Abstract
The present study is based on a follow-up of a survey carried out in 2000, consisting in the revisitation of ten sites, with the scope of assessing changes in the composition and richness of epiphytic macrolichens within Cluj-Napoca city over the past 24 years. Within this period most of the polluting factories from the city outskirts were closed but in turn, the number of registered cars increased almost six-fold. An increasing compositional homogenization by contribution of generalist, stress-tolerant species was detected over time while total lichen taxa richness declined, which is mostly imputable to the synergic effects of intense car traffic and warmer/drier summers. Most sites displayed a compositional change along a weak, mixed gradient of eutrophication and xerophitization. Only two sites (located on the windy, Someș valley bottom) experienced a compositional change from higher to lower trophicity levels. Other two sites (positioned on more sheltered hillsides) displayed unfavourable dynamics in terms of lost species. Unexpectedly, the number of epiphytic lichen taxa at site level has, on average, increased over time, but the main contributors were poleotolerant species. The warming trend, due to climate change and local heat sources, is expected to worsen the negative effects of air-borne pollutants on the composition of the epiphytic lichen species pool within the Cluj-Napoca urban area.
1. Introduction
Urbanization is an ongoing, generalized phenomenon throughout Europe; it has been determined that almost 75% of people reside in cities, and over 80% of them are predicted to do so during the next ten years [1,2].
One of the most pressing environmental challenges in cities is atmospheric pollution, which is caused by a combination of local and background factors. Urban areas are ecosystems affected by strong man-made impacts, mostly due to housing and transportation [2,3,4,5,6]. It has been long believed that urban settings have a detrimental effect on biotic communities. This is because habitat fragmentation or loss, physiological stress (such as air pollution and urban heat island effect) and disturbance are some of the ways through which urbanization can lead to a decrease in biodiversity [7]. Both human health and ecosystem functioning may be impacted by these problems [1,8,9]. In addition to causing local species extinctions, urbanization changes the natural environment in different ways. Numerous studies showed that epiphytic lichen species richness gradually declines along a gradient from rural to urban areas [6,10].
In a variety of landscape types, such as urban, woodland, and agricultural settings, lichens—symbiotic organisms that arise from the interaction of a fungal partner with a photosynthetic partner (algae or cyanobacteria)—have proven to be a useful tool for evaluating changes in air quality [2,11,12]. Specifically, tree bark epiphytic macrolichens are highly effective bioindicators of air pollution [13]. For instance, they have extended lifespans and are supplied with moisture and nutrients via the thalli surface. Because they lack cuticles and deciduous parts, lichens can also concentrate contaminants. Thus, since the discovery of lichen dieback in Paris in 1866 [14], epiphytic macrolichens have been employed as bioindicators of air pollution [15,16,17,18,19]. Lichens are known for their strong hygroscopicity, which enables their thallus to absorb water from the surrounding air. Lichens are more resilient than plants to conditions like high temperatures, dry spells, and brief growing seasons, but many of them are also more vulnerable to air pollution [20,21].
Early reports of lichen loss in urban settings date back to Europe’s industrialization in the XIXth century [14]. Industry and traffic-generated SOx were the primary cause of this drop. The 1960s and 1970s saw a rise in SOx, and high emission levels were observed in urban lichen deserts [2,6]. However, lately, the conditions in the economically developed countries have changed and pollution has decreased, as the main pollutants have changed [17]. Thus, a decrease in SO2 emissions has been noticed in recent decades as a result of tighter emission control laws and socio-economic shifts. Consequently, many European cities and urban agglomerations, including London, Munich, Paris, Rome and Turin (but also smaller cities), have all reported improvements in lichen diversity [22,23,24,25]. Given the abundance of studies on lichen recovery in the rest of Europe, the present study represents an attempt to fill in part the information gap on urban areas from Romania.
The first available data on the epiphytic macrolichens within the Cluj-Napoca city were obtained after an inventory carried out in 1960 but were limited to the area of the Alexandru Borza Botanic Garden [26]. In 2000, a more extended survey was conducted in the urban and adjacent peri-urban areas of Cluj-Napoca [27] in order to evaluate the distribution of the epiphytic macrolichen taxa in both larger or smaller green spots. Road traffic, population density, exposure, and the existence of lakes or streams with moving or still water were among the criteria used to choose the sampling locations. The inventory revealed that foliose and fruticose lichens were prevalent in the study area, reaching a total of 31 species. Nevertheless, the lack of comparable data from previous studies rendered these results inconclusive in regard to the extent to which trees were repopulated with macrolichens as a result of the presumed decrease in air pollution. In fact, the positive trend, due to the cessation of many industrial activities, may have been counterbalanced by an increase in urban car traffic. To date, there are no studies conducted exclusively within urban areas of Romanian cities that should be aimed at revealing relationships between air pollution and lichen composition/diversity. However, various investigations were carried out in the surroundings of industrial facilities or urban areas in order to evaluate the intensity of pollution based on lichen occurrence [28,29,30,31].
The present study was designed as a follow-up to a survey carried out in 2000 with the scope of assessing the change reflected by the epiphytic macrolichens over the past 24 years in terms of both compositional resemblance and taxon richness. In particular, we aimed at answering the following questions: (i) Are there significant, compositional dissimilarities and taxonomic richness differences between paired sites surveyed in 2000 and 2024? (ii) If yes, which are the main environmental drivers of these changes in the epiphytic macrolichen species pool? (iii) Which lichen taxa are mainly responsible for the observed differences between the two survey years?
We expected to detect an increase in the frequency/richness of eutrophic taxa along with a decline in the taxonomic richness of epiphytic lichens, given the increasing car traffic and mean temperatures in recent decades.
2. Materials and Methods
2.1. Study Area
Cluj-Napoca is currently the second largest city in Romania, home to about 287,000 people. The municipality is located on the valley of the river Someșul Mic, in the northern part of the Transylvanian Depression and close to the Apuseni Mountains. The total municipality area is 179.5 km2, of which 36 km2 is built-up. Surrounded by hills that range in height from 500 m (Hoia Hill) to 700 m (Feleac Hill), the city centre is situated at an elevation of 363 m a.s.l. The climate is moderate continental, with an average annual temperature of 8.3 °C and about 582 mm of yearly precipitations [32]. An increase in mean temperatures (both in winter and summer), attributable to global warming, was documented between 1961 and 2016 [33].
There is uncertainty regarding the extension of urban green areas; authorities estimate 18.59 m2 per person, whereas sociologists estimate 16.44 m2. The city is crossed by the highly trafficked European road E60 (Bucharest, Romania—Vienna, Austria), while the number of petrol/diesel vehicles registered in the city of Cluj-Napoca has increased almost six-fold in the last 30 years, reaching about 220,000 in 2023 [34]. The development of airplane traffic from the local airport located in the eastern city outskirts was spectacular, increasing from 75,750 passengers in 2000 to almost 3,250,000 in 2023 [35]. A relative improvement in air quality occurred after the closure of the most polluting industrial units after the year 2000. Nowadays, NOx pollution in Cluj-Napoca is mainly caused by car traffic, infrastructure deficiencies, apartment power plants, airplanes landing/taking-off over the main axis of the city, etc. In addition, the city’s landfill, which is now partly green, has been a major source of pollution over the past 30 years.
The ten study sites consist of green areas and (street) tree rows situated within the city administrative borders from the central to peripheral urban zones (Table 1). The Hațieganu Park and Central Park are located at the lowest elevation, in the Someș river floodplain, whereas the Hoia Forest and Cetățuia Park lie on the adjacent hill ridge (Figure 1). The Hoia Forest is presumably the least disturbed area and hosts the highest density of native trees. All others sites are composed of scattered, aligned and/or clumped trees of different native and exotic species. Based on previous estimates of the intensity of air pollution throughout the Cluj-Napoca urban area [36,37], the potentially most exposed sites considered herein are the M. Kogălniceanu and Turzii streets, both being affected by the high traffic within the city centre and, respectively, along the European road E60 (Table 1).

Table 1.
Characteristics of the sites sampled for epiphytic macrolichens within the Cluj-Napoca city. The label numbers match those reported on the map in Figure 1.
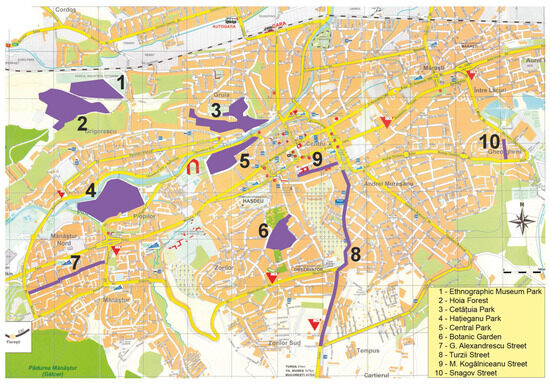
Figure 1.
Distribution of surveyed sites within Cluj-Napoca urban area (the street tree rows and green areas are represented through violet lines and, respectively, polygons). The label numbers match their counterparts and site names reported in Table 1.
2.2. Data Collection
All ten sites surveyed by Crișan and Pop [27] in the year 2000 (Figure 1 and Table 1) were revisited during the spring and early summer of 2024. In order to carry out a paired systematic inventory, the same methodologic protocol adopted in 2000 was also used in 2024. Thus, the same pathways walked 24 years ago were followed again at each site, and all trees rooted within a strip of 30 m along these pathways were fully examined for the presence of corticolous macrolichens on the trunk and low accessible branches (up to 2 m height). A minimum amount of tallus of each macrolichen species, discernible to the naked eye, was harvested irrespective of vital status (healthy, wounded or withered), bark smoothness, trunk/branch side or other criteria. Subsequently, the lichen taxa were determined in the lab at species level by using various identification keys [38,39,40]. The ecological preferences with respect to eutrophication and aridity, as well as the poleotolerance to NOx and SOx of the recorded lichen species, was drawn from the literature [41]. In particular, we made use of their scaled ecological indicator values, ranging from oligotrophic (1) to very eutrophic (5), from hygrophytic (1) to very xerophytic (5) and from very sensitive (0) to highly tolerant (3) of air pollution.
The complete list of lichen taxa, along with some of their ecological preferences/tolerance and their distribution by site in the years 2000/2024, is reported in Table A1 (see Appendix A).
2.3. Numerical Analyses
The specific dissimilarities between sites were assessed by the complement of Sørensen index calculated from binary (presence-absence) data. The ordination of sites surveyed both in 2000 and 2024 was performed through non-metric multidimensional scaling (NMDS).
The correlations between the ordination scores and species-related variables (taxon counts or presence) were calculated through the Spearman’s rho index and the point biserial correlation index, respectively.
The statistical significance of change at site level between 2000 and 2024 due to gained/lost taxa at each site was estimated through the McNemar exact test, whose null hypothesis is that the odds ratio equals 1.
The statistical significance of the mean difference in site-pairwise, taxonomic dissimilarities/lichen taxon richness between 2000 and 2024 was estimated by means of a permutation-based, two-sided, paired t-test for unequal variances. The null hypothesis of zero mean difference was tested via 9999 permutations. The number of taxa was log-transformed prior to analysis in order to normalize the distribution of the response variable.
All numerical analyses were performed in an R environment by employing the packages ’vegan’ [42], ’exact2 × 2’ [43] and ’MKinfer’ [44].
3. Results
The ordination of sites revealed a rough separation of the urban parks/woods, in the lower part, from the tree rows along streets, in the upper part (Figure 1 and Table 1). This pattern is obviously linked to the expected larger number of epiphytic lichen species in larger green areas, which are usually characterized by higher densities of trees.
Most sites displayed a compositional change toward the negative end of the second NMDS axis (Figure 2) overlapping a mixed, weak gradient of increasing eutrophication and xerophitization. That was suggested by the significant, negative correlations between the axis scores on one side and the number of lichen taxa (Spearman’s rho = −0.6730; p = 0.0011) and the occurrence of two eumesotrophilous—mesoxerophilous species, namely Polycauliona candelaria and Physcia tenella (Table 2), on the other side.
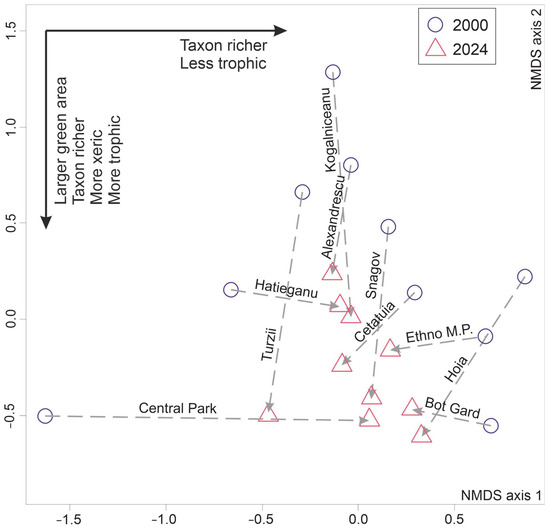
Figure 2.
Ordination of sites in the bidimensional NMDS space determined by the occurring lichen species in 2000 and 2024. The dashed arrows show the direction of within-site change in lichen species composition over time. The solid arrows indicate the inferred environmental/structural gradients along the two axes.

Table 2.
Correlations between the NMDS scores of sites and the lichen taxon occurrences. Only significant correlations larger than 0.5 in absolute values are displayed. The range of ecological indicator values associated with each lichen taxon is given for reference (Ard—aridity; Eutr—eutrophication; Ptol—poleotolerance).
The first NMDS axis was instead significantly positively correlated with lichen richness (Spearman’s rho = +0.7742; p < 0.0001) and, in addition, the occurrence of Xanthoria parietina, a common mesotrophilous—mesoxerophilous lichen species (Table 2).
Only two sites (the Central and Hațieganu parks) experienced a compositional change from higher to lower trophicity levels (Figure 2), but only the former showed a significant increase in lichen taxon richness (Table 3). On the contrary, two sites (Ethnographic Museum Park and Botanic Garden) displayed unfavourable dynamics in terms of both lost species (Table 3) and eutrophication (Figure 2).

Table 3.
Number of gained and lost lichen taxa between 2020 and 2024 at each site along with the statistics of the exact tests (the sites are listed in descending order of the odd ratio values).
Overall, the observed change over 24 years has led to increasing compositional homogenization of the epiphytic lichen species pool, as revealed by the conspicuously less dispersed cluster of sites investigated in 2024 compared with the cluster of sites surveyed in 2000 (Figure 2), as well as by the significant, negative mean difference in site-pairwise, taxonomic dissimilarities (Figure 3).
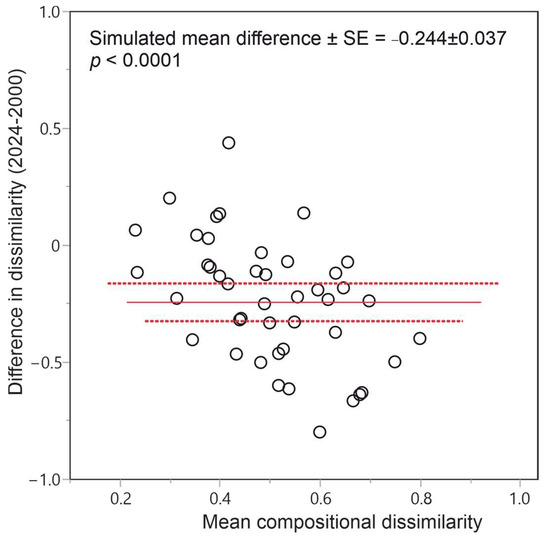
Figure 3.
Distribution of differences in site-pairwise taxonomic dissimilarities between 2024 and 2000. The observed mean difference and its 95% confidence interval are represented through the solid line and dashed lines, respectively.
The total number of macrolichen taxa recorded in 2024 was only 20, compared with 25 taxa found back in 2000. At the site level, the number of lichen taxa has instead increased on average from 2000 to 2024, as indicated by the significant, positive mean difference in taxonomic richness (Figure 4).
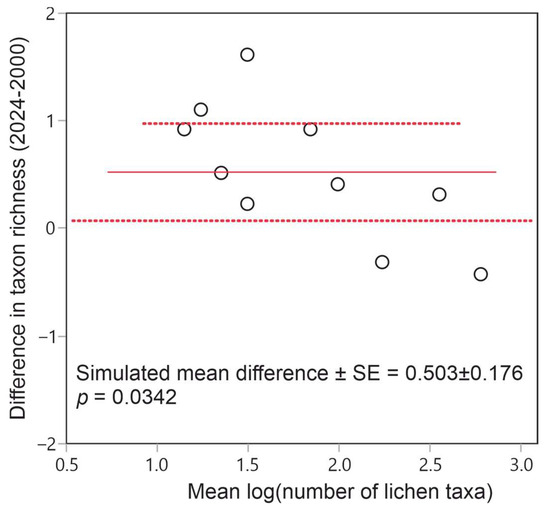
Figure 4.
Distribution of differences in site-pairwise lichen richness between 2024 and 2000. The observed mean difference and its 95% confidence interval are represented through the solid line and dashed lines, respectively.
4. Discussion
A general trend of compositional change, mediated by the increasing occurrence of several eumesotrophilous and xerophilous lichen species, was observed at almost all sites. This trend was also responsible for the increase in lichen taxon richness at site level. Sites that were presumably the most affected by pollution due to a high volume of car traffic (e.g., Kogălniceanu and Turzii streets) showed the biggest change in species composition, as also observed in other European cities, e.g., Tallinn [4], Lisbon [17] and Košice [19]. Unexpectedly, the temporal change observed in the Hoia Forest, which should be the least exposed site to car exhaust fumes, suggests an increasing eutrophication, probably determined by a nearby, recently built-up residential area. In the almost absence of emissions originated from industrial factories, the main sources of air-borne pollutants in Cluj-Napoca seem to be the numerous house heating systems and petrol/diesel vehicles.
Polycauliona candelaria, Xanthoria parietina, Parmelia sulcata and Physcia tenella, which were the most important species supporting the floristic change over time, are known for their tolerance to both moderate drought and pollutants [41,45]. These findings are consistent with numerous recent investigations performed in other urban areas, e.g., Toruń [46], Seoul [16], Cracow [25], Ferrara [8], Łomża [11], Lisbon [17], Portland [7] and Münich [47]. All these studies, along with the present one, emphasized the high occurrence of poleotolerant lichens, suggesting a saturation of eutrophilous lichen species in urban ecosystems and the recruitment of new (nitro)-xerophilous species. This is in accordance with the increased mean temperatures recorded in the Cluj-Napoca city in recent decades [33]. Besides, some studies have revealed that the urban climate had a greater impact on lichen diversity than the air pollution [1,48].
As expected, the total number of epiphytic lichen taxa declined over time, but, surprisingly, this was combined with an increase in the number of lichen taxa at site level. That translates to lower beta-diversity (i.e., compositional homogenization) and higher alpha-diversity. Such a pattern might have been determined by the locally improved air quality after the closure of big, polluting factories from the city outskirts that had already started around the year 2000. The same trend, with higher local lichen species richness as compared to earlier surveys, was found in Rome [23], Seoul [16], Cracow [25], Lisbon [17] and Tampere [22]. Pollutant levels have been steadily declining in socio-economically developed countries in recent decades, and the improvement in air quality within cities has been reflected in the rise in epiphytic lichen diversity [22,24,25,46]. Nevertheless, poleotolerant lichen taxa (e.g., Polycauliona candelaria and Xanthoria parietina), which are tolerant of various pollutants released in urban areas, were the main contributors to the observed taxonomic enrichment in the Cluj-Napoca urban area as well as in other cities, e.g., Trabzon [3], Toruń [46], Seoul [16], Cracow [25], Łomża [11], Lisbon [17] and Portland [7]. On the contrary, the oligotrophilous lichens are at the highest risk of potential local extinction in urban settings [23].
The favourable change in epiphytic lichen composition/richness in the Hațieganu Park and, especially, in Central Park was probably related to several factors, among which were their location on the Someș valley bottom (featuring higher air circulation and humidity), the pedestrian exclusive zone established in the historical city centre in 2007 and, probably, the reduced traffic congestion along the confined one-way streets. These findings are also supported by other studies, e.g., [23,24,25], which found that lichen species that can withstand low levels of eutrophication are less common in traffic areas but are more frequent in green areas. On the other hand, the unexpected, unfavourable dynamics of epiphytic lichen composition in two large green areas (Ethnographic Museum Park and Botanic Garden) lying on hillsides is probably determined by the weaker circulation of polluted air masses compared with that registered along the Someș valley bottom, where the Central and Hațieganu parks are located. With reference to the even older inventory performed exclusively within the Botanic Garden in 1960 [26], 60% and 75% of corticolous lichen taxa were shared with the epiphytic lichen species pool revealed at the same site after conducting the 2000 and 2024 surveys, respectively.
5. Limitations and Synthesis
The present study has some inherent limitations that stemmed from (i) slight differences in the intensity of sampling between 2000 and 2024 because several trees, damaged by wind or withering, were removed in the meantime; (ii) overlooked, non-conspicuous lichens; (iii) uncontrolled factors (e.g., herbivores, pesticides sprayed by gardeners) that may have influenced the lichen establishment and survival.
The general trend observed on the epiphytic lichen species pool over 24 years suggests increasing between-site homogenization, eutrophication and xerophitization, which are mostly imputable to the synergic effects of air pollution and warmer/drier summers. The increase in mean lichen taxon richness detected at site level between 2024 and 2000 was exclusively determined by poleotolerant species. Keeping the hollowed or partly wilted standing trees, especially in large green areas, is important for maintaining epiphytic lichen diversity in big cities.
The warming trend due to climate change and local heat sources is expected to worsen the negative effects of air-borne pollutants on epiphytic lichen composition/richness within the urban area of Cluj-Napoca city.
Author Contributions
Conceptualization, F.C. and D.G.; methodology, D.G. and F.C.; software, D.G.; validation, F.C. and I.G.; formal analysis, D.G.; investigation, F.C.; resources, F.C.; data curation, D.G. and F.C.; writing—original draft preparation, D.G.; writing—review and editing, D.G., F.C. and I.G.; visualization, I.G.; supervision, D.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All original floristic data used in this article are contained within the Appendix A (Table A1).
Acknowledgments
We thank Andreea Iulia Drăgan for the assistance provided in collecting the lichen samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Occurrence of the epiphytic macrolichen taxa recorded in the ten study sites by survey year (2000/2024; 1—presence; 0—absence). The range of ecological indicator values associated with each lichen taxon is given for reference (Ard—aridity; Eutr—eutrophication; Ptol—poleotolerance).
Table A1.
Occurrence of the epiphytic macrolichen taxa recorded in the ten study sites by survey year (2000/2024; 1—presence; 0—absence). The range of ecological indicator values associated with each lichen taxon is given for reference (Ard—aridity; Eutr—eutrophication; Ptol—poleotolerance).
| Site | M. Kogălniceanu Street | Ethnographic Museum Park | Cetățuia Park | Botanic Garden | Hațieganu Park | Central Park | Turzii Street | Hoia Forest | Grigore Alexandrescu Street | Snagov Street | Ecological Preference (https://dryades.units.it (accessed on 28 January 2025) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lichen taxon | Ard | Eutr | Ptol | ||||||||||
| Candelaria concolor (Dicks.) Stein | 0/0 | 0/0 | 0/1 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3–4 | 3–5 | 1–3 |
| Candelariella aurella (Hoffm.) Zahlbr. | 0/0 | 0/1 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3–5 | 2–4 | 1–3 |
| Candelariella vitellina (Hoffm.) Müll. Arg. | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 | 0/1 | 3–4 | 2–5 | 1–3 |
| Cladonia fimbriata (L.) Fr. | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 2–3 | 1–3 | 1–2 |
| Evernia prunastri (L.) Ach. | 0/0 | 1/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 2–3 | 1–3 | 1–2 |
| Flavoparmelia caperata (L.) Hale | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | 3 | 1–3 | 1–2 |
| Lepra albescens (Huds.) Hafellner | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2–3 | 1–2 | 1–2 |
| Lepraria sp. Ach. | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | NA | NA | NA |
| Melanelixia glabratula (Lamy) Sandler and Arup | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 | 2–3 | 2–3 | 1–3 |
| Melanohalea exasperata (De Not.) O. Blanco, A. Crespo, Divakar, Essl., D. Hawksw. and Lumbsch | 0/0 | 1/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 3–4 | 2–3 | 1–2 |
| Melanohalea olivacea (L.) O. Blanco, A. Crespo, Divakar, Essl., D. Hawksw. and Lumbsch | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | 3 | 1 | 1 |
| Parmelia saxatilis (L.) Ach. | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/1 | 0/0 | 0/1 | 0/0 | 0/1 | 2–3 | 1–3 | 1–2 |
| Parmelia sulcata Taylor | 0/0 | 1/1 | 1/0 | 1/1 | 0/0 | 0/1 | 0/0 | 1/1 | 0/0 | 0/0 | 2–3 | 1–3 | 1–3 |
| Parmelina tiliacea (Hoffm.) Hale | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3 | 2–3 | 1–3 |
| Parmeliopsis ambigua (Hoffm.) Nyl. | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 3–4 | 1 | 1–2 |
| Phaeophyscia nigricans (Flörke) Moberg | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 3–4 | 4 | 1–2 |
| Phaeophyscia orbicularis (Neck.) Moberg | 0/0 | 1/1 | 1/1 | 1/1 | 0/1 | 0/0 | 0/1 | 0/1 | 0/1 | 1/1 | 3–4 | 4–5 | 1–3 |
| Phlyctis agelaea (Ach.) Flot. | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2–3 | 1–2 | 1–2 |
| Physcia adscendens H. Olivier | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/0 | 1/1 | 1/1 | 1/1 | 3–4 | 3–5 | 1–3 |
| Physcia aipolia (Humb.) Fürnr. | 0/0 | 1/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 | 3–4 | 2–4 | 1–3 |
| Physcia caesia (Hoffm.) Fürnr. | 1/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 | 0/0 | 4 | 4–5 | 1–2 |
| Physcia dubia (Hoffm.) Lettau | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/0 | 0/1 | 0/1 | 0/1 | 4 | 4–5 | 1–3 |
| Physcia stellaris (L.) Nyl. | 0/1 | 1/1 | 0/1 | 1/1 | 0/0 | 0/1 | 0/0 | 1/1 | 0/0 | 0/1 | 3–4 | 2–4 | 1–2 |
| Physcia tenella (Scop.) DC. | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/0 | 0/1 | 3–4 | 3–4 | 1–2 |
| Physconia grisea (Lam.) Poelt | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3–4 | 3–5 | 1–3 |
| Polycauliona candelaria (L.) Frödén, Arup and Søchting | 0/0 | 0/0 | 0/1 | 1/1 | 1/0 | 1/1 | 0/1 | 0/1 | 0/0 | 0/1 | 4 | 4–5 | 1–2 |
| Pseudevernia furfuracea (L.) Zopf | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3–4 | 1–2 | 1–2 |
| Punctelia subrudecta (Nyl.) Krog | 0/0 | 1/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3 | 1–3 | 1–3 |
| Ramalina farinacea (L.) Ach. | 0/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1–2 | 1–3 | 1–2 |
| Xanthomendoza fallax (Hepp) Søchting, Kärnefelt and S.Y. Kondr. | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 | 1/0 | 0/0 | 0/1 | 4 | 3–4 | 1–2 |
| Xanthoria parietina (L.) Th. Fr. | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 3–4 | 3–4 | 1–3 |
References
- Munzi, S.; Correia, O.; Silva, P.; Lopes, N.; Freitas, C.; Branquinho, C.; Pinho, P. Lichens as ecological indicators in urban areas: Beyond the effects of pollutants. J. Appl. Ecol. 2014, 51, 1750–1757. [Google Scholar] [CrossRef]
- Llop, E.; Pinho, P.; Ribeiro, C.; Pereira, M.J.; Branquinho, C. Traffic represents the main source of pollution in small Mediterranean urban areas as seen by lichen functional groups. Environ. Sci. Pollut. Res. 2017, 24, 12016–12025. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.; Aslan, A. Distribution of epiphytic lichens and air pollution in the city of Trabzon, Turkey. Bull. Environ. Contam. Toxicol. 2006, 7, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Marmor, L.; Randlane, T. Effects of road traffic on bark pH and epiphytic lichens in Tallinn. Folia Cryptogam. Est. 2007, 43, 23–37. [Google Scholar]
- Dymytrova, L. Epiphytic lichens and bryophytes as indicators of air pollution in Kyiv city (Ukraine). Folia Cryptogam. Est. 2009, 46, 33–44. [Google Scholar]
- Lättman, H.; Bergman, K.-O.; Rapp, M.; Tälle, M.; Westerberg, L.; Milberg, P. Decline in lichen biodiversity on oak trunks due to urbanization. Nord. J. Bot. 2014, 32, 518–528. [Google Scholar] [CrossRef]
- Prather, H.M.; Eppley, S.M.; Rosenstiel, T.N. Urban forested parks and tall tree canopies contribute to microlichen epiphyte biodiversity in urban landscapes. Urban For. Urban Green. 2018, 32, 133–142. [Google Scholar] [CrossRef]
- Gerdol, R.; Marchesini, R.; Iacumin, P.; Brancaleoni, L. Monitoring temporal trends of air pollution in an urban area using mosses and lichens as biomonitors. Chemosphere 2014, 108, 388–395. [Google Scholar] [CrossRef]
- Crişan, F. The significance of epiphytic lichens as bioindicators of air pollution for human health. Proc. Rom. Acad. (Ser. B) 2023, 25, 167–174. [Google Scholar]
- Parzych, A.; Zduńczyk, A.; Astel, A. Epiphytic lichens as bioindicators of air pollution by heavy metals in an urban area (northern Poland). J. Elem. 2016, 21, 781–795. [Google Scholar] [CrossRef]
- Matwiejuk, A.; Chojnowska, P. Lichens as indicators of air pollution in Łomża. Steciana 2016, 20, 63–72. [Google Scholar] [CrossRef]
- Llewellyn, T.; Gaya, E.; Murrell, D.J. Are urban communities in successional stasis? A case study on epiphytic lichen communities. Diversity 2020, 12, 330. [Google Scholar] [CrossRef]
- Moreno-Palacios, M.; Torres-Benitez, A.; Soto-Medina, E.; Sánchez, M.; Divakar, P.K.; Pereira, I.; Gómez-Serranillos, M.P. Corticolous lichen communities and their bioindication potential in an urban and peri-urban ecosystem in the central region of Colombia. Land 2024, 13, 932. [Google Scholar] [CrossRef]
- Nylander, W. Les lichens du Jardin du Luxembourg. Bull. Soc. Bot. Fr. 1866, 13, 364–372. [Google Scholar] [CrossRef]
- Loppi, S.; Ivanov, D.; Boccardi, R. Biodiversity of epiphytic lichens and air pollution in the town of Siena (Central Italy). Environ. Pollut. 2002, 116, 123–128. [Google Scholar] [CrossRef]
- Ahn, C.-R.; Chang, E.; Kang, H. Epiphytic macrolichens in Seoul: 35 years after the first lichen study in Korea. J. Ecol. Field Biol. 2011, 34, 381–391. [Google Scholar] [CrossRef]
- Sérgio, C.; Carvalho, P.; Garcia, C.A.; Alameida, E.; Novais, V.; Sim-Sim, M.; Jordão, H.; Sousa, A.J. Floristic changes of epiphytic flora in the Metropolitan Lisbon area between 1980–1981 and 2010–2011 related to urban air quality. Ecol. Indic. 2016, 67, 839–852. [Google Scholar] [CrossRef]
- Varela, Z.; López-Sánchez, G.; Yáñez, M.; Pérez, C.; Fernández, J.A.; Matos, P.; Branquinho, C.; Aboal, J.R. Changes in epiphytic lichen diversity are associated with air particulate matter levels: The case study of urban areas in Chile. Ecol. Indic. 2018, 91, 307–314. [Google Scholar] [CrossRef]
- Marcinčinová Varholova, M.; Tuptová, V. Epiphytic lichen diversity in the urban area of Košice (E Slovakia) with some notes on its air quality. Thaiszia 2022, 32, 91–108. [Google Scholar] [CrossRef]
- Paoli, L.; Munzi, S.; Guttová, A.; Senko, D.; Sardellad, G.; Loppi, S. Lichens as suitable indicators of the biological effects of atmospheric pollutants around a municipal solid waste incinerator (S Italy). Ecol. Indic. 2015, 52, 362–370. [Google Scholar] [CrossRef]
- Perlmutter, G.B. Bioassessing air pollution effects with epiphytic lichens in Raleigh, North Carolina, U.S.A. Bryologist 2010, 113, 39–50. [Google Scholar] [CrossRef]
- Ranta, P. Changes in urban lichen diversity after a fall in sulphur dioxide levels in the city of Tampere, SW Finland. Ann. Bot. Fenn. 2001, 38, 295–304. [Google Scholar]
- Munzi, S.; Ravera, S.; Caneva, G. Epiphytic lichens as indicators of environmental quality in Rome. Environ. Pollut. 2007, 146, 350–358. [Google Scholar] [CrossRef]
- Lisowska, M. Lichen recolonisation in an urban-industrial area of southern Poland as a result of air quality improvement. Environ. Monit. Assess. 2011, 179, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Slaby, A.; Lisowska, M. Epiphytic lichen recolonization in the centre of Cracow (Southern Poland) as a result of air quality improvement. Pol. J. Ecol. 2012, 60, 225–240. [Google Scholar]
- Codoreanu, V.; Țiu, R.; Micle, F. Licheni corticoli din grădina botanică din Cluj. Contrib. Bot. 1960, 2, 97–107. [Google Scholar]
- Crişan, F.; Pop, I. Die epiphytischen Grosflechten als Bioindikatoren der Luftverschmutzung in Klausenburg. Naturwissenschaftliche Forschungen Über Sieben. 2000, 6, 135–145. [Google Scholar]
- Bartók, K. Influenţa poluării atmosferice asupra florei lichenologice din zona industrială a Zlatnei. Contrib. Bot. 1980, 20, 195–199. [Google Scholar]
- Ștefănescu, G.; Bartók, K. Noi date privind cartarea lichenologică a intensităţii poluării atmosferice din bazinul industrial Baia Mare. Bul. Şt. Baia Mare (Ser. B Fasc. Chim.-Biol.) 1998, 13, 133–137. [Google Scholar]
- Györkös, L.; Bartók, K. Diversity, distribution and ecology of the lichens in the Aiud town area (Alba County). Contrib. Bot. 2006, 41, 13–22. [Google Scholar]
- Vicol, I. The sinstructure of epiphytic lichens within forests from the eastern part of Bucharest Municipality (Romania). Bot. Serbica 2012, 36, 131–137. [Google Scholar]
- Sevianu, E. Rezervaţia de orbeţi de la Apahida. Ocrotirea Nat. 2010, 46, 85–94. [Google Scholar]
- Banc, Ș.; Croitoru, A.E.; David, N.A.; Scripcă, A.S. Changes detected in five bioclimatic indices in large Romanian cities over the period 1961–2016. Atmosphere 2020, 11, 819. [Google Scholar] [CrossRef]
- Monitorul de Cluj. Available online: www.monitorulcj.ro (accessed on 22 December 2024).
- Aeroportul Internațional Avram Iancu Cluj. Available online: www.airportcluj.ro (accessed on 22 December 2024).
- Bartók, K.; Rusu, A.-M.; Kozma, A. Caracterizarea gradului de poluare al oraşului Cluj-Napoca prin componentul lichenologic. Environ. Prog. 2003, 1, 29–33. [Google Scholar]
- Oroian, I.; Odagiu, A.; Covrig, I.; Safirescu, C.O. The current status of traffic nitrogen oxides pollution in Cluj–Napoca. Bull. USAMV (Ser. Agric.) 2015, 72, 457–462. [Google Scholar] [CrossRef]
- Purvis, O.W.; Coppins, B.J.; Hawksworth, D.L.; James, P.W.; Moore, D.M. The Lichen Flora of Great Britain and Ireland; Natural History Museum Publications; British Lichen Society: London, UK, 1992; pp. 1–710. [Google Scholar]
- Wirth, V. Die Flechten Baden-Württembergs; Ulmer: Stuttgart, Germany, 1995; pp. 1–1006. [Google Scholar]
- Ciurchea, M. Determinatorul lichenilor din România; Editura Bit: Iași, Romania, 2004; pp. 1–488. [Google Scholar]
- Wirth, V. Ecological indicator values of lichens—Enlarged and updated species list. Herzogia 2010, 23, 229–248. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package v2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 8 May 2024).
- Fay, M.P. Exact Tests and Confidence Intervals for 2 × 2 Tables. R Package Version v1.6.9. 2024. Available online: https://cran.r-project.org/web/packages/exact2x2/index.html (accessed on 8 May 2024).
- Kohl, M. MKinfer Inferential Statistics. R Package v1.2. 2024. Available online: https://cran.r-project.org/web/packages/MKinfer/index.html (accessed on 8 May 2024).
- Nimis, P.L.; Conti, M.; Martellos, S. ITALIC—The Information System on Italian Lichens, version 8.0; University of Trieste, Department of Biology. 2024. Available online: https://dryades.units.it (accessed on 23 January 2025).
- Adamska, E. Lichen recolonization in the city of Toruń. Ecol. Quest. 2011, 15, 119–125. [Google Scholar] [CrossRef][Green Version]
- Sebald, V.; Goss, A.; Ramm, E.; Gerasimova, J.V.; Werth, S. NO2 air pollution drives species composition, but tree traits drive species diversity of urban epiphytic lichen communities. Environ. Pollut. 2022, 308, 119678. [Google Scholar] [CrossRef]
- Coffey, H.M.P.; Fahrig, L. Relative effects of vehicle pollution, moisture and colonization sources on urban lichens. J. Appl. Ecol. 2012, 49, 1467–1474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).