1. Introduction
The jaguar (
Panthera onca), the largest felid in the Americas, plays a crucial role as an apex predator in maintaining the ecological balance of various animal and plant species [
1,
2,
3]. Despite its status as a predator, the species is considered vulnerable due to its risk of extinction in Brazil [
4], facing direct and indirect threats from human activities that lead to habitat fragmentation and reduction, prey scarcity, and decreased genetic diversity (inbreeding), as well as population decline [
2,
5,
6] and retaliatory hunting [
7,
8]. Conservation strategies aim to conserve original habitats and recover viable species through natural means and anthropogenic actions, focusing on conservation through captive populations [
9]. The management of these animals requires the use of sedatives and/or anesthetics to ensure the safety of both the team and the specimens [
10].
Currently, the combination of α
2-adrenergic agonist drugs with ketamine has shown efficacy in immobilizing both in situ and ex situ carnivores [
11,
12,
13]. Activation of α
2-adrenergic receptors promotes sedation, analgesia, hypotension, and bradycardia [
14]. Medetomidine, a representative of this pharmacological class, induces dose-dependent sedative and anxiolytic effects, leading to loss of vigilance and reflexes in animals [
15], as well as vasopressor effects associated with reduced heart rate [
14]. The use of atipamezole, an α
2-adrenergic antagonist, allows for greater anesthetic control in the presence of adverse effects [
10,
12,
14,
15]. Another desirable effect of medetomidine is the relaxation of the smooth muscle of the vas deferens, facilitating semen collection through pharmacological ejaculation in domestic cats and large felids [
16,
17,
18,
19].
However, the interaction between medetomidine and ketamine can overload the cardiovascular system due to peripheral vasoconstriction, resulting in bradycardia [
10,
12]. According to Luczinski et al. [
20], mitral valve insufficiency may occur, although ketamine has dissociative analgesic and anesthetic effects in animals and humans [
21].
The conservation of endangered species is essential for maintaining biomes and advancing knowledge about the physiology and pathology of these species [
22]. Previous studies have identified cardiopathies in wild mammals and adverse effects of some anesthetics [
23,
24]. Given the need for safer anesthetic protocols for the management and conservation of wild animals, it is crucial to analyze the impacts of these drugs on cardiovascular function, considering the known effects of these substances.
Based on these principles, it is hypothesized that the anesthetic protocol used may cause (i) alterations in cardiovascular parameters, such as bradycardia and hypotension; (ii) pro-arrhythmic atrioventricular effects; and (iii) induction of mitral and aortic valve insufficiency. This study aimed to determine the cardiovascular parameters in clinically healthy jaguars anesthetized with medetomidine and ketamine, evaluating cardiac morphology and function through echocardiography, analyzing electrocardiographic parameters of rhythm and wave morphology and heart rate variability through conventional and Holter electrocardiography, and measuring systemic blood pressure.
2. Materials and Methods
Fifteen healthy jaguars, sourced from the non-governmental conservation center Instituto Onça Pintada–IOP (Mineiros, GO, Brazil; 17°54′00.5”S 53°00′21.7”W), were selected for routine clinical, semen collection, and cardiological evaluation. The animals were housed in suitable enclosures, with free access to water and a diet based on beef. Ages were estimated at 4.9 ± 3.1 years, according to the keeper’s records. These evaluations were conducted alongside a jaguar reproduction study linked with SISBIO/ICMBio/MMA under number 57293-5 and approved by the Animal Use Ethics Committee of the Faculty of Veterinary Medicine and Animal Science at the University of São Paulo under protocol number 9300241019.
After 12 h of fasting, the animals were chemically restrained using anesthetic darts containing medetomidine (0.08–0.1 mg/kg, IM; Precision Pharmacy, Bakersfield, CA, USA) and ketamine (5 mg/kg, IM; Dopalen, CEVA, Paulínia, SP, Brazil) [
13,
25]. Once the effects of the drugs were observed, the specimens were transferred to an outpatient setting for clinical analyses and biological sample collection for routine tests. Subsequently, the animals were returned to their original enclosures, and anesthesia was reversed with atipamezole (0.25 mg/kg, IM; Precision Pharmacy, Bakersfield, CA, USA).
For Holter electrocardiography, adhesive electrodes were attached to previously shaved skin (
Figure 1). Two negative electrodes were positioned: one on the left thoracic wall and another on the manubrium; two positive electrodes were placed on the left thoracic wall and over the xiphoid cartilage. A three-channel digital recorder (Cardioflash digital, Cardios Sistemas, São Paulo, SP, Brazil) was connected, and recording was conducted throughout the experimental period, from capture to release, for approximately 60 min.
Cardiac cycle analysis was performed using CardioManager S540 software (Cardios Sistemas, São Paulo, SP, Brazil) and manually. The R-R interval was considered normal only when the rhythm was sinus. Heart rate variability was assessed in the time domain, considering the standard deviation of all R-R intervals (SDNN), the standard deviation of the mean R-R intervals every five minutes (SDANN), the mean of the standard deviations of R-R intervals every five minutes (SDNNIDX), the root mean square of the successive differences (rMSSD), and the percentage of adjacent R-R intervals with a difference greater than 50 ms (pNN > 50).
After Holter placement, the jaguars were positioned in right lateral recumbency for a five-minute digital electrocardiographic recording of twelve simultaneous leads (Delta Life, São José dos Campos, SP, Brazil). Bipolar leads (I, II, and III) and augmented unipolar leads (aVR, aVF, and aVL) were recorded according to the standard limb electrode placement [
26]. Precordial electrodes were positioned in the first right intercostal space (V1) near the sternum and in the sixth left intercostal space (V2 to V6) from the sternal border towards the cardiac base [
27]. After recording, standardized electrocardiographic variables, including heart rate,
p wave duration and amplitude, PR interval duration, QRS complex duration, Q, R, and S wave amplitudes, QT interval duration, ST segment, and T wave amplitude and polarity, were evaluated in lead II. The electrical axis of the QRS complex and
p and T waves were also determined. For each precordial lead, the morphological parameters of
p wave amplitude and QRS complex amplitude and duration were measured. Each measurement was performed in three randomly selected cycles throughout the recording, with the average used for analysis.
Systolic blood pressure was assessed using the oscillometric method (BPScan; Inpulse Animal Health, Florianópolis, SC, Brazil) at the beginning of the protocol during non-invasive anesthetic monitoring. Cuff n° 5 was placed at the base of each animal’s tail, and blood pressure was measured through seven sequential evaluations, with extreme values discarded and the five remaining values averaged for analysis.
Echocardiographic evaluation was performed using an ultrasound machine equipped with a 1-5 MHz sector scan transducer (Esaote, MyLab SigmaVET, Genoa, Italy) and simultaneous electrocardiography on the monitor. The examination included two-dimensional mode (B-mode), motion mode (M-mode), pulsed Doppler, continuous Doppler, color flow Doppler, and tissue Doppler imaging. After shaving segments of the right and left thoracic regions, the animals were positioned in the right and left lateral recumbency, allowing the acquisition of imaging planes for cardiac structure measurement according to the American College of Veterinary Internal Medicine guidelines [
28], as well as those established by Boon [
29] and the American Society of Echocardiography [
30]. From the right parasternal window, in right lateral recumbency, cardiac chambers were evaluated in B-mode and M-mode. Conventionally, from M-mode images obtained from transverse cuts of the left ventricle at the chordal plane, the following parameters were obtained: left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs), ejection fraction (EF), and fractional shortening (FS) calculated by the Teichholz method; additionally, aortic and pulmonary planes were examined. According to Boon [
29], the left atrial morphology was assessed in two-dimensional mode in both transverse aortic and longitudinal planes, both after the end of the T wave on the electrocardiogram, determining the ratio between left atrial and aortic diameters (LA/Ao).
Qualitative pulmonary flow evaluation with color Doppler and determination of pulmonary arterial flow velocity using pulsed Doppler were conducted; similarly, the aorta was assessed in an apical five-chamber view from the left parasternal window [
29].
Still, in left lateral recumbency, mitral flow velocities were determined from an apical four-chamber view, with the sample positioned centrally at the tips of the anterior and posterior cusps. In this curve, the peak velocities of the E wave (rapid filling of the left ventricle) and A wave (atrial contraction) were analyzed, obtaining the E:A ratio and the deceleration time of rapid filling velocity (DT) [
29]. Additionally, systolic velocity (S’), early diastolic velocity (E’), and late diastolic velocity (A’) were measured with the tissue Doppler sample positioned over the mitral annulus at its lateral and septal portions; for right ventricular assessment, only the lateral portion of the tricuspid annulus was considered, with the same parameters obtained [
29].
Isovolumetric relaxation time (IVRT) was determined using color Doppler, with the sample positioned at the transition between the left ventricular outflow tract and the septal mitral cusp in the apical five-chamber view, measuring the interval between the end of aortic flow and the start of mitral inflow using pulsed Doppler. Additionally, the ratio between the peak velocity of rapid filling and the isovolumetric relaxation time (E/IVRT) was obtained.
Left ventricular volumetric measurements using the biplane Simpson method were performed in apical four- and two-chamber images during systolic and diastolic cycles, using simultaneous electrocardiography. For this purpose, the borders of the mitral annulus and the endocardial border at the apical tip of the left ventricle were established to quantify the left ventricular volume at end-diastole (immediately before the QRS complex) and end-systole (end of the T wave). Echocardiographic measurements were averaged over three consecutive cardiac cycles [
29].
For statistical analysis, the animals were divided by weight and sex into four groups: Group 1 (G1) consisted of males, Group 2 (G2) of females, Group 3 (G3) of animals with body mass up to 45 kg, and Group 4 (G4) of specimens over 45 kg. The Shapiro–Wilk test was used to assess normal data distribution, with parametric data analyzed using the Student’s t-test and non-parametric data compared with the Mann–Whitney test. Statistical analyses were conducted using Statistix version 10.0.1.5. Statistical significance was set at 95% (p < 0.05).
3. Results
Fifteen adult, clinically healthy jaguars with good body condition scores and normal hematological tests (complete blood count, renal and liver function) were evaluated. Of these, eight were females, and seven were males, with an overall average weight of 51.3 ± 18.0 kg and an average age of 4.9 ± 3.1 years. Group 1 (G1) consisted of seven males with an average weight of 59.4 ± 19.9 kg and an average age of 4.4 ± 3.7 years. Group 2 (G2) included eight females with an average weight of 43.6 ± 11.1 kg and an average age of 5.1 ± 2.3 years. Group 3 (G3) consisted of specimens weighing up to 45 kg, including two males and five females, with an average weight of 38.9 ± 7.8 kg and an age of 3 ± 1.9 years. Group 4 (G4) included animals weighing more than 45 kg, with five males and three females averaging 61.9 ± 14.5 kg and 6 ± 2.8 years of age.
The jaguars remained under anesthesia for an average of 69.3 ± 7.5 min, including the time from dart administration to the administration of the reversal agent. The induction time after dart administration was 17.1 ± 4.3 min. The execution of the echocardiogram in the right (25.8 ± 4.7 min) and left (26.4 ± 2.8 min) parasternal windows totaled 52.2 ± 7.5 min. The average heart rate was 72 ± 18 beats per minute, with a sinus rhythm in ten animals and sinus arrhythmia in five jaguars (
Figure 2).
Anatomically, jaguars have dense fur, thick skin, and more developed thoracic musculature compared with domestic carnivores. Cardiac auscultation of the specimens in this study was considered more muffled compared with that of dogs and cats, although a grade II/VI systolic murmur was detected at the mitral focus in seven specimens.
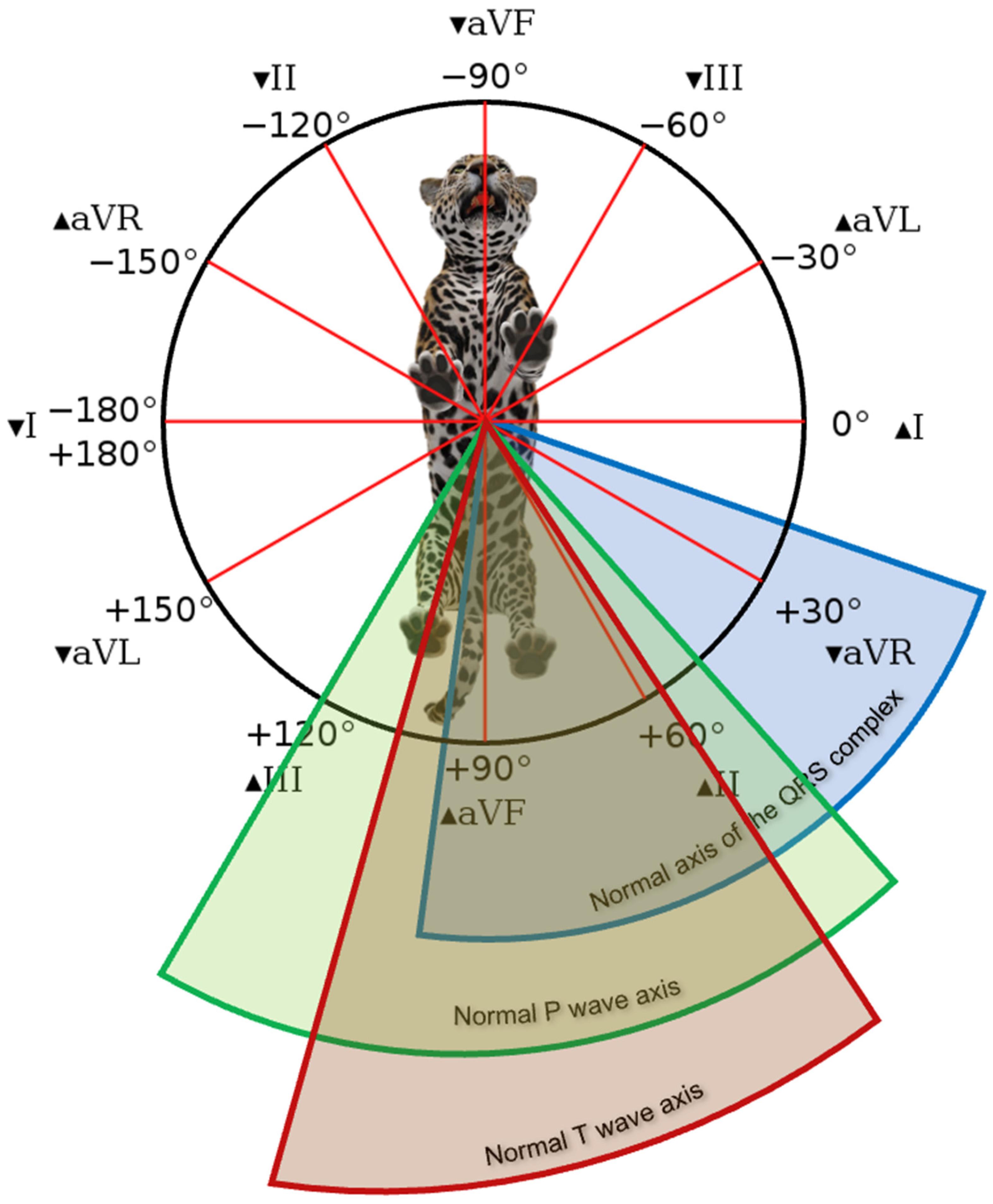
In jaguars, the
p wave was positive in lead II, with an average duration of 49 ms (
Table 1). In precordial leads, the
p wave was negative in V1 and V2, becoming slightly positive in V3 and stabilizing between V4, V5, and V6 (
Table 2). The average electrical axis of the frontal plane of the
p wave showed variability of 81 ± 23 degrees (
Table 1), ranging from 50 to 120 degrees among the 15 animals studied.
Regarding the morphology of the QRS complex, an R and Rs pattern was observed in leads II, III, and aVF, with peak amplitude in lead II (
Table 3) and a duration of 54 ms. In precordial leads, the QRS complex exhibited an rS morphology in V1 and r from V2 to V6 (
Table 2), with an average electrical axis of 82 ± 13 degrees (
Table 1) and variation from 20 to 110 degrees in the specimens studied (
Figure 3).
The T wave amplitude showed a variation greater than 25% compared with the R wave due to the latter’s low amplitude, presenting as positive in all animals, without amplitude increase or polarity change during the monitoring period.
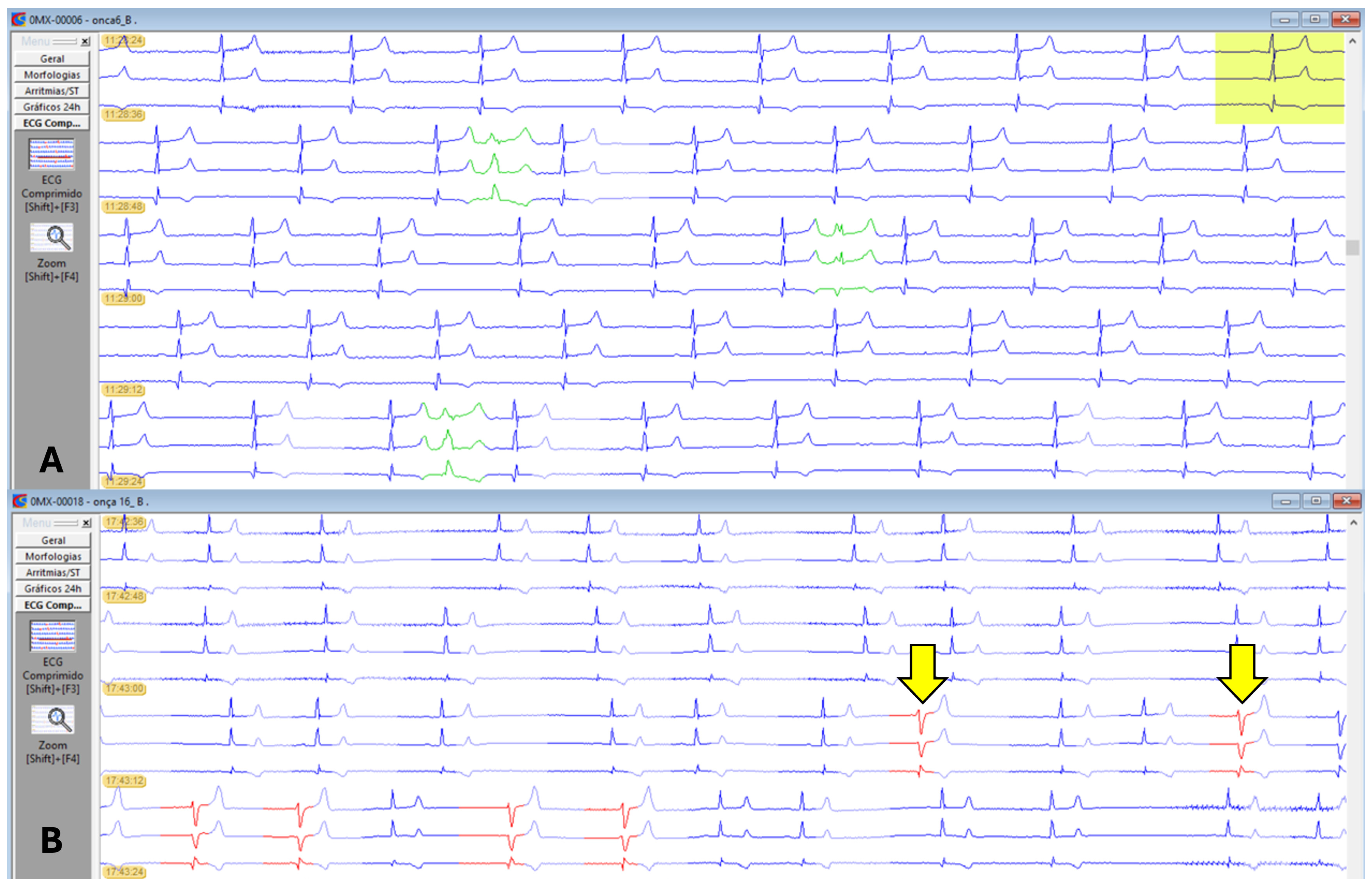
A second-degree atrioventricular block, Mobitz type II (
Figure 4), was observed in six animals, one male and five females. Extrapolating the reference value used in dogs and animals in G1 and G4, as well as the overall average, showed a PR interval greater than 140 ms, indicating a first-degree atrioventricular block (
Table 1).
All parameters evaluated in Holter electrocardiography are presented in
Table 4. There was no significant difference between the groups evaluated. The animals’ profiles indicated a predominance of the parasympathetic autonomic nervous system, with greater heart rate variability, evidenced by elevated rMSSD and pNN > 50% values, as well as SDNN, SDANN, and SDNNIDX, which indicate greater variability as they deviate from zero. However, five animals, two females and three males, exhibited a sympathetic profile, contributing to the reduction of rMSSD in G1 and G4. The dominance of patients with a tachogram profile is represented by image (B) in
Figure 5.
One male, belonging to both G1 and G4, presented 35 events of premature supraventricular complexes. One female, included in G2 and G3, exhibited a short period of ventricular bigeminy (4 events) and 17 isolated episodes of monomorphic premature ventricular complexes (PVCs) of left ventricular origin (
Figure 6).
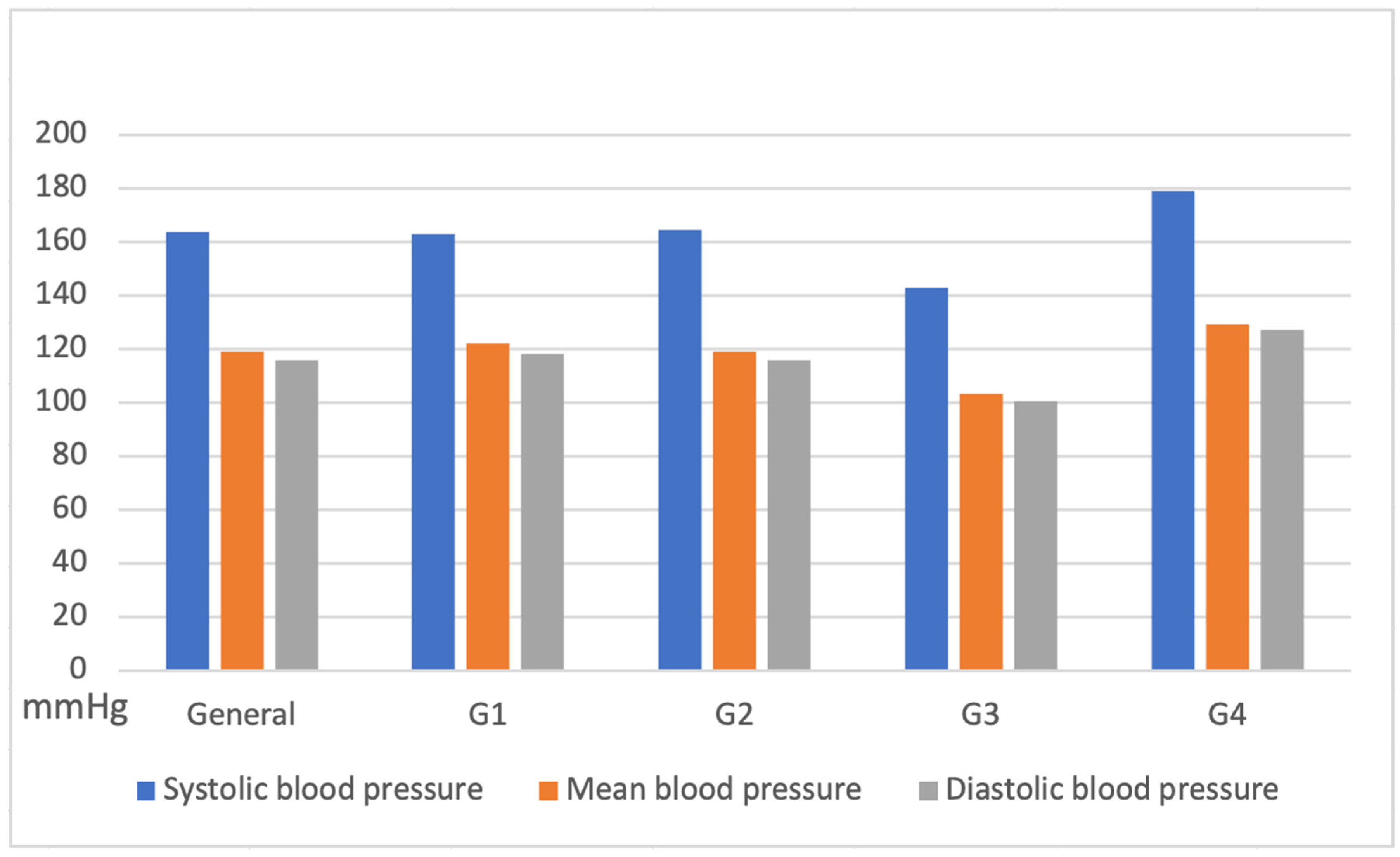
Non-invasive systemic blood pressure remained stable across all groups during the procedure, with systolic pressure values (163 ± 29 mmHg), diastolic (116 ± 53 mmHg), and mean (119 ± 77 mmHg) (
Figure 7).
In the echocardiographic evaluation, ventricular morphometry data indicated no significant variation in the left ventricular internal diameter between males and females, nor in relation to the weight range of the individuals studied. A larger left ventricular diameter was found among G1 and G4, associated with an average fractional shortening of 25% and an average ejection fraction of 48% in the jaguars (
Table 5).
As shown in
Table 6, specimens from G1 and G4 had higher atrial and aortic morphometric values in the two-dimensional mode. In the volumetric evaluation of the left chambers using the biplane Simpson method, the left ventricular ejection fraction of all groups showed higher values in the apical two-chamber window compared to the apical four-chamber window. However, the left atrial ejection fraction showed higher values in the latter window compared with the apical two-chamber (
Table 7).
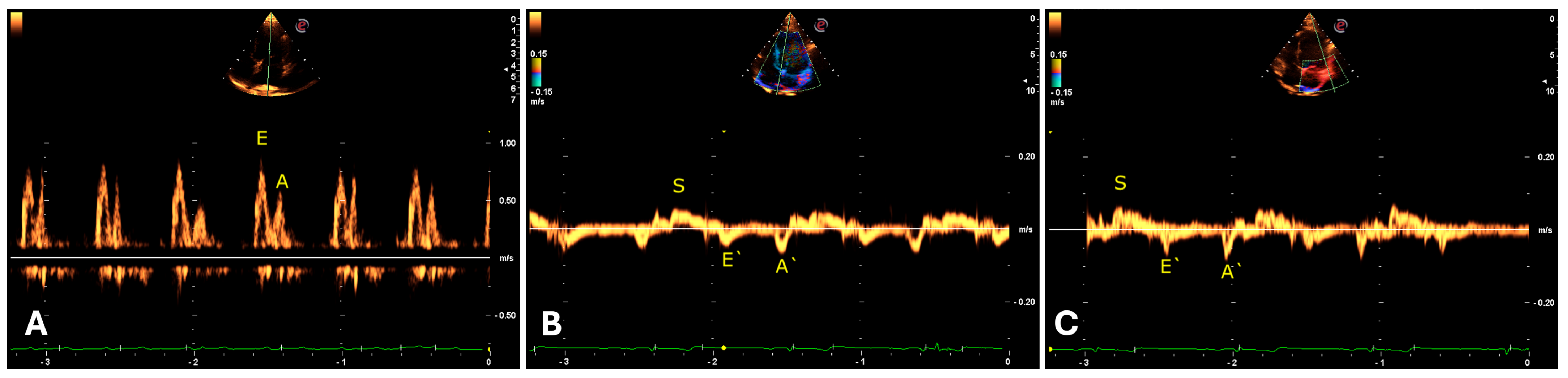
Regarding the transmitral flow measured by the pulsed Doppler method, an E wave pattern with greater amplitude than the A wave was identified (
Table 8). In the evaluation of the mitral septal and parietal valve annuli using tissue Doppler, an E’ wave pattern smaller than A’ was observed, suggesting an abnormal type II (pseudonormal) ventricular filling pattern (
Figure 8). The same patterns were observed in the right chambers (
Table 9).
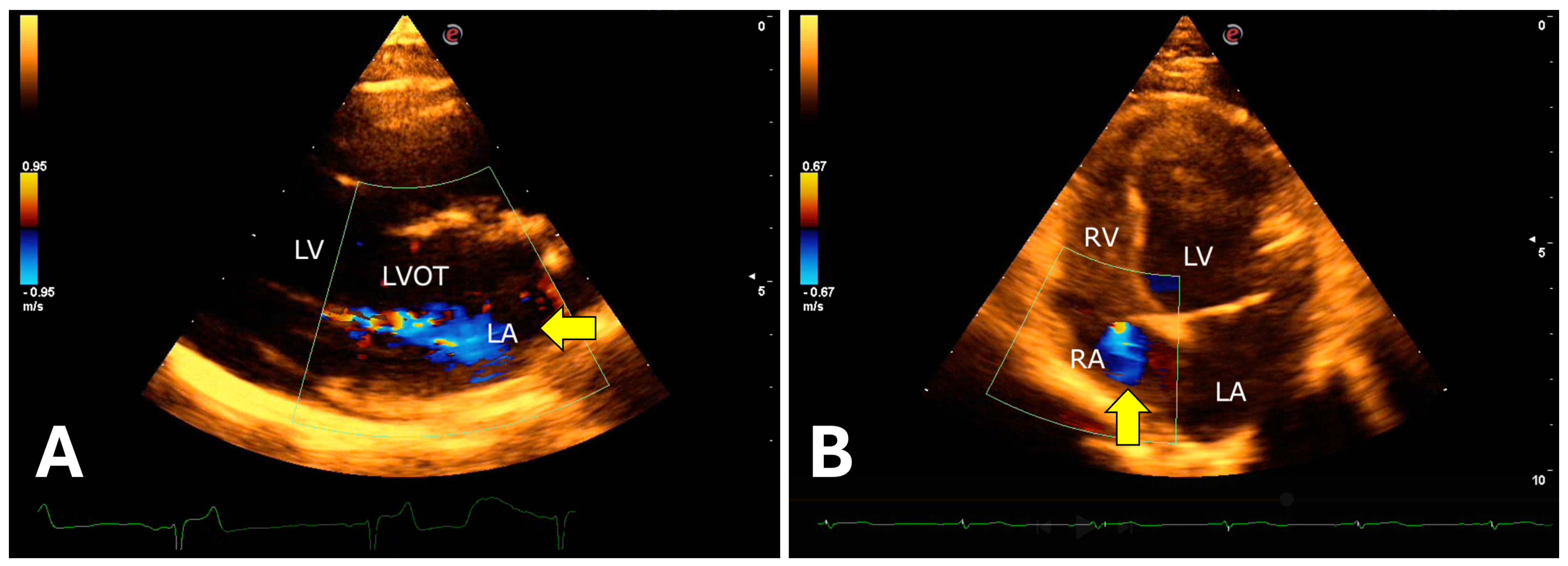
The isovolumetric relaxation time was prolonged, associated with a reduced E wave peak, resulting in a decreased E/IVRT ratio. Color Doppler revealed mitral valve insufficiency (80%), aortic (73%), tricuspid (47%), and pulmonary (13%) in the 15 animals evaluated (
Figure 9).
4. Discussion
The jaguar, the largest feline in the Americas, requires chemical restraint for safe handling, both for the technicians and the animal, even when kept in captivity. In free-ranging jaguars, where environmental variables and field conditions are unpredictable, it is crucial that chemical restraint is rapid and that the animal’s recovery from anesthesia is efficient [
31,
32]. Unlike companion animals, where complementary tests are usually performed before anesthesia, this is more challenging in jaguars. The weight of captive animals, used to calculate the anesthetic dose, is often based on the last restraint, which may not reflect the current weight. In free-ranging jaguars, weight is visually estimated, which can result in anesthetic overdosing or underdosing. Therefore, it is necessary to use anesthetic protocols with a wide safety margin, reduced volume, and the ability to be quickly reversed after handling.
One of the main anesthetic protocols for restraining jaguars, both in captivity and in the wild, is the combination of tiletamine and zolazepam [
33,
34,
35,
36,
37,
38,
39], presented in a lyophilized form, facilitating the manipulation of drug concentration. However, a significant disadvantage of this protocol is its high cost and the lack of a reversal agent. Currently, the combination of medetomidine and ketamine is widely used in captive and free-ranging jaguars [
13,
16,
20,
40,
41,
42].
This study is pioneering in investigating the pharmacological effects of the medetomidine and ketamine combination on the cardiological parameters of healthy captive jaguars. The results demonstrated the safety of this protocol for the reproductive management of the specimens, contributing to ex situ conservation. Additionally, the study enriches the database on echocardiographic, conventional electrocardiographic, Holter, and systemic blood pressure parameters, serving as a model for evaluating potential subclinical cardiovascular diseases in this species. According to Napier et al. [
43], in a previous study with leopards, the cardiovascular system was identified as the fourth most common organ system associated with mortality, and the presence of low-grade murmurs is common in felids, including young individuals.
During the evaluation among the groups (G1, G2, G3, and G4) in this research, no significant differences were identified in the animal segmentation criteria, nor adverse effects such as hypothermia, hypotension, emesis, and myoclonus during the induction period until the medicated reversal, as mentioned by Sinclair [
15].
Oscillometric systemic blood pressure measurement showed no significant variation in jaguars, as described by Caramalac et al. [
44], who also reported episodes of arrhythmias in pumas anesthetized with medetomidine and ketamine associated with propofol. This study observed 35 episodes of premature complexes of supraventricular origin and 17 isolated and monomorphic premature ventricular complexes, with four events of ventricular bigeminy in two different specimens.
Additionally, the average PR interval duration in animals from groups G1 and G4 exceeded 140 ms, characterizing first-degree atrioventricular block (AVB) compared with reference values in dogs [
45]. Oliveira et al. [
46] also observed first-degree AVB in pumas (
Puma concolor) anesthetized with sevoflurane or isoflurane. Six animals in this study exhibited a second-degree atrioventricular block, Mobitz type II, possibly due to increased vagal tone induced by the drugs. Holter electrocardiography parameters rMSSD, pNN > 50%, SDNN, SDANN, and SDNNIDX suggest greater heart rate variability, indicating parasympathetic autonomic nervous system activity.
In electrocardiography, there was an increase in PR and QT intervals compared with domestic cats, inversely proportional to heart rate [
26,
47]. In Holter electrocardiography, elevated mean NN values were observed, also inversely proportional to heart rate.
In tigers subjected to the same anesthetic protocol, Smith et al. [
48] reported ST segment elevation and/or depression, increased T wave amplitude, and T wave polarity changes. In the present study, no significant changes were observed in these parameters, corroborating previous studies that did not identify significant adverse effects in tigers [
49].
The evaluation of cardiac chambers using the echocardiographic technique recommended in dogs and cats by Thomas et al. [
28] and Boon [
29] is suitable for investigating possible cardiopathies in jaguars. In this study, the application of color Doppler revealed mild mitral and tricuspid valve insufficiency, as well as aortic and pulmonary insufficiency, which have been described in other felids anesthetized with the same protocol [
50,
51], similar to what is observed in domestic cats [
52]. However, Chai et al. [
50] did not attribute these findings to the anesthetic protocol itself. The identified valve regurgitations may have a subclinical nature, considering the technical limitations of echocardiographic evaluation in wild felids.
Regarding the mitral and tricuspid valve apparatus, no morphological or echogenicity alterations were observed, similar to what occurs in degenerative processes in dogs [
29]. The aortic and pulmonary semilunar valves also showed no signs of structural alterations, although macroscopic and histopathological analysis could provide greater detail, which is not the focus of this study. Despite the valve regurgitations, no signs of hemodynamic repercussions were observed in the specimens studied.
According to Wang et al. [
53], in healthy dogs medicated with dexmedetomidine, there was an increase in left ventricular internal diameter during the systolic and diastolic phases due to the drug’s vasoconstrictor effect, with reduced ejection and shortening fractions, as well as significant mitral valve insufficiency. These findings partially validate the hemodynamic data of this study, which also observed valve insufficiency in jaguars anesthetized with ketamine and medetomidine. However, due to the wild nature of the animals and the need for chemical restraint for handling, obtaining baseline values is limited, preventing observation of increased left ventricular size. The echocardiographic study of tigers anesthetized with medetomidine, ketamine, midazolam, and isoflurane [
51] indicated similar left chamber morphometric values to those obtained in jaguars.
Data obtained from pulsed Doppler in transmitral flow (E and A) and tissue Doppler in the septal and parietal valve annulus of the left and right ventricles (E’ and A’) exhibited an abnormal type II (pseudonormal) ventricular filling pattern differing from healthy and non-anesthetized domestic species [
29]. This finding may be related to the anesthetic protocol’s effect, resulting in a longer E wave deceleration time (DT) in the echocardiogram, inversely proportional to heart rate, in addition to increased ventricular filling pressures observed in transmitral flow. Carvalho et al. (2019) documented similar results in domestic cats subjected to continuous dexmedetomidine infusion.
Volumetric values obtained using the biplane Simpson method were equivalent to those described in domestic animals [
29,
54]. Volumetric measurements of the atrium and left ventricle are important for monitoring cardiovascular diseases, such as mitral valve disease and dilated cardiomyopathy in dogs, being preferable to the Teichholz method due to its greater sensitivity [
54]. Lower ejection fraction values were observed when compared by the Simpson and Teichholz methods; the second technique may underestimate these values due to its segmental analysis.
For many years, tiletamine and zolazepam (Zoletil, Virbac, Franca ou Telazol, Zoetis, Spain) have been recommended as the protocol of choice for jaguars, both in veterinary practices and field research, especially in reproductive evaluations [
36]. The growing need for information on jaguars and the development of reproductive biotechnologies have encouraged the adoption of new anesthetic protocols, which offer more advantages for the veterinary team and animal health [
13,
16]. These advantages include cost reduction, the use of anesthetic reversers, and rapid anesthetic recovery, allowing animal monitoring until complete recovery.
It is important to highlight some limitations of this study. The small sample size limits the establishment of reference standards, as individual variations have a greater impact on a small sample. However, as it involves research with wild animals, the reported sample size is larger compared with isolated reports and other similar studies. Additionally, the interpretation of echocardiographic evaluation data under the influence of anesthetic drugs, which impact cardiac physiology, should be made in the context of the effects promoted by the drug combination used.
In summary, the morphometric echocardiographic data obtained indicate a cardiovascular response in jaguars similar to that observed in domestic cats and wild felids subjected to the same anesthetic protocol. The tissue Doppler pattern, indicating altered ventricular filling, may be associated with the vasoconstrictor effect of the drug in jaguars.