Abstract
Jaguars play a crucial role in population control across multiple biomes. They are endangered and protected by in situ and ex situ conservation mechanisms to ensure their conservation. Cardiovascular diseases in wild mammals, including jaguars, often have unclear etiopathogenies, underscoring the need for research into novel hemodynamic parameters. This study evaluates the cardiovascular health of fifteen clinically healthy jaguars using conventional and Holter electrocardiography, non-invasive systemic blood pressure measurement, and echocardiography. Chemical restraint was achieved with medetomidine (0.08–0.1 mg/kg) and ketamine (5 mg/kg), with anesthesia reversed using atipamezole (0.25 mg/kg). The average heart rate was 72 ± 18 bpm, with sinus rhythm in ten animals and sinus arrhythmia in five. Six animals exhibited first and second-degree atrioventricular blocks, one had supraventricular complexes, and another had premature ventricular complexes. Non-invasive systolic blood pressure remained stable at 163 ± 29 mmHg during anesthesia. Echocardiographic examination revealed mitral, tricuspid, pulmonary, and aortic valve insufficiencies via color Doppler. The transmitral flow showed a normal E/A ratio and E` < A`, suggesting a pseudonormal ventricular filling pattern. No significant anesthetic complications were observed, affirming the protocol’s safety. This study provides valuable data, validating the anesthetic protocol and establishing reference cardiovascular values for jaguars, thus paving the way for future research in other veterinary species.
1. Introduction
The jaguar (Panthera onca), the largest felid in the Americas, plays a crucial role as an apex predator in maintaining the ecological balance of various animal and plant species [1,2,3]. Despite its status as a predator, the species is considered vulnerable due to its risk of extinction in Brazil [4], facing direct and indirect threats from human activities that lead to habitat fragmentation and reduction, prey scarcity, and decreased genetic diversity (inbreeding), as well as population decline [2,5,6] and retaliatory hunting [7,8]. Conservation strategies aim to conserve original habitats and recover viable species through natural means and anthropogenic actions, focusing on conservation through captive populations [9]. The management of these animals requires the use of sedatives and/or anesthetics to ensure the safety of both the team and the specimens [10].
Currently, the combination of α2-adrenergic agonist drugs with ketamine has shown efficacy in immobilizing both in situ and ex situ carnivores [11,12,13]. Activation of α2-adrenergic receptors promotes sedation, analgesia, hypotension, and bradycardia [14]. Medetomidine, a representative of this pharmacological class, induces dose-dependent sedative and anxiolytic effects, leading to loss of vigilance and reflexes in animals [15], as well as vasopressor effects associated with reduced heart rate [14]. The use of atipamezole, an α2-adrenergic antagonist, allows for greater anesthetic control in the presence of adverse effects [10,12,14,15]. Another desirable effect of medetomidine is the relaxation of the smooth muscle of the vas deferens, facilitating semen collection through pharmacological ejaculation in domestic cats and large felids [16,17,18,19].
However, the interaction between medetomidine and ketamine can overload the cardiovascular system due to peripheral vasoconstriction, resulting in bradycardia [10,12]. According to Luczinski et al. [20], mitral valve insufficiency may occur, although ketamine has dissociative analgesic and anesthetic effects in animals and humans [21].
The conservation of endangered species is essential for maintaining biomes and advancing knowledge about the physiology and pathology of these species [22]. Previous studies have identified cardiopathies in wild mammals and adverse effects of some anesthetics [23,24]. Given the need for safer anesthetic protocols for the management and conservation of wild animals, it is crucial to analyze the impacts of these drugs on cardiovascular function, considering the known effects of these substances.
Based on these principles, it is hypothesized that the anesthetic protocol used may cause (i) alterations in cardiovascular parameters, such as bradycardia and hypotension; (ii) pro-arrhythmic atrioventricular effects; and (iii) induction of mitral and aortic valve insufficiency. This study aimed to determine the cardiovascular parameters in clinically healthy jaguars anesthetized with medetomidine and ketamine, evaluating cardiac morphology and function through echocardiography, analyzing electrocardiographic parameters of rhythm and wave morphology and heart rate variability through conventional and Holter electrocardiography, and measuring systemic blood pressure.
2. Materials and Methods
Fifteen healthy jaguars, sourced from the non-governmental conservation center Instituto Onça Pintada–IOP (Mineiros, GO, Brazil; 17°54′00.5”S 53°00′21.7”W), were selected for routine clinical, semen collection, and cardiological evaluation. The animals were housed in suitable enclosures, with free access to water and a diet based on beef. Ages were estimated at 4.9 ± 3.1 years, according to the keeper’s records. These evaluations were conducted alongside a jaguar reproduction study linked with SISBIO/ICMBio/MMA under number 57293-5 and approved by the Animal Use Ethics Committee of the Faculty of Veterinary Medicine and Animal Science at the University of São Paulo under protocol number 9300241019.
After 12 h of fasting, the animals were chemically restrained using anesthetic darts containing medetomidine (0.08–0.1 mg/kg, IM; Precision Pharmacy, Bakersfield, CA, USA) and ketamine (5 mg/kg, IM; Dopalen, CEVA, Paulínia, SP, Brazil) [13,25]. Once the effects of the drugs were observed, the specimens were transferred to an outpatient setting for clinical analyses and biological sample collection for routine tests. Subsequently, the animals were returned to their original enclosures, and anesthesia was reversed with atipamezole (0.25 mg/kg, IM; Precision Pharmacy, Bakersfield, CA, USA).
For Holter electrocardiography, adhesive electrodes were attached to previously shaved skin (Figure 1). Two negative electrodes were positioned: one on the left thoracic wall and another on the manubrium; two positive electrodes were placed on the left thoracic wall and over the xiphoid cartilage. A three-channel digital recorder (Cardioflash digital, Cardios Sistemas, São Paulo, SP, Brazil) was connected, and recording was conducted throughout the experimental period, from capture to release, for approximately 60 min.

Figure 1.
Electrode placement for Holter monitoring: negative electrodes on the left thoracic wall and manubrium; positive electrodes on the left thoracic wall and xiphoid cartilage.
Cardiac cycle analysis was performed using CardioManager S540 software (Cardios Sistemas, São Paulo, SP, Brazil) and manually. The R-R interval was considered normal only when the rhythm was sinus. Heart rate variability was assessed in the time domain, considering the standard deviation of all R-R intervals (SDNN), the standard deviation of the mean R-R intervals every five minutes (SDANN), the mean of the standard deviations of R-R intervals every five minutes (SDNNIDX), the root mean square of the successive differences (rMSSD), and the percentage of adjacent R-R intervals with a difference greater than 50 ms (pNN > 50).
After Holter placement, the jaguars were positioned in right lateral recumbency for a five-minute digital electrocardiographic recording of twelve simultaneous leads (Delta Life, São José dos Campos, SP, Brazil). Bipolar leads (I, II, and III) and augmented unipolar leads (aVR, aVF, and aVL) were recorded according to the standard limb electrode placement [26]. Precordial electrodes were positioned in the first right intercostal space (V1) near the sternum and in the sixth left intercostal space (V2 to V6) from the sternal border towards the cardiac base [27]. After recording, standardized electrocardiographic variables, including heart rate, p wave duration and amplitude, PR interval duration, QRS complex duration, Q, R, and S wave amplitudes, QT interval duration, ST segment, and T wave amplitude and polarity, were evaluated in lead II. The electrical axis of the QRS complex and p and T waves were also determined. For each precordial lead, the morphological parameters of p wave amplitude and QRS complex amplitude and duration were measured. Each measurement was performed in three randomly selected cycles throughout the recording, with the average used for analysis.
Systolic blood pressure was assessed using the oscillometric method (BPScan; Inpulse Animal Health, Florianópolis, SC, Brazil) at the beginning of the protocol during non-invasive anesthetic monitoring. Cuff n° 5 was placed at the base of each animal’s tail, and blood pressure was measured through seven sequential evaluations, with extreme values discarded and the five remaining values averaged for analysis.
Echocardiographic evaluation was performed using an ultrasound machine equipped with a 1-5 MHz sector scan transducer (Esaote, MyLab SigmaVET, Genoa, Italy) and simultaneous electrocardiography on the monitor. The examination included two-dimensional mode (B-mode), motion mode (M-mode), pulsed Doppler, continuous Doppler, color flow Doppler, and tissue Doppler imaging. After shaving segments of the right and left thoracic regions, the animals were positioned in the right and left lateral recumbency, allowing the acquisition of imaging planes for cardiac structure measurement according to the American College of Veterinary Internal Medicine guidelines [28], as well as those established by Boon [29] and the American Society of Echocardiography [30]. From the right parasternal window, in right lateral recumbency, cardiac chambers were evaluated in B-mode and M-mode. Conventionally, from M-mode images obtained from transverse cuts of the left ventricle at the chordal plane, the following parameters were obtained: left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs), ejection fraction (EF), and fractional shortening (FS) calculated by the Teichholz method; additionally, aortic and pulmonary planes were examined. According to Boon [29], the left atrial morphology was assessed in two-dimensional mode in both transverse aortic and longitudinal planes, both after the end of the T wave on the electrocardiogram, determining the ratio between left atrial and aortic diameters (LA/Ao).
Qualitative pulmonary flow evaluation with color Doppler and determination of pulmonary arterial flow velocity using pulsed Doppler were conducted; similarly, the aorta was assessed in an apical five-chamber view from the left parasternal window [29].
Still, in left lateral recumbency, mitral flow velocities were determined from an apical four-chamber view, with the sample positioned centrally at the tips of the anterior and posterior cusps. In this curve, the peak velocities of the E wave (rapid filling of the left ventricle) and A wave (atrial contraction) were analyzed, obtaining the E:A ratio and the deceleration time of rapid filling velocity (DT) [29]. Additionally, systolic velocity (S’), early diastolic velocity (E’), and late diastolic velocity (A’) were measured with the tissue Doppler sample positioned over the mitral annulus at its lateral and septal portions; for right ventricular assessment, only the lateral portion of the tricuspid annulus was considered, with the same parameters obtained [29].
Isovolumetric relaxation time (IVRT) was determined using color Doppler, with the sample positioned at the transition between the left ventricular outflow tract and the septal mitral cusp in the apical five-chamber view, measuring the interval between the end of aortic flow and the start of mitral inflow using pulsed Doppler. Additionally, the ratio between the peak velocity of rapid filling and the isovolumetric relaxation time (E/IVRT) was obtained.
Left ventricular volumetric measurements using the biplane Simpson method were performed in apical four- and two-chamber images during systolic and diastolic cycles, using simultaneous electrocardiography. For this purpose, the borders of the mitral annulus and the endocardial border at the apical tip of the left ventricle were established to quantify the left ventricular volume at end-diastole (immediately before the QRS complex) and end-systole (end of the T wave). Echocardiographic measurements were averaged over three consecutive cardiac cycles [29].
For statistical analysis, the animals were divided by weight and sex into four groups: Group 1 (G1) consisted of males, Group 2 (G2) of females, Group 3 (G3) of animals with body mass up to 45 kg, and Group 4 (G4) of specimens over 45 kg. The Shapiro–Wilk test was used to assess normal data distribution, with parametric data analyzed using the Student’s t-test and non-parametric data compared with the Mann–Whitney test. Statistical analyses were conducted using Statistix version 10.0.1.5. Statistical significance was set at 95% (p < 0.05).
3. Results
Fifteen adult, clinically healthy jaguars with good body condition scores and normal hematological tests (complete blood count, renal and liver function) were evaluated. Of these, eight were females, and seven were males, with an overall average weight of 51.3 ± 18.0 kg and an average age of 4.9 ± 3.1 years. Group 1 (G1) consisted of seven males with an average weight of 59.4 ± 19.9 kg and an average age of 4.4 ± 3.7 years. Group 2 (G2) included eight females with an average weight of 43.6 ± 11.1 kg and an average age of 5.1 ± 2.3 years. Group 3 (G3) consisted of specimens weighing up to 45 kg, including two males and five females, with an average weight of 38.9 ± 7.8 kg and an age of 3 ± 1.9 years. Group 4 (G4) included animals weighing more than 45 kg, with five males and three females averaging 61.9 ± 14.5 kg and 6 ± 2.8 years of age.
The jaguars remained under anesthesia for an average of 69.3 ± 7.5 min, including the time from dart administration to the administration of the reversal agent. The induction time after dart administration was 17.1 ± 4.3 min. The execution of the echocardiogram in the right (25.8 ± 4.7 min) and left (26.4 ± 2.8 min) parasternal windows totaled 52.2 ± 7.5 min. The average heart rate was 72 ± 18 beats per minute, with a sinus rhythm in ten animals and sinus arrhythmia in five jaguars (Figure 2).
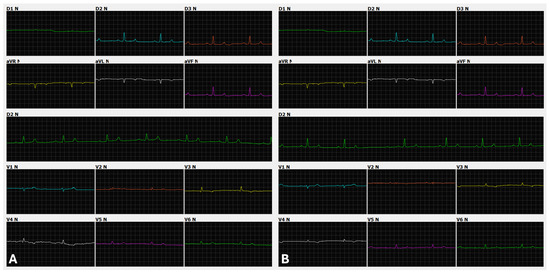
Figure 2.
Appearance of the P-QRS-T complex in limb and precordial leads in two jaguar specimens anesthetized with medetomidine and ketamine. (A): Male, 61.3 kg, 4 years old. Twelve leads (I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, and V6), speed 50 mm/s, sensitivity 10 mm/mV. Sinus rhythm with a heart rate of 66 beats per minute. (B): Female, 44.6 kg, 8 years old. Twelve leads (I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, and V6), speed 50 mm/s, sensitivity n. Sinus arrhythmia with a heart rate ranging from 44 to 71 beats per minute.
Anatomically, jaguars have dense fur, thick skin, and more developed thoracic musculature compared with domestic carnivores. Cardiac auscultation of the specimens in this study was considered more muffled compared with that of dogs and cats, although a grade II/VI systolic murmur was detected at the mitral focus in seven specimens.
In jaguars, the p wave was positive in lead II, with an average duration of 49 ms (Table 1). In precordial leads, the p wave was negative in V1 and V2, becoming slightly positive in V3 and stabilizing between V4, V5, and V6 (Table 2). The average electrical axis of the frontal plane of the p wave showed variability of 81 ± 23 degrees (Table 1), ranging from 50 to 120 degrees among the 15 animals studied.

Table 1.
Electrocardiographic variables obtained from lead DII in jaguars anesthetized with medetomidine and ketamine.

Table 2.
Electrocardiographic variables related to precordial leads (V1, V2, V3, V4, V5, and V6) recorded in jaguars anesthetized with medetomidine and ketamine.
Regarding the morphology of the QRS complex, an R and Rs pattern was observed in leads II, III, and aVF, with peak amplitude in lead II (Table 3) and a duration of 54 ms. In precordial leads, the QRS complex exhibited an rS morphology in V1 and r from V2 to V6 (Table 2), with an average electrical axis of 82 ± 13 degrees (Table 1) and variation from 20 to 110 degrees in the specimens studied (Figure 3).

Table 3.
Amplitude (mV) of the electrocardiographic waves Q, R, and S in bipolar leads (I, II, and III) and augmented unipolar leads (aVR, aVL, and aVF) recorded in jaguars anesthetized with medetomidine and ketamine.
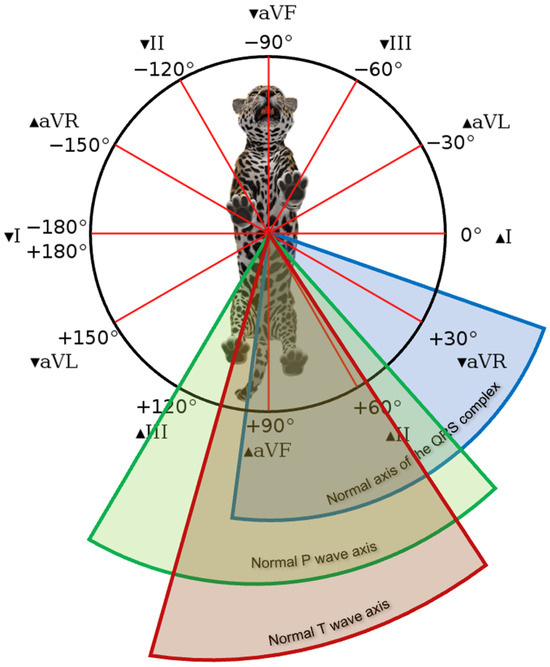
Figure 3.
Mean electrical axis interval of the QRS complex (blue), p wave (green), and T wave (red) in the frontal plane of jaguar specimens anesthetized with a combination of medetomidine and ketamine.
The T wave amplitude showed a variation greater than 25% compared with the R wave due to the latter’s low amplitude, presenting as positive in all animals, without amplitude increase or polarity change during the monitoring period.
A second-degree atrioventricular block, Mobitz type II (Figure 4), was observed in six animals, one male and five females. Extrapolating the reference value used in dogs and animals in G1 and G4, as well as the overall average, showed a PR interval greater than 140 ms, indicating a first-degree atrioventricular block (Table 1).
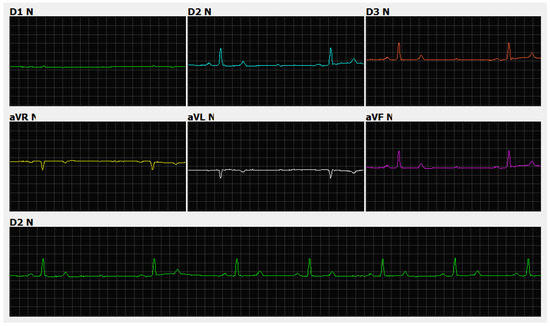
Figure 4.
Electrocardiographic recording. Sinus rhythm with a heart rate of 62 beats per minute and second-degree atrioventricular block, Mobitz type II, gain n, and speed 50 ms. Note the presence of a non-conducted p wave (yellow line) in jaguars anesthetized with medetomidine and ketamine.
All parameters evaluated in Holter electrocardiography are presented in Table 4. There was no significant difference between the groups evaluated. The animals’ profiles indicated a predominance of the parasympathetic autonomic nervous system, with greater heart rate variability, evidenced by elevated rMSSD and pNN > 50% values, as well as SDNN, SDANN, and SDNNIDX, which indicate greater variability as they deviate from zero. However, five animals, two females and three males, exhibited a sympathetic profile, contributing to the reduction of rMSSD in G1 and G4. The dominance of patients with a tachogram profile is represented by image (B) in Figure 5.

Table 4.
Holter electrocardiography parameters of jaguars anesthetized with medetomidine and ketamine.
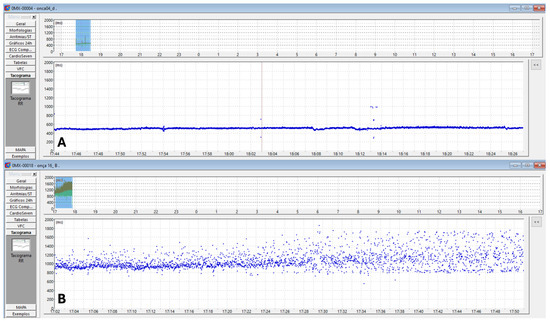
Figure 5.
Two-dimensional tachogram in jaguars anesthetized with medetomidine and ketamine. The time of day is on the X-axis, and the R-R interval (ms) is on the Y-axis. Each R-R interval is represented by a blue dot over time. In (A), the blue dots are more condensed and linearly arranged, indicating lower heart rate variability. In (B), the blue dots are more dispersed, illustrating greater heart rate variability due to increased variation in duration (ms) between R-R intervals.
One male, belonging to both G1 and G4, presented 35 events of premature supraventricular complexes. One female, included in G2 and G3, exhibited a short period of ventricular bigeminy (4 events) and 17 isolated episodes of monomorphic premature ventricular complexes (PVCs) of left ventricular origin (Figure 6).
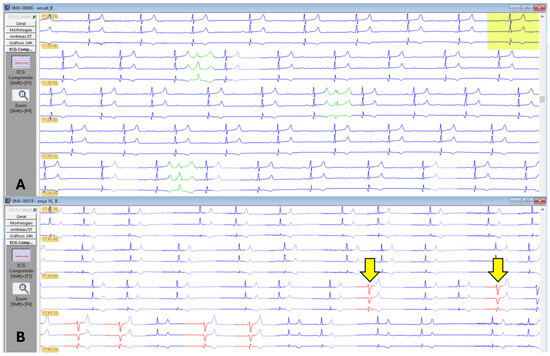
Figure 6.
Holter electrocardiographic recordings in jaguars anesthetized with medetomidine and ketamine. (A): Sinus rhythm represented in three channels (C1, C2, and C3) in blue. Presence of three supraventricular ectopic complexes in green. (B): Sinus arrhythmia represented in three channels (C1, C2, and C3) in blue. Presence of two isolated, monomorphic premature ventricular complexes of left ventricular origin (yellow arrows) and bigeminy events in other beats highlighted in red in the last line of the trace.
Non-invasive systemic blood pressure remained stable across all groups during the procedure, with systolic pressure values (163 ± 29 mmHg), diastolic (116 ± 53 mmHg), and mean (119 ± 77 mmHg) (Figure 7).
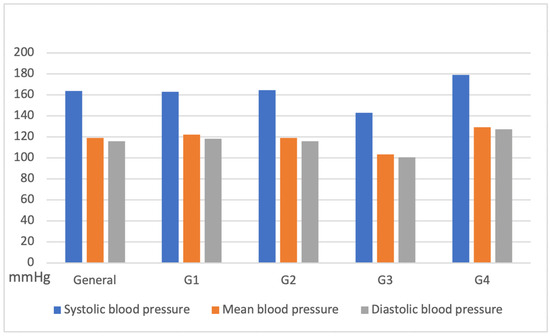
Figure 7.
Variation in systolic, diastolic, and mean blood pressure obtained by oscillometry in jaguars anesthetized with medetomidine and ketamine, subdivided into groups: G1: Males; G2: Females; G3: weight less than 45 kg; G4: weight over 45 kg).
In the echocardiographic evaluation, ventricular morphometry data indicated no significant variation in the left ventricular internal diameter between males and females, nor in relation to the weight range of the individuals studied. A larger left ventricular diameter was found among G1 and G4, associated with an average fractional shortening of 25% and an average ejection fraction of 48% in the jaguars (Table 5).

Table 5.
Morphometric and functional echocardiographic parameters obtained by M-mode in jaguars anesthetized with medetomidine and ketamine.
As shown in Table 6, specimens from G1 and G4 had higher atrial and aortic morphometric values in the two-dimensional mode. In the volumetric evaluation of the left chambers using the biplane Simpson method, the left ventricular ejection fraction of all groups showed higher values in the apical two-chamber window compared to the apical four-chamber window. However, the left atrial ejection fraction showed higher values in the latter window compared with the apical two-chamber (Table 7).

Table 6.
Morphometric echocardiographic parameters of the left atrium and aorta obtained using M-mode and two-dimensional (B-mode) in jaguars anesthetized with medetomidine and ketamine.

Table 7.
Volumetric parameters (mL) of the left ventricle and left atrium using the biplane Simpson method in jaguars anesthetized with medetomidine and ketamine.
Regarding the transmitral flow measured by the pulsed Doppler method, an E wave pattern with greater amplitude than the A wave was identified (Table 8). In the evaluation of the mitral septal and parietal valve annuli using tissue Doppler, an E’ wave pattern smaller than A’ was observed, suggesting an abnormal type II (pseudonormal) ventricular filling pattern (Figure 8). The same patterns were observed in the right chambers (Table 9).

Table 8.
Echocardiographic parameters obtained using pulsed and tissue Doppler (m/s) of the left ventricle in jaguars anesthetized with medetomidine and ketamine.
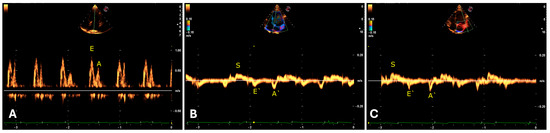
Figure 8.
Evaluation of transmitral flow by pulsed Doppler in a four-chamber apical window indicating E and A waves (A). Tissue Doppler evaluation of the mitral annulus from apical images indicating inversion of E’ and A’ waves in the septal (B) and lateral (C) regions in jaguars anesthetized with medetomidine and ketamine. S = Systole.

Table 9.
Echocardiographic parameters obtained using pulsed and tissue Doppler (m/s) of the right ventricle in jaguars anesthetized with medetomidine and ketamine.
The isovolumetric relaxation time was prolonged, associated with a reduced E wave peak, resulting in a decreased E/IVRT ratio. Color Doppler revealed mitral valve insufficiency (80%), aortic (73%), tricuspid (47%), and pulmonary (13%) in the 15 animals evaluated (Figure 9).
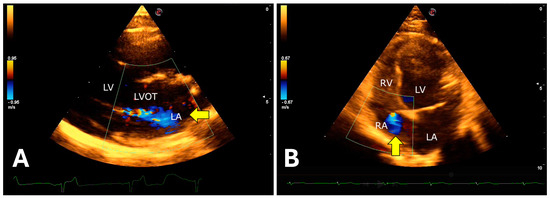
Figure 9.
Color Doppler evaluation demonstrating atrioventricular valvular insufficiency in jaguars anesthetized with medetomidine and ketamine. (A): right parasternal window showing turbulent systolic flow in the anterograde direction (arrow) over the mitral valve apparatus. (B): left apical window showing tricuspid systolic regurgitation (arrow). LVOT = Left ventricular outflow tract. LV = Left ventricle. RV = Right ventricle. LA = Left atrium. RA = Right atrium.
4. Discussion
The jaguar, the largest feline in the Americas, requires chemical restraint for safe handling, both for the technicians and the animal, even when kept in captivity. In free-ranging jaguars, where environmental variables and field conditions are unpredictable, it is crucial that chemical restraint is rapid and that the animal’s recovery from anesthesia is efficient [31,32]. Unlike companion animals, where complementary tests are usually performed before anesthesia, this is more challenging in jaguars. The weight of captive animals, used to calculate the anesthetic dose, is often based on the last restraint, which may not reflect the current weight. In free-ranging jaguars, weight is visually estimated, which can result in anesthetic overdosing or underdosing. Therefore, it is necessary to use anesthetic protocols with a wide safety margin, reduced volume, and the ability to be quickly reversed after handling.
One of the main anesthetic protocols for restraining jaguars, both in captivity and in the wild, is the combination of tiletamine and zolazepam [33,34,35,36,37,38,39], presented in a lyophilized form, facilitating the manipulation of drug concentration. However, a significant disadvantage of this protocol is its high cost and the lack of a reversal agent. Currently, the combination of medetomidine and ketamine is widely used in captive and free-ranging jaguars [13,16,20,40,41,42].
This study is pioneering in investigating the pharmacological effects of the medetomidine and ketamine combination on the cardiological parameters of healthy captive jaguars. The results demonstrated the safety of this protocol for the reproductive management of the specimens, contributing to ex situ conservation. Additionally, the study enriches the database on echocardiographic, conventional electrocardiographic, Holter, and systemic blood pressure parameters, serving as a model for evaluating potential subclinical cardiovascular diseases in this species. According to Napier et al. [43], in a previous study with leopards, the cardiovascular system was identified as the fourth most common organ system associated with mortality, and the presence of low-grade murmurs is common in felids, including young individuals.
During the evaluation among the groups (G1, G2, G3, and G4) in this research, no significant differences were identified in the animal segmentation criteria, nor adverse effects such as hypothermia, hypotension, emesis, and myoclonus during the induction period until the medicated reversal, as mentioned by Sinclair [15].
Oscillometric systemic blood pressure measurement showed no significant variation in jaguars, as described by Caramalac et al. [44], who also reported episodes of arrhythmias in pumas anesthetized with medetomidine and ketamine associated with propofol. This study observed 35 episodes of premature complexes of supraventricular origin and 17 isolated and monomorphic premature ventricular complexes, with four events of ventricular bigeminy in two different specimens.
Additionally, the average PR interval duration in animals from groups G1 and G4 exceeded 140 ms, characterizing first-degree atrioventricular block (AVB) compared with reference values in dogs [45]. Oliveira et al. [46] also observed first-degree AVB in pumas (Puma concolor) anesthetized with sevoflurane or isoflurane. Six animals in this study exhibited a second-degree atrioventricular block, Mobitz type II, possibly due to increased vagal tone induced by the drugs. Holter electrocardiography parameters rMSSD, pNN > 50%, SDNN, SDANN, and SDNNIDX suggest greater heart rate variability, indicating parasympathetic autonomic nervous system activity.
In electrocardiography, there was an increase in PR and QT intervals compared with domestic cats, inversely proportional to heart rate [26,47]. In Holter electrocardiography, elevated mean NN values were observed, also inversely proportional to heart rate.
In tigers subjected to the same anesthetic protocol, Smith et al. [48] reported ST segment elevation and/or depression, increased T wave amplitude, and T wave polarity changes. In the present study, no significant changes were observed in these parameters, corroborating previous studies that did not identify significant adverse effects in tigers [49].
The evaluation of cardiac chambers using the echocardiographic technique recommended in dogs and cats by Thomas et al. [28] and Boon [29] is suitable for investigating possible cardiopathies in jaguars. In this study, the application of color Doppler revealed mild mitral and tricuspid valve insufficiency, as well as aortic and pulmonary insufficiency, which have been described in other felids anesthetized with the same protocol [50,51], similar to what is observed in domestic cats [52]. However, Chai et al. [50] did not attribute these findings to the anesthetic protocol itself. The identified valve regurgitations may have a subclinical nature, considering the technical limitations of echocardiographic evaluation in wild felids.
Regarding the mitral and tricuspid valve apparatus, no morphological or echogenicity alterations were observed, similar to what occurs in degenerative processes in dogs [29]. The aortic and pulmonary semilunar valves also showed no signs of structural alterations, although macroscopic and histopathological analysis could provide greater detail, which is not the focus of this study. Despite the valve regurgitations, no signs of hemodynamic repercussions were observed in the specimens studied.
According to Wang et al. [53], in healthy dogs medicated with dexmedetomidine, there was an increase in left ventricular internal diameter during the systolic and diastolic phases due to the drug’s vasoconstrictor effect, with reduced ejection and shortening fractions, as well as significant mitral valve insufficiency. These findings partially validate the hemodynamic data of this study, which also observed valve insufficiency in jaguars anesthetized with ketamine and medetomidine. However, due to the wild nature of the animals and the need for chemical restraint for handling, obtaining baseline values is limited, preventing observation of increased left ventricular size. The echocardiographic study of tigers anesthetized with medetomidine, ketamine, midazolam, and isoflurane [51] indicated similar left chamber morphometric values to those obtained in jaguars.
Data obtained from pulsed Doppler in transmitral flow (E and A) and tissue Doppler in the septal and parietal valve annulus of the left and right ventricles (E’ and A’) exhibited an abnormal type II (pseudonormal) ventricular filling pattern differing from healthy and non-anesthetized domestic species [29]. This finding may be related to the anesthetic protocol’s effect, resulting in a longer E wave deceleration time (DT) in the echocardiogram, inversely proportional to heart rate, in addition to increased ventricular filling pressures observed in transmitral flow. Carvalho et al. (2019) documented similar results in domestic cats subjected to continuous dexmedetomidine infusion.
Volumetric values obtained using the biplane Simpson method were equivalent to those described in domestic animals [29,54]. Volumetric measurements of the atrium and left ventricle are important for monitoring cardiovascular diseases, such as mitral valve disease and dilated cardiomyopathy in dogs, being preferable to the Teichholz method due to its greater sensitivity [54]. Lower ejection fraction values were observed when compared by the Simpson and Teichholz methods; the second technique may underestimate these values due to its segmental analysis.
For many years, tiletamine and zolazepam (Zoletil, Virbac, Franca ou Telazol, Zoetis, Spain) have been recommended as the protocol of choice for jaguars, both in veterinary practices and field research, especially in reproductive evaluations [36]. The growing need for information on jaguars and the development of reproductive biotechnologies have encouraged the adoption of new anesthetic protocols, which offer more advantages for the veterinary team and animal health [13,16]. These advantages include cost reduction, the use of anesthetic reversers, and rapid anesthetic recovery, allowing animal monitoring until complete recovery.
It is important to highlight some limitations of this study. The small sample size limits the establishment of reference standards, as individual variations have a greater impact on a small sample. However, as it involves research with wild animals, the reported sample size is larger compared with isolated reports and other similar studies. Additionally, the interpretation of echocardiographic evaluation data under the influence of anesthetic drugs, which impact cardiac physiology, should be made in the context of the effects promoted by the drug combination used.
In summary, the morphometric echocardiographic data obtained indicate a cardiovascular response in jaguars similar to that observed in domestic cats and wild felids subjected to the same anesthetic protocol. The tissue Doppler pattern, indicating altered ventricular filling, may be associated with the vasoconstrictor effect of the drug in jaguars.
5. Conclusions
The results of this study, obtained through conventional and Holter electrocardiography, echocardiography, and non-invasive blood pressure measurement, indicate that jaguars kept in artificial environments did not exhibit significant adverse effects during anesthesia. The combination of medetomidine and ketamine at the specified doses resulted in minor hemodynamic changes, such as mitral, tricuspid, pulmonary, and aortic valve insufficiencies, as well as modifications in left ventricular filling patterns and electrical conduction disturbances (first- and second-degree atrioventricular blocks) and premature supraventricular and ventricular complexes. No significant anesthetic complications were identified that would increase the risk of the anesthetic protocol. This study provides novel and valuable information, validating the technique and establishing reference values for jaguars while also paving the way for future research in other species of veterinary interest.
Author Contributions
Conceptualization, M.D.K., M.F.S., R.A.N.A. and A.A.C.; data curation, M.D.K.; Formal analysis, M.D.K., M.F.S., R.A.N.A. and A.A.C.; investigation, M.D.K., M.F.S., R.A.N.A., A.P.R.S., F.A., G.R.d.A., P.N.J.-N., S.R.P. and J.P.S.; methodology, M.D.K., M.F.S., R.A.N.A. and A.A.C.; project administration, M.D.K.; resources, M.D.K., M.F.S., R.A.N.A. and A.A.C.; supervision, A.A.C.; validation, M.D.K.; visualization, M.D.K., M.F.S., R.A.N.A., A.P.R.S., F.A., G.R.d.A., P.N.J.-N., S.R.P., J.P.S., C.S.P., T.D.-S. and A.A.C.; writing—review and editing, G.R.d.A., P.N.J.-N., C.S.P. and T.D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001. This study was financed in part by the Universidade Federal de Mato Grosso do Sul–UFMS/MEC–Brazil. This study was financed in part by the Foundation to Support the Development of Education, Science and Technology in the State of Mato Grosso do Sul. This study was financed in part by the National Council for Scientific and Technological Development. This study was financed in part by the Reprocon Institute.
Institutional Review Board Statement
This manuscript adheres to the ethical standards as outlined in the 1964 Declaration of Helsinki and its later amendments. It does not require approval from an animal use ethics committee in Brazil, as the study was conducted to monitor the animals during reproductive procedures. Approval for these reproductive procedures was obtained from the Ethics Committee of the Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo (FMVZ-USP) under protocol number 9300241019. Additionally, authorization for scientific activities related to these procedures was issued by SISBIO / ICMBio / MMA under no. 57293-2.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors acknowledge the following institutions and persons: Instituto Onça Pintada, Leandro Silveira, Anah Tereza de Almeida Jácomo, and the Fundação Universidade Federal de Mato Grosso do Sul.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| A’ | Peak velocity of diastolic mitral annular motion as determined by pulsed wave Doppler |
| Ao | Aorta |
| aVF | Augmented Unipolar Limb Led Left Leg |
| aVL | Augmented Unipolar Limb Led Left Arm |
| aVR | Augmented Unipolar Limb Led Right Arm |
| B-mode | Bidimensional Mode |
| bpm | Beats per minute |
| DT | E wave deceleration time |
| E:A | Ratio of E to A |
| E’ | Peak velocity of early diastolic mitral annular motion as determined by pulsed wave Doppler |
| EF | Ejection fraction |
| FS | Fractional shortening |
| HR | Heart rate |
| IM | Intramuscular |
| IVRT | Isovolumetric relaxation time |
| LA | Left atrium |
| LA:Ao | Ratio of the left atrial dimension to the aortic annulus dimension |
| LVIDs | Left ventricular internal dimension at end-systole |
| M-mode | Movement mode |
| mmHg | Millimetres of mercury |
| P | P wave |
| pNN > 50 | Percentage of adjacent RR intervals with a duration difference greater than 50 ms |
| PR | PR interval |
| QRS | QRS complex |
| QT | QT segment |
| rMSSD | Square root of the mean of the square of the differences between adjacent RR intervals |
| S | Peak velocity of systolic pulmonary vein flow |
| S’ | peak velocity of systolic mitral annular motion as determined by pulsed wave Doppler |
| SDANN | Standard deviation of the means of the RR intervals obtained every five minutes |
| SDNN | Standard deviation of all RR intervals |
| SDNNIDX | The average of the standard deviations of the RR intervals every five minutes |
| ST | ST segment |
| T | T wave |
| V1 | Precordial lead V1 |
| V2 | Precordial lead V2 |
| V6 | Precordial lead V6 |
| LVIDd | Left ventricular internal dimension at end-diastole |
References
- Deco-Souza, T.; Araújo, G.R.; Pizzutto, C.S.; Requena, L.A.; Jorge-Neto, P.N. In Situ and Ex Situ Jaguar (Panthera onca) Reproduction: What Do We Have so Far? Theriogenology Wild 2024, 4, 100070. [Google Scholar] [CrossRef]
- Silveira, L. Ecologia Comparada e Conservação Da Onça-Pintada (Panthera onca) e Onça-Parda (Puma concolor), No Cerrado e Pantanal. Ph.D. Thesis, Universidade de Brasília, Brasília, DF, Brazil, 2004. [Google Scholar]
- De Azevedo, F.C.C. Food Habits and Livestock Depredation of Sympatric Jaguars and Pumas in the Iguaçu National Park Area, South Brazil. Biotropica 2008, 40, 494–500. [Google Scholar] [CrossRef]
- Brasil Portaria MMA no 148, de 7 de Junho de 2022; 2022; Vol. 108, p. 74. Available online: https://unbciencia.unb.br/images/Noticias/2022/06-Jun/PORTARIA_MMA_No148_7_DE_JUNHO_DE_2022.pdf (accessed on 10 December 2024).
- Luczinski, T.C.; Jorge-Neto, P.N.; Ribeiro, R.M.; De Jesus, R.S.; Pizzutto, C.S.; De Deco-Souza, T.; De Araújo, G.R.; Morgado, T.O.; Corrêa, S.H.R.; Peixer, M.A.S.; et al. One Conservation Concept in Practice. Theriogenology Wild 2023, 2, 100024. [Google Scholar] [CrossRef]
- Requena, L.A.; Luczinski, T.C.; Traldi, A.d.S.; Deco-Souza, T.d.; de Araújo, G.R.; Pizzutto, C.S.; Miranda, G.M.; Porto, M.R.; da Silva, M.C.C.; Jorge-Neto, P.N. Reproductive Evaluation of Luisa, the Last Jaguar of the Caatinga. Anim. Reprod. 2023, 20, e20230090. [Google Scholar] [CrossRef]
- Zimmermann, A.; Walpole, M.J.; Leader-Williams, N. Cattle Ranchers’ Attitudes to Conflicts with Jaguar Panthera onca in the Pantanal of Brazil. Oryx 2005, 39, 406. [Google Scholar] [CrossRef]
- Csermak, A.C.; De Araújo, G.R.; Pizzutto, C.S.; De Deco-Souza, T.; Jorge-Neto, P.N. GPS Collars as a Tool to Uncover Environmental Crimes in Brazil: The Jaguar as a Sentinel. Anim. Conserv. 2023, 26, 137–139. [Google Scholar] [CrossRef]
- Pizzutto, C.S.; Colbachini, H.; Jorge-Neto, P.N. One Conservation: The Integrated View of Biodiversity Conservation. Anim. Reprod. 2021, 18, e20210024. [Google Scholar] [CrossRef]
- Gunkel, C.; Lafortune, M. Felids. In Zoo Animal and Wildlife Immobilization and Anesthesia; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-0-8138-2566-3. [Google Scholar]
- Morris, P.J. Chemical Immobilization of Felids, Ursids, and Small Ungulates. Vet. Clin. N. Am. Exot. Anim. Pract. 2001, 4, 267–298. [Google Scholar] [CrossRef]
- Caulkett, N.A.; Arnemo, J.M. Comparative Anesthesia and Analgesia of Zoo Animals and Wildlife. In Veterinary Anesthesia and Analgesia; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 764–776. ISBN 978-1-118-52623-1. [Google Scholar]
- Araujo, G.R.; Deco-Souza, T.; Morato, R.G.; Crawshaw, P.G.; Silva, L.C.; Jorge-Neto, P.N.; Csermak-Jr, A.C.; Bergo, L.C.F.; Kantek, D.L.Z.; Miyazaki, S.S.; et al. Use of Foot Snares to Capture Large Felids. Methods Ecol. Evol. 2021, 12, 322–327. [Google Scholar] [CrossRef]
- Scheinin, H.; Virtanen, R.; Macdonald, E.; Lammintausta, R.; Scheinin, M. Medetomidine—A Novel A2-Adrenoceptor Agonist: A Review of Its Pharmacodynamic Effects. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 635–651. [Google Scholar] [CrossRef]
- Sinclair, M.D. A Review of the Physiological Effects of Alpha2-Agonists Related to the Clinical Use of Medetomidine in Small Animal Practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar] [PubMed]
- de Araujo, G.R.; de Paula, T.A.R.; de Deco-Souza, T.; Morato, R.G.; Bergo, L.C.F.; da Silva, L.C.; Costa, D.S.; Braud, C. Comparison of Semen Samples Collected from Wild and Captive Jaguars (Panthera onca) by Urethral Catheterization after Pharmacological Induction. Anim. Reprod. Sci. 2018, 195, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.R.; Paula, T.A.R.; Deco-Souza, T.; Morato, R.G.; Bergo, L.C.F.; Silva, L.C.; Jorge-Neto, P.N.; Sampaio, B.F.B. Colheita farmacológica de sêmen de onças-pardas (Puma concolor: Mammalia: Carnivora: Felidae). Arq. Bras. Med. Veterinária Zootec. 2020, 72, 437–442. [Google Scholar] [CrossRef]
- De Araújo, G.R.; Jorge-Neto, P.N.; Csermak-Jr, A.C.; Pizzutto, C.S.; Luczinski, T.C.; de Deco-Souza, T. Avanços na andrologia de grandes felinos neotropicais. Rev. Bras. Reprodução Anim. 2021, 45, 219–228. [Google Scholar] [CrossRef]
- Da Silva, M.C.C.; Ullony, K.M.; de Araújo, G.R.; Jorge-Neto, P.N.; Albuquerque, V.B.; Caramalac, S.M.; de Oliveira, A.R.; Zanella, R.; Marques, M.G.; Csemark Junior, A.C.; et al. Can Detomidine Replace Medetomidine for Pharmacological Semen Collection in Domestic Cats? Anim. Reprod. 2021, 18, e20210017. [Google Scholar] [CrossRef]
- Luczinski, T.C.; de Araújo, G.R.; Silveira, M.F.; Kirnew, M.D.; Navarrete, R.A.; Salomão-Jr, J.A.; Requena, L.A.; dos Santos, J.A.M.; Koshiyama, M.H.; Pizzutto, C.S.; et al. Medetomidine May Cause Heart Murmur in Cougars and Jaguars: Case Report. J. Threat. Taxa 2020, 12, 17000–17002. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Pizzutto, C.S.; de Araújo, G.R.; Csermak-Jr, A.C.; Jorge-Neto, P.N.; Luczinski, T.C.; de Deco-Souza, T. Uma visão integrada das biotecnologias reprodutivas com o conceito de One Conservation. Rev. Bras. Reprodução Anim. 2021, 45, 241–245. [Google Scholar] [CrossRef]
- Stern, J.A.; Ueda, Y. Inherited Cardiomyopathies in Veterinary Medicine. Pflüg. Arch.-Eur. J. Physiol. 2019, 471, 745–753. [Google Scholar] [CrossRef]
- Horowitz, B.N.; Kutinsky, I.B.; Linde, A. Species-Spanning Echocardiography: Cardiovascular Insights from across the Animal Kingdom. Curr. Cardiol. Rep. 2020, 22, 165. [Google Scholar] [CrossRef]
- Jorge-Neto, P.N.; Requena, L.A.; De Araújo, G.R.; Traldi, A.D.S.; Luczinski, T.C.; De Deco-Souza, T.; Pizzutto, C.S.; Baldassarre, H. Efficient Recovery of in Vivo Mature and Immature Oocytes from Jaguars (Panthera onca) and Pumas (Puma concolor) by Laparoscopic Ovum Pick-Up (LOPU). Theriogenology Wild 2023, 3, 100042. [Google Scholar] [CrossRef]
- Tilley, L.P. Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1992; ISBN 978-0-8121-1443-0. [Google Scholar]
- Santilli, R.A.; Porteiro Vázquez, D.M.; Gerou-Ferriani, M.; Lombardo, S.F.; Perego, M. Development and Assessment of a Novel Precordial Lead System for Accurate Detection of Right Atrial and Ventricular Depolarization in Dogs with Various Thoracic Conformations. Am. J. Vet. Res. 2019, 80, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Vet, M.; Moise, N.S.; Moses, B.L. Recommendations for Standards in Transthoracic Two-dimensional Echocardiography in the Dog and Cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef]
- Boon, J.A. Acquired Valvular Disease. In Veterinary Echocardiography; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 267–334. ISBN 978-0-470-95891-9. [Google Scholar]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- da Silva, M.C.C.; Jorge-Neto, P.N.; Miranda, G.M.; Csermak-Jr, A.C.; Zanella, R.; Pizzutto, C.S.; Colbachini, H.; Silva, A.R.; de Araújo, G.R.; de Deco-Souza, T. Reproductive Parameters of Male Crab-Eating Foxes (Cerdocyon thous) Subjected to Pharmacological Semen Collection by Urethral Catheterization. Theriogenology Wild 2022, 1, 100004. [Google Scholar] [CrossRef]
- Carneiro, J.D.S.; Motheo, T.F. Pharmacological Semen Collection in Domestic and Wild Canids and Felids: Literature Review. Anim. Reprod. 2023, 20, e20230036. [Google Scholar] [CrossRef]
- Morato, R.G.; Guimarães, M.A.d.B.V.; Nunes, A.L.V.; Carciofi, A.C.; Ferreira, F.; Barnabe, V.H.; Barnabe, R.C. Colheita e Avaliação Do Sêmen Em Onça Pintada (Panthera onca). Braz. J. Vet. Res. Anim. Sci. 1998, 35, 178–181. [Google Scholar] [CrossRef]
- Morato, R.G.; Conforti, V.A.; Azevedo, F.C.C.; Jacomo, A.T.A.; Silveira, L.; Sana, D.; Nunes, A.L.V.; Guimarães, M.A.B.V.; Barnabe, R.C. Reproductive Characteristics of Captive Male Jaguar. Braz. J. Vet. Res. Anim. Sci. 1999, 36, 262–266. [Google Scholar] [CrossRef]
- Morato, R.G.; Conforti, V.A.; Azevedo, F.C.; Jacomo, A.T.A.; Silveira, L.; Sana, D.; Nunes, A.L.V.; Guimarães, M.A.B.V.; Barnabe, R.C. Comparative Analyses of Semen and Endocrine Characteristics of Free-Living versus Captive Jaguars (Panthera onca). Reproduction 2001, 122, 745–751. [Google Scholar] [CrossRef]
- Morato, R.G.; Moura, C.A.; Crawshaw, P.G., Jr. Inmovilización Química de Jaguares Libres Con Una Combinación de Tiletamina y Zolazepam. In El jaguar en el Nuevo Milenio; Fondo de Cultura Economica: San Diego, CA, USA, 2004; pp. 91–99. ISBN 968-16-6617-8. [Google Scholar]
- Morato, R.G.; Bueno, M.G.; Malmheister, P.; Verreschi, I.T.N.; Barnabe, R.C. Changes in the Fecal Concentrations of Cortisol and Androgen Metabolites in Captive Male Jaguars (Panthera onca) in Response to Stress. Braz. J. Med. Biol. Res. 2004, 37, 1903–1907. [Google Scholar] [CrossRef]
- Morato, R.G.; Verreschi, I.T.N.; Guimarães, M.A.B.V.; Cassaro, K.; Pessuti, C.; Barnabe, R.C. Seasonal Variation in the Endocrine–Testicular Function of Captive Jaguars (Panthera onca). Theriogenology 2004, 61, 1273–1281. [Google Scholar] [CrossRef]
- Toniollo, G.H.; Faria, D.d., Jr.; Lega, E.; Batista, C.M.; Nunes, N. Piômetra na espécie felina—Relato de um caso em Panthera onca. Braz. J. Vet. Res. Anim. Sci. 2000, 37, 166–168. [Google Scholar] [CrossRef]
- Jorge-Neto, P.N.; da Silva, M.C.C.; Csermak-Júnior, A.C.; Salmão-Júnior, J.A.; de Araújo, G.R.; de Oliveira, G.; Leuzinger, L.; Pizzutto, C.S.; de Deco-Souza, T. Cryptorchidism in Free-Living Jaguar (Panthera onca): First Case Report. Anim. Reprod. 2020, 17, e20200555. [Google Scholar] [CrossRef]
- Jorge-Neto, P.N.; Luczinski, T.C.; de Araújo, G.R.; Salomão Júnior, J.A.; Traldi, A.d.S.; dos Santos, J.A.M.; Requena, L.A.; Gianni, M.C.M.; de Deco-Souza, T.; Pizzutto, C.S.; et al. Can Jaguar (Panthera onca) Ovulate without Copulation? Theriogenology 2020, 147, 57–61. [Google Scholar] [CrossRef]
- Jorge-Neto, P.N.; Luczinski, T.C.; de Araújo, G.R.; Requena, L.A.; de Jesus, R.S.; Souza, L.S.B.; Zanella, R.; Pizzutto, C.S. Cryopreservation of Jaguar (Panthera onca) Sperm Cells Using Different Cryoprotectants and Different Thawing Temperatures. Anim. Reprod. 2023, 20, e20230009. [Google Scholar] [CrossRef]
- Napier, J.E.; Lund, M.S.; Armstrong, D.L.; McAloose, D. A Retrospective Study of Morbidity and Mortality in the North American Amur Leopard (Panthera pardus Orientalis) Population in Zoologic Institutions from 1992 to 2014. J. Zoo Wildl. Med. 2018, 49, 70–78. [Google Scholar] [CrossRef]
- Caramalac, S.M.; Oliveira, A.R.; Albuquerque, V.B.; Deco-Souza, T.; Frazílio, F.O. Efeitos Cardiovasculares Da Medetomidina e Cetamina Em Puma Concolor e Tempo de Recuperação Após Aplicação de Ioimbina Ou Atipamezole. Arq. Bras. Med. Veterinária E Zootec. 2020, 72, 1666–1674. [Google Scholar] [CrossRef]
- Santilli, R.; Moïse, S.; Pariaut, R.; Perego, M. Electrocardiography of the Dog and Cat: Diagnosis of Arrhythmias, 2nd ed.; Edra: Coral Gables, FL, USA, 2019; ISBN 978-88-214-4785-3. [Google Scholar]
- Oliveira, A.R.; Silva, K.F.; Palumbo, M.I.P.; Souza, A.I.; Souza, T.D.; Albuquerque, V.B.; Araújo, M.A.; Frazílio, F.O. Eletrocardiografia Em Onças-Pardas (Puma concolor) Anestesiadas Com Sevoflurano Ou Isoflurano. Arq. Bras. Med. Veterinária E Zootec. 2016, 68, 1613–1620. [Google Scholar] [CrossRef][Green Version]
- Kittleson, M.D. Electrocardiography: Basic Concepts, Diagnosis of Chamber Enlargement, and Intraventricular Conduction Disturbances. In Small Animal Cardiovascular Medicine; Mosby: St. Louis, MO, USA, 1998; p. 603. ISBN 978-0-8151-5140-1. [Google Scholar]
- Smith, C.K.; Seddighi, R.; Zhu, X.; Tepe, A.J.; Ramsay, E.C.; Cushing, A.C. Use of Plethysmographic Variability Index and Perfusion Index to Evaluate Changes in Arterial Blood Pressure in Anesthetized Tigers (Panthera tigris). Am. J. Vet. Res. 2018, 79, 845–851. [Google Scholar] [CrossRef]
- Miller, M.; Weber, M.; Neiffer, D.; Mangold, B.; Fontenot, D.; Stetter, M. Anesthetic Induction of Captive Tigers (Panthera tigris) Using a Medetomidine–Ketamine Combination. J. Zoo Wildl. Med. 2003, 34, 307–308. [Google Scholar] [CrossRef]
- Chai, N.; Petit, T.; Kohl, M.; Bourgeois, A.; Gouni, V.; Trehiou-Sechi, E.; Misbach, C.; Petit, A.; Damoiseaux, C.; Garrigou, A.; et al. Prevalence of Valvular Regurgitations in Clinically Healthy Captive Leopards and Cheetahs: A Prospective Study from the Wildlife Cardiology (WLC) Group (2008–2013). J. Zoo Wildl. Med. 2015, 46, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.T.; Gompf, R.E.; Smith, C.K.; Price, J.M.; Cushing, A.C. Echocardiographic Parameters in Eight Adult Tigers (Panthera tigris) during Two Phases of an Anesthetic Protocol. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2018, 49, 875–880. [Google Scholar] [CrossRef]
- Carvalho, E.R.; Champion, T.; Ambrosini, F.; Da Silva, G.A.; Freitas, G.C.; D’Otaviano De Castro Vilani, R.G. Dexmedetomidine Low Dose Followed by Constant Rate Infusion and Antagonism by Atipamezole in Isoflurane-Anesthetized Cats: An Echocardiographic Study. Vet. Anaesth. Analg. 2019, 46, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hung, C.; Lee, W.; Chang, K.; Chen, K. Effects of Intravenous Dexmedetomidine on Cardiac Characteristics Measured Using Radiography and Echocardiography in Six Healthy Dogs. Vet. Radiol. Ultrasound 2016, 57, 8–15. [Google Scholar] [CrossRef]
- Wess, G.; Bauer, A.; Kopp, A. Echocardiographic Reference Intervals for Volumetric Measurements of the Left Ventricle Using the Simpson’s Method of Discs in 1331 Dogs. J. Vet. Intern. Med. 2021, 35, 724–738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).