M.; Hawkins, M.T.R.; Frasier, C.L.; Culligan, R.R.; Sefczek, T.M.; Randriamampionona, R.; Louis, E.E., Jr. Behaviour and Ecology of Males Aye-Ayes (Daubentonia madagascariensis) in the Kianjavato Classified Forest, South-eastern Madagascar. Folia Primatol. 2018, 82, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ancrenaz, M.; Ancrenaz, I.L.; Mundy, N. Field Observations of Aye-Aye (Daubentonia madagascariensis) in Madagascar. Folia Primatol. 1994, 62, 22–36. [Google Scholar] [CrossRef]
- Petter, J.J. The Aye-Aye. In Primate Conservation; Grimaldi, R., III, Bourne, G.H., Eds.; Academic Press Inc: New York, NY, USA, 1977. [Google Scholar]
- Fuller, G.; Raghanti, M.A.; Dennis, P.M.; Kuhar, C.W.; Willis, M.A.; Schook, M.W.; Lukas, K.E. A Comparison of Nocturnal Primate Behaviour in Exhibits Illuminated with Red and Blue Light. Appl. Anim. Behav. Sci. 2016, 184, 126–134. [Google Scholar] [CrossRef]
- McKinley, M.B. (Zoological Society of London, London, UK). Personal Communications, 2024.
- Killick, R. (Bristol Zoological Society, Bristol, UK). Personal Communications, 2020.
- Baines, F.; Chattell, J.; Dale, J.; Garrick, D.; Gill, I.; Skelton, T.; Swatman, M. How Much UV-B Does My Reptile Need? The UV-Tool, a Guide to the Selection of UV Lighting for Reptiles and Amphibians in Captivity. J. Zool. Res. 2016, 4, 42–63. [Google Scholar]
- Hendricks, J.C.; Finn, S.M.; Panckeri, K.A.; Williams, J.A.; Sehgal, A.; Pack, A.I. Rest in Drosophila Is a Sleep-like State. Neuron 2000, 25, 129–138. [Google Scholar] [CrossRef]
- Rattenborg, N.C.; de la Iglesia, H.O.; Kempenaers, B.; Lesku, J.A.; Meerlo, P.; Scriba, M.F. Sleep Research Goes Wild: New Methods and Approaches to Investigate the Ecology, Evolution and Functions of Sleep. Phil. Trans. R. Soc. B Biol. Sci. 2017, 372, 1–14. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R Studio, version 4.2.1; Posit: Boston, MA, USA, 2022; Available online: https://posit.co/download/rstudio-desktop/ (accessed on 26 August 2022).
- Wickham, H.; Francois, R.; Henry, L.; Muller, K.; Vaughn, D. dplyr: A Grammar of Data Manipulation. R Package, Version 1.1.3. Posit: Boston, MA, USA, 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 1 June 2022).
- Kassambara, A. rstatixR Package, Version 0.7.2. Posit: Boston, MA, USA, 2022. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 1 June 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubrR Package, Version 0.6.0. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 June 2022).
- Masquelier, B.; Waltisperger, D.; Ralijaona, O.; Pison, G.; Ravelo, A. The Epidemiological Transition in Antananarivo, Madagascar: An Assessment Based on Death Registers. Glob. Health Action 2024, 7, 23237. [Google Scholar] [CrossRef] [PubMed]
- Portafaix, T.; Lamy, K.; Amellie, V.; Rakotoniaina, S.; Moulin, L.; Ranaivombola, M. 5 years of UV Index Measurements in the Western Indian Ocean. AIP Publ. Conf. Proc. 2024, 2988, 0900041–0900044. [Google Scholar]
- Edwards, W.M.; Griffiths, R.A.; Bungard, M.J.; Rakotondrasoa, E.F.; Razafimanahaka, J.H.; Razafindraibe, P.; Andriantsimanarilafy, R.R.; Randrianantoandro, J.C. Microhabitat Preference of the Critically Endangered Golden Mantella Frog in Madagascar. Herpotol. J. 2019, 29, 207–213. [Google Scholar] [CrossRef]
- Goetz, M. EAZA Best Practice Guidelines for the Ploughshare Tortoise of Angonoka (Astrochelys yniphora), 1st ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Rivas, A.E.; Mitchell, M.A.; Flower, J.; Welle, K.R.; Whittington, J.K. Effects of Ultraviolet Radiation on Serum 25 hydroxyvitamin D Concentrations in Captive Chinchillas. J. Exot. Pet Med. 2014, 23, 270–276. [Google Scholar] [CrossRef]
- Southworth, L.O.; Holick, M.F.; Chen, T.C.; Kunz, T.H. Effects of Sunlight on Behaviour and 25-Hydroxyvitamin D Levels in Two Species of Old World Fruit Bats. Dermatoendocrynoly 2013, 5, 192–198. [Google Scholar] [CrossRef]
- ZIMS. Animal Advanced Search. Available online: https://zims.species360.org/Main.aspx (accessed on 27 April 2024).
- Maclaughlin, J.; Hollick, M.F. Aging Decreases the Capacity of Human Skin to Produce Vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Iruzubieta, P.; Terán, Á.; Crespo, J.; Fábrega, E. Vitamin D deficiency in Chronic Liver Disease. World J. Hepatol. 2014, 6, 901–915. [Google Scholar] [CrossRef]
- Mosekilde, L. Vitamin D and the Elderly. Clin. Endocrinol. 2005, 62, 265–281. [Google Scholar] [CrossRef]
- Jakins, R. (Bristol Zoological Society, Bristol, UK). Personal Communications, 2021.
- Boccia, M.L.; Laudenslager, M.; Reite, M. Food Distribution, Dominance and Aggressive Behaviours in Bonnet Macaques. Am. J. Primatol. 1988, 16, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rakotondrazandry, J.N.; Ravelonmandrato, F.; Sefczek, T.M.; Andriamalala, Y.R. Developmental Timeline of Wild Aye-aye (Daubemtonia madagascariensis) Infants in Kianjavato and Torotorofotsy, Madagascar. Int. J. Primatol. 2021, 42, 344–348. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral Thermoregulation in Mammals: A Review. Front. Biosc. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.A.; Jablonski, N.G.; Chaplin, G.; Sussman, R.W.; Kamilar, J.M. Behavioral Thermoregulation in Lemur catta: The significance of Sunning and Huddling Behaviors. Am. J. Primatol. 2016, 78, 745–754. [Google Scholar] [CrossRef]
- Kappeler, P.M. Nets, Tree Holes, and the Evolution of Primate Life Histories. Am. J. Primatol. 1998, 46, 7–43. [Google Scholar] [CrossRef]
- Brown, K.J.; Downs, C.T. Basking Behaviour in the Rock Hyrax (Procavia capensis) during winter. Afr. Zool. 2007, 42, 70–79. [Google Scholar] [CrossRef]
- Pavey, C.R.; Geiser, F. Basking and Diurnal Foraging in the Dasyurid Marsupial Pseudantechinus macdonnellensis. Aust. J. Zool. 2008, 56, 129–135. [Google Scholar] [CrossRef]
- Gonfalone, A.A.; Jha, S.K. The Influence of Gravity on REM sleep. Open Access Anim. Physiol. 2015, 7, 65–72. [Google Scholar] [CrossRef]
- Warnecke, L.; Schleucher, E.; Geiser, F. Basking Behaviour in Relation to Energy Use and Food Availability in One of the Smallest Marsupials. Physiol. Behav. 2010, 5, 389–393. [Google Scholar] [CrossRef]
- Rambeloson, E.; Andriambeloson, J.B.; Rasoanaivo, H.A.; Ramarokoto, E.E.; Prosper; de Foucault, C.; Greene, L.K.; Blanco, M.B. Initial Introduction of the Aye-aye (Daubentonia Madagascariensis) in Anjajavy Reserve, Northwestern Madagascar. Folia Primatol. 2021, 92, 284–295. [Google Scholar] [CrossRef]
- Gupta, K.K.; Attri, J.P.; Singh, A.; Kaur, H.; Kaur, G. Basic Concepts for Sample Size Calculation: Critical Step for any Clinical Trials. Saudi J. Anaesth. 2016, 10, 328–331. [Google Scholar] [CrossRef]
- Jiang, X.; Kiel, D.P.; Kraft, P. Genetics of Vitamin D. Bone 2019, 126, 59–77. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight, Vitamin D and Health: A D-lightful Story. In Solar Radiation and Human Health; Bjertness, E., Ed.; Norwegian Academy of Science and Letters: Oslo, Norway, 2008; pp. 147–166. [Google Scholar]

| Test Subject ID | Sex | Birth Status | Date of Birth | Age * |
|---|---|---|---|---|
| A | Male | Captive Born | 29 June 2016 | 2 |
| B | Female | Captive Born | 17 May 2015 | 3 |
| C | Female | Captive Born | 17 May 2015 | 3 |
| D | Male | Wild Caught | Unknown | ~28 |
| E | Male | Captive Born | 29 November 2020 | 0 |
| Test Subject ID | Experimental Phase | Duration (Days) |
|---|---|---|
| A | 1.1 | 119 |
| 1.2 | 302 | |
| 1.3 | 574 | |
| B | 1.1 | 102 |
| 1.2 | 234 | |
| C | 1.1 | 102 |
| D | 1.1 | 58 |
| 1.3 | 878 | |
| E | 1.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, D.; Bwye, P.; Richdon, S. The Effects of Artificial UV-B Provision on Positional Sleeping Behaviour and Vitamin D3 Metabolites of Captive Aye-Ayes (Daubentonia madagascariensis). J. Zool. Bot. Gard. 2025, 6, 39. https://doi.org/10.3390/jzbg6030039
Walker D, Bwye P, Richdon S. The Effects of Artificial UV-B Provision on Positional Sleeping Behaviour and Vitamin D3 Metabolites of Captive Aye-Ayes (Daubentonia madagascariensis). Journal of Zoological and Botanical Gardens. 2025; 6(3):39. https://doi.org/10.3390/jzbg6030039
Chicago/Turabian StyleWalker, Danielle, Paige Bwye, and Sarah Richdon. 2025. "The Effects of Artificial UV-B Provision on Positional Sleeping Behaviour and Vitamin D3 Metabolites of Captive Aye-Ayes (Daubentonia madagascariensis)" Journal of Zoological and Botanical Gardens 6, no. 3: 39. https://doi.org/10.3390/jzbg6030039
APA StyleWalker, D., Bwye, P., & Richdon, S. (2025). The Effects of Artificial UV-B Provision on Positional Sleeping Behaviour and Vitamin D3 Metabolites of Captive Aye-Ayes (Daubentonia madagascariensis). Journal of Zoological and Botanical Gardens, 6(3), 39. https://doi.org/10.3390/jzbg6030039





