Abstract
The Rio Pescado stubfoot toad (Atelopus balios) is a critically endangered member of the struggling Atelopus genus which has been ravaged by the fungal pathogen Batrachochytridium dendrobatidis. Captive management of this species is crucial to improve survival outcomes in their native range in Ecuador. Here, we talk about reproductive successes with A. balios at The Dallas World Aquarium, which represents the first successful reproduction of this species outside of Ecuador. We document five clutches of eggs that were deposited in the adult breeding tank and the developmental rates of the offspring. We also provide observations on husbandry regimes that support the recovery of the females’ postdeposition along with observed time between reproductive events for females. We also document the habitat requirements of the adults of the species and the offspring with notes on feeding practices once toadlets emerge from the water. Documentation on the reproduction of Atelopus species is critical to understanding habitat preferences and cues needed to influence reproduction in the wild and thus give insights into the path of captive propagation in the conservation of the focal species.
1. Introduction
Species within the diverse and mysterious genus Atelopus exist throughout the tropics of Central and South America. Made up of approximately 113 species, Atelopus exhibit remarkably reserved genetics with limited phylogenetic differentiation [1], leading to the development of two major clades, the Andean–Choco clade and the Amazonian–Guianan clade. These clades are made up of many species complexes that group populations with diverse physical appearances but lack the ecological data and genetic information to cleave species from them [2,3], although certain groups have been separated from one another on the basis of morphology [4]. Species within this historically megadiverse genus have suffered major population declines in the last 50 years. All of the highland species populations are in decline, with 75% of species disappearing from the wild and 38% of lowland species disappearing in the last 20 years [5].
Many factors have been proposed to explain this mass die-off; climate change, human encroachment, and invasive pathogens have all been pointed out as contributing causes [6,7,8,9]. Understanding the ecology and natural history of this genus is made difficult by our lack of knowledge regarding the divergence of species within the genus and the lack of observation in the wild. Many of the members of this genus are in need of ex-situ reproductive efforts to ensure the species do not go extinct [10,11].
The Rio Pescado stubfoot toad (Atelopus balios) is a critically endangered species of tropical anurans native to only a few lowland valleys in southwestern Ecuador [12]. This is a smaller species with SVL measuring 30–38 mm in males and 40–50 mm in females and is physically distinguishable from other Atelopus species by the light green skin covered dorsally with small black spots (Figure 1). Although it was thought to be extinct just two decades ago, the discovery of relict populations has provided an opportunity to preserve this species by creating ex-situ breeding populations to increase the total population size. It is normally found near streams running through tropical lowland forests from 200 to 460 m above sea level [13]. This species is threatened by human encroachment and changing biotic factors such as climate, air quality, and pathogen infections, of which their primary threat is the widespread chytrid fungus, Batrachochytridium dendrobatidis (Bd) [14], which leads to chytridiomycosis. Due to their nature as a riparian dwelling species and Bd zoospores being transported through water sources, A. balios is at particularly high risk from this pathogen, and comparative investigations into population densities before and after outbreaks highlight the vulnerability of Atelopus species to this invasive pathogen [15]. As a critically endangered species, captive management has become a priority for all institutions in order to provide a genetic reservoir through captive breeding programs. When developing care guidelines for a species, it is important to look at all available literature to investigate the factors that have led to the evolution and survival of the species. This publication, focusing on an adult group of Atelopus balios housed at The Dallas World Aquarium (DWA), is meant to serve as a first step, an all-inclusive guide to keeping and successfully reproducing Atelopus balios. This publication and others like it [16] exist to aid in the captive husbandry of rare amphibians, and they are a key to moving the role of zoos and aquariums towards a more functional and scientific end [17,18].

Figure 1.
Sexual dimorphism. Atelopus balios is easily sexable, by comparative size, with females being two or three times the mass of a male and a snout-vent length (SVL) up to 50% longer than males.
2. Materials and Methods
2.1. Habitat
2.1.1. Enclosure Dimensions
Depending on the sex ratios of the population you will be working with, the dimensions of the housing enclosure must meet certain criteria. Each housing enclosure should have 10–15 gallons of space per frog. This will increase areas for foraging, decrease instances of aggression between specimens, and provide plenty of areas to hide and deposit eggs. For a group of four frogs, irrespective of sex, a 40-gallon breeder (36″ × 18″ × 17″) should be the minimum tank size provided. The stream section of the enclosure should run the length of the enclosure with a depth of about 4–6″. There are many ways to increase usable space for the animals within the same enclosure, such as constructing a background that is able to be planted and filling the empty space with driftwood or other wooden elements that allow for climbing space.
2.1.2. Substrate
Atelopus balios inhabit the areas near lowland mountain streams on the western side of the Andes Mountains in Ecuador [12]. The high abundance of moss growth on the rocky borders of these streams can be attributed to moisture from the streams and stable climate variables. These mossy stones serve as the call sites for males year-round and breeding sites where females return during the breeding season. At DWA, we provide our breeding group with various rocky structures and fallen logs covered in moss bordering a constructed stream (Figure 2). In the back part of the enclosure, we offer a flat moss substrate covered in leaf litter to serve as shelter for the microfauna species that clean the waste and fungus in the tank. These microfauna also serve as an intermittent food source and encourage natural foraging behavior. Giving variable heights and types of substrates also allows the Atelopus to choose areas of optimum temperature and moisture. Pea gravel, river rocks, cork bark, leaf litter, and live mosses are all great options to use as substrates and physical elements of the captive enclosure for this species.

Figure 2.
The large stream enclosure served as the first male–female introduction site. A large filter pumps the water left to right with multiple ramp-like areas to access the stream and heavy vegetation for shelter and call sites.
2.1.3. Water Parameters
The lowland streams running through the forests inhabited by Atelopus balios are mountain run-offs with an elevated pH of ~7.7 and fairly low temperatures between 17 and 20 °C [19]. Holding areas for this species should have a stream of moving water with high filtration, allowing adults to descend to the water to lay eggs. Normal room temperatures will keep the water within the ideal parameters in the enclosures. Obtaining the proper pH of ~7.7 is the hardest parameter to adjust for in a closed captive enclosure without introducing complex chemical compounds, which may create more problems than it fixes. Sticking to these parameters is important for the health of the adults but really becomes important for the progeny of the captive adults, both eggs and tadpoles. Because Atelopus balios inhabit areas around fast-moving streams, dissolved oxygen (DO) content in the water must be high, both for adults preparing to reproduce and for tadpole health.
2.1.4. Vegetation
As stated in the introduction, A. balios inhabits areas of high vegetative cover, so their enclosures should be heavily planted, offering hiding places, perching areas, and spaces for foraging. Assorted Philodendron spp. on the ground and in wood decorations within the enclosure will allow the specimens to avoid each other if necessary and to hide upon the leaves when they feel threatened. Large creeping vines like the Pothos spp. or Monstera spp. will produce nice foraging areas and light cover. Adding logs, cork bark, or driftwood will provide a more vertical area for plants to grow and add cover for the Atelopus.
2.1.5. Environmental
Light
When considering lighting for any ectothermic organism, four main factors must be taken into account: heat/temperature, brightness, UV application, and photoperiod. Atelopus balios naturally inhabit lowland valleys near streams in southwestern Ecuador, where ground-level ecosystems are exposed to limited, if any, direct sunlight. Because its home range is so near to the equator, a photoperiod of 12-h-on/12-h-off is ideal year-round for the species. While some Atelopus species inhabit, at least seasonally, riparian environments that are not provided complete solar cover, most species inhabit areas with high cover and low levels of direct sunlight or radiation. Female Atelopus balios are known to move away from streams after breeding and settle in the leaf litter of the surrounding forest, another habitat with low direct light exposure. When considering UV lighting, it must be remembered that Atelopus balios are generally located in environments where they are sheltered from direct sunlight and can be spared the negative repercussions of UV radiation. The negative effects of lack of UV radiation can be offset by providing food items that are supplemented or gut-loaded, which will allow the animals to obtain cholecalciferol (Vitamin D3) for use in skeletal development.
Brightness can be altered in a few different ways with varying success. Low-intensity lights are one avenue that allows the cautious Atelopus to feel comfortable venturing out to forage and explore. This does, however, present an issue with plant growth, which is an important factor for healthy behavior in this species (See Section 2.1.4). The other way the light intensity of an enclosure can be manipulated is to make it heavily planted. Making use of LED lighting is ideal due to high light intensity, natural light spectrum, and low emission of excess heat. An enclosure that is adequately planted not only provides cover from these high-output lights to allow specimens to choose their exposure but also provides microclimates and hiding spots to reduce unfavorable interactions and, again, provides comfort for the animal.
Temperatures in the tropical lowlands of southwestern Ecuador are fairly stable and range from around 30 °C to 22 °C throughout the year [20]. Warmer average temperatures are associated with higher rainfall and humidity in the area and can be used to manipulate the breeding activity of Atelopus balios. These temperatures are quite a bit higher than optimal temperatures of other tropical species of anurans and can be readily achieved by introducing an infrared (IR) heat emitter above the enclosure.
2.1.6. Humidity and Water
Water vapor and precipitation are major factors in most amphibians’ breeding cycles. Humidity should vary during breeding cycle stages with some significance as the rainy season in southwestern Ecuador consistently brings high levels of humidity to the area. The return of high humidity levels, along with a drop in air pressure to the environment, precludes an increase in rainfall and triggers most anurans into reproductive mode. Some Atelopus species have peculiar cycling needs and are more reliant on a dry period to trigger breeding behaviors [21]. Keeping a humidity level around 40–50% during the dry season for A. balios mimics the air moisture in their home range and ensures that they will not dry out or be uncomfortable. Because A. balios inhabits environments near streams, it is beneficial to replicate a stream in their enclosure which will add to humidity and can also be used to trigger breeding activity and act as a deposition site for eggs. Humidity can be maintained at 50% by hand-spraying, but for ease of use and consistency, a misting system (MistKing, Jungle Hobbies Ltd., Windsor, ON, Canada) can be used and set to low frequency and duration of activity (2× at 20 s per day). The major use of the system lies when humidity is increased to ~100% during the rainy season simulation. This resting humidity of 50% will also benefit the plants in the enclosure and the microfauna to be used in enclosures of tropical fauna. An increase in humidity and misting will trigger the Atelopus to seek out mates and enter into amplexus. Closing the open areas of the top of the enclosure and misting more frequently will quickly raise the humidity and the effect on the frogs should be quickly noticeable. If you are choosing to replicate the separation of male and female specimens, which occurs naturally as females move into the nearby forests, placing the females in with the males just before you raise the humidity would be the most beneficial course of action; see more about this in the Section 2.2. As you keep the humidity high, misting more frequently while keeping an eye on the plants, make sure that the frogs can find a dry area to escape the constant moisture and threat of bacterial infection. Be sure to watch for a return to foraging behavior as a signal to return to a dry season of the cycle to make sure that females are not gravid and at risk of being egg-bound.
2.1.7. Temperature
Temperatures in the native ecosystems of Atelopus balios are very stable, ranging from 22–30 °C year-round. These temperatures are high for most other groups of frogs from the tropics and because Atelopus are a delicate genus of frog, caution must be taken when altering temperatures in enclosed spaces such as glass or acrylic enclosures. In conjunction with lighting, the use of ceramic heat emitters can aid in producing the normal temperature range of the species. The temperature should be checked daily to make sure that it is stable and not putting stress on the Atelopus. Keeping habitat enclosures between 23 and 29 °C provides a stable environmental temperature that will allow natural behavior in the specimens. Water does not need to be heated since the streams where this species comes from are much colder because they descend from high mountain peaks.
2.1.8. Microfauna
Vivariums for Atelopus must be constructed with the goal of creating a complete environment for the health of the species. A key factor in this endeavor is to add various microfauna to aid in waste management, fungal control, and nutrient supplementation for the inhabitants. Various species of these microfauna, such as springtails (Genus: Collembola) (Figure 3) and isopods (Order: Isopoda), are available for sale online and in reptile specialty stores. There are two ways to introduce these animals, and for the best results, doing both is highly recommended. Firstly, seeding the enclosures with microfauna before you add the Atelopus species allows the isopods and springtails to colonize the enclosure and will keep the vivarium clean and create a more stable environment inside your tank. The other strategy is to start cultures of these microfauna outside of the enclosures, which allows you to add them to the enclosures as you deem necessary. Both of the strategies will individually benefit your animals, but using both is the best way to achieve prolonged tank and microecosystem health.

Figure 3.
Springtails after being harvested from their colonies. Flooding the colony with water allows for the easy harvesting of the springtails for application to the enclosure or to provide food for the toadlets.
2.1.9. Diet
Prey Items
As with most ex-situ populations, Atelopus balios benefits from a varied diet supplemented with a vitamin regime to ensure total nutrition for the animals. Food should be offered every day with a light supplementation of vitamins added to prey items three times a week. Since we have been keeping this species, we have noticed that adults display a high affinity for coleopteran species such as bean beetles (Callosobruchus maculatus), flour beetles (Tribolium spp.), and skin beetles (Dermestes maculatus). Cultures of these species, as well as various others, are readily available and easy to maintain in a 32 oz container. A. balios also display a strong feeding response to fruit flies (Drosophila spp.) with D. hydei preferable to D. melanogaster due to their larger size making them better nutritionally per individual. Adult A. balios seem to be apprehensive about responding to one-eighth-inch common house crickets (Acheta domesticus) and are more interested in pinhead (PH) or 10-day-old A. domesticus, although they do not have the same response as they do towards the Coleopteran spp. or Drosophila spp. This caution may be due to human error in the manner in which prey items are added to the enclosure. Wild-caught termites (Isoptera) are a favorite of the A. balios at DWA and a great superfood to get any amphibian species primed for the breeding season but they should be offered in a container to ensure they are not loose in the enclosure.
2.1.10. Schedule
As with most species in captivity, Atelopus species can quickly become overweight if the keepers do not pay close attention to the feeding schedule. At DWA, our feeding schedule is tied closely to the reproductive cycling in the adults and is sustained constantly for the offspring. When the adults are outside breeding mode, females are offered 15–20 PH crickets or Drosophila spp. per frog with available Coleoptera species mixed in every two days. Our males receive a similar diet of 10–15 PH crickets per frog with added Coleoptera spp. every two days. This diet provides both groups with the ability to maintain healthy body conditions while gaining the nutrition to prepare them for the breeding season. Caution must be taken to avoid overfeeding, which can lead to many known health issues, such as systemic diseases leading to decreased lifespan [22] and possibly unknown or undocumented issues. A decrease in reproductive health due to obesity and a sedentary lifestyle in reptiles and amphibians has been observed by caretakers in zoos (pers. comm) but has not been directly investigated.
Application of the food item is also very important. Providing the prey item in a single area can decrease the amount of natural foraging behaviors of the Atelopus. Therefore, scattering the insects in the enclosure will make sure that the animal cannot just sit in a place and become engorged unless that is the objective of the care regime of the specimen in question. Enticing natural behavior must be a long-term priority in the captive management of any animal. Feeding stations, which in this case are set up as mini breeding colonies for fruit flies, can be set up conditionally for specimens under quarantine, medical observation, or postpartum females to ensure these at-risk specimens are able to access nutrition more readily to improve their condition.
2.1.11. Supplementation
The choice of supplementation for the species is a key variable. In other ex-situ populations of Atelopus, complications from vitamin deficiencies or improper diet were linked to the cause of death in a majority of both short- and long-term captive residents [23]. Captive amphibians regularly suffer from deficiencies in Vitamin A, Vitamin E, and Vitamin D3, which can lead to necrosis of the reproductive, cardiovascular, neurological, and skeletal systems in amphibians [24,25,26,27]. These issues are mostly attributable to deficiencies in the natural nutritional value of captive feeder insects [22]. All feeder insects need supplementation to fill these nutritional gaps and there are a wide variety of supplements to enhance feeder insects. At DWA, we supplement with a host of multivitamins for reptiles and amphibians, which together, fulfill all of the shortcomings the feeders have.
There are two main types of supplementation that can be used to improve the nutrition of feeder insects: dusting and gut loading. When dusting, feeders normally must be consumed quickly (within 20 min) since the insects will begin to clean themselves once finding a perch. Scattering the supplemented feeders near the Atelopus will ensure they get full nutrition if they choose to engage. Crickets and fruit flies are supplemented with every other feeding to avoid hypervitaminosis. Gut loading is simply the provisioning of supplementation for consumption by the feeder insects so that these supplements are consumed and made available to the amphibians. This can be equally important as dusting in instances where some Atelopus do not immediately react to food being provided and may then miss the dusted prey items.
2.2. Breeding
2.2.1. Group Dynamics
Atelopus balios groups in captivity function best when separated by sex, with males staying in the breeding tank and females moved to a tank with leaf litter and plant cover. While separated, a long period of heavy misting should follow with the group being reunited after misting has been reduced to a minimum and feeding events are increased. This follows the same pattern of natural movements and creates an urgency to reproduce when males and females are in the same stream-side enclosure at the beginning of the dry season [28]. A breeding group of A. balios can be kept in a well-planted paludarium-style tank, having adequate space to forage, hide, and lay eggs in the stream. Male-on-male aggression is documented in the presence of females or the part of the cycle simulating the dry season but is generally nonexistent by the groups of males otherwise, especially when given lots of foliage. Females can exist together indefinitely with very little, if any, aggression.
While male–male aggression is not physically threatening to the health of the combatants, it can be detrimental to the stress it exerts on the frogs, which can lead to negative physiological effects. Persistent aggression may cause males to avoid other intra-specific interactions. When the breeding group is separated into male and female groups, following the pattern of safe exhibit construction by placing both groups into large, planted enclosures can diminish the probability of direct interaction between the frogs.
The primary concern with captive reproduction of endangered species is maintaining healthy genetic variation within the satellite populations. Identifying individuals in the collection using dorsal spotting patterns and documenting offspring as they emerge from the water, which may serve as potential mates to produce the F2 generation, is critical to maintaining this genetic diversity.
2.2.2. Cycle
It is well known amongst caretakers that most tropical amphibians rely on specific cues from the environment to begin breeding behaviors. Atelopus balios have a peculiar environmental cue in that the females leave the breeding areas at the outset of the wet season to take refuge in the leaf litter of the surrounding forests. Females swell with eggs when they are ready to breed and this change is something that can be readily observed given enough experience (Figure 4). When the climate begins to dry out and heat up, the females return to the streams after feeding on the insects that emerge after the rains and are met by the males. This sort of reverse cycling, where the onset of lower precipitation and warmer temperatures trigger a breeding response (Table 1), has been reported in other Atelopus species [21,29] but is considered a rare trend among neotropical anurans.

Figure 4.
Female A. balios with egg mass visible from the dorsal side of the animal. This egg mass developed after 1.5 months of heavy feeding on a diverse array of prey items on leaf litter.

Table 1.
Environmental parameters for naturally cycling Atelopus balios.
Using a ceramic heat emitter can bring the high temperatures up from 26 to 29 °C, and shutting off the MistKing will keep the humidity in the proper range of 40–50% (Table 1). Males participate in mate guarding, jumping into amplexus long before an actual breeding event (Figure 5A,B). Females will continue to feed during this time but will hold off on feeding when they are close to laying eggs. After the eggs have been deposited, the male will detach, and they will go their separate ways. At this time, the female can be removed and placed in the leaf litter tank to be fed and recover with a light rainy season. Recovery time can fluctuate but should be substantial enough to relieve stress on females and to allow males time to build body reserves after being off scheduled feeding while in amplexus.
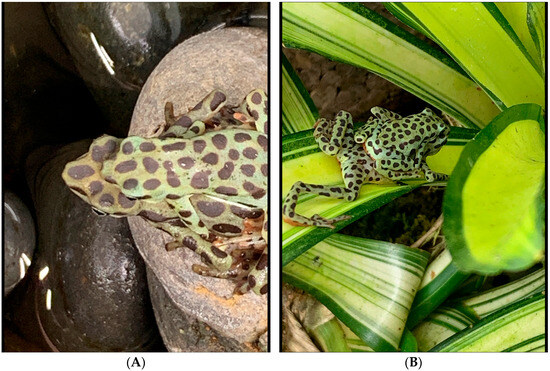
Figure 5.
Two amplectic pairs of A. balios. (A) Identifying the spot patterning can help to determine the parents of clutches. The comparative size difference between males and females of this species is evident. (B) Pair seen with the female foraging in the large stream enclosure.
2.2.3. Egg Deposition
Many male specimens of Atelopus species participate in mate guarding, where males cling to females long before she is ready to deposit eggs. This guarding endeavor can last for weeks or even months. What we have observed here at DWA is that as females near oviposition, carrying their attached male, they begin to spend more time in the water, even submerging for several minutes (Figure 6). This behavior may be females searching for an oviposition site or perhaps a way to trigger the female to prepare to deposit her eggs physiologically. Atelopus balios deposit 150–600 eggs (Figure 7) in typical Bufonid strings, which fall within the broadly variable observed clutch sizes within the genus Atelopus [30]. Eggs in this species are approximately 1 mm in diameter (Figure 8B). Atelopus balios deposit these eggs in sheltered areas within the streams, sometimes even within the rocky bottom of the stream. Once the eggs are deposited, the male will usually separate and leave the water, although sometimes the male holds in amplexus until after they leave the water (See Section 3).
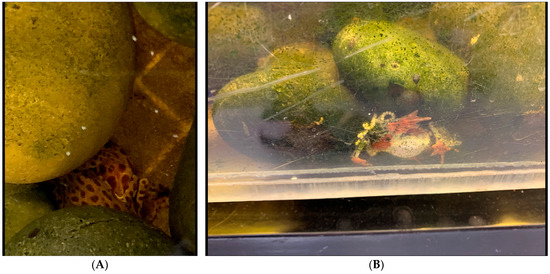
Figure 6.
(A) A pair of A. balios depositing their eggs underneath a small rock cavern on the bottom of the stream. (B) beginning of deposition process. This pair stayed submerged for over 6 h and deposited over 250 eggs.
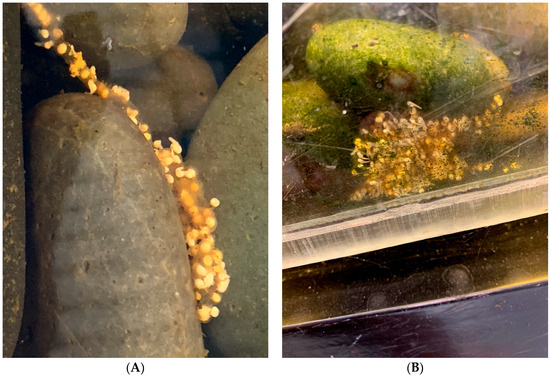
Figure 7.
Development of clutches 2 and 3 (A) Clutch 2, laid in the small auxiliary tank on 5th day after deposition with fertilized embryos at stage 19 [31]. (B) Clutch 3, six days after being deposited in the large stream tank at stage 21 [31].
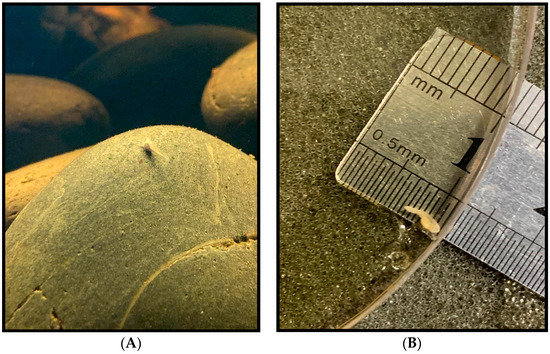
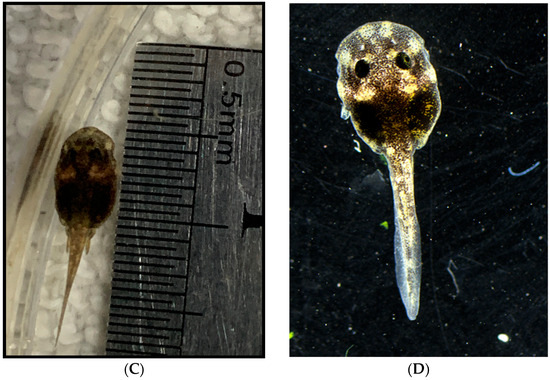
Figure 8.
Tadpole development stages. (A,B) Just after emergence from the egg at stage 22, with Total Length (TL) at under 4.0 mm. (C) 26 days after emergence, stage 25 and TL at 8.5 mm. (D) 97 days after emergence at stage 36 with TL measuring 13.0 mm. Pigmentation change is apparent, and digits on lower limbs are slightly visible.
At DWA, we have observed and partially recorded a pair depositing eggs fully submerged in water for over 6 h, indicating that gas exchange through the skin can be heavily relied on for respiration in this species. In the clutch deposition that was observed, (Clutch 3), the pair wedged themselves underneath the rocks on the bottom of the stream in the enclosure. The strings of eggs were placed on the bottom side of a rock that created a cavity in the stream (Figure 7A,B). We believe this cavity provides cover from light as well as protection from rapid stream torrents while still allowing for water flow. We suspect that the eggs of Atelopus balios are both flow- and light-sensitive (Pers. Obs.). Clutches near the surface, exposed to light, seem to culture fungi within a few days after deposition (See Section 3). It also seems that eggs with limited exposure to water flow (Perhaps low DO %) culture fungi, which generally will not spread to the rest of the clutch if water flow is increased. For these reasons, we recommend offering multiple ideal egg-laying areas for the successful emergence of tadpoles from deposited eggs of this species.
2.2.4. Embryo and Tadpole Development
A description of the tadpole of Atelopus balios at various stages has been done in great detail [19], but such a description of the larval development of this species with a corresponding timeline in all Gosner stages of development (GSD) [31] has not yet been done. In fact, to date, very few descriptions of the timeline of larval development of any Atelopus spp. exists in the literature; however, Gawor et al. (2006) designed a great table of the timeline of larval development of Atelopus flavescens. The development time for A. balios species ranges from 115 to 200+ days (Table 2), which reflects the wide range of development times seen in other Atelopus species [16]. The cause for the differentiation of metamorphosis rates in clutch mates has been investigated before in other anurans [32], where genetic predetermination, epigenetic shifts, and environmental manipulation have been proposed as possible explanations. Phenotypic plasticity has been accurately applied to explain this occurrence; however, the specific causes for the variation in developmental times remain unknown and require more investigation.

Table 2.
Description of larval development with the accompanied timeline for Clutch 1 of Atelopus balios at DWA. Ranges of development times for this clutch are too varied to portray neatly in one table so the largest group of offspring were used to attain development rates.
Once the eggs are deposited, development happens quickly with tadpoles rushing through the first 19 stages of amphibian larval development [31] and emerging from the egg in 8–10 days after being deposited. Upon emergence from the egg, the tadpoles are a light beige color, measuring only ~3.5 mm long (Figure 8A). An ontogenetic shift in coloration occurs from a pale neonate to a dark and cryptic tadpole to a brighter adult color phase. Accompanying this shift is the development of a 2/3 labial tooth row formula at Gosner stage 24 that occurs in various other Atelopodids [33,34]. Tadpoles at all stages of development are shy and avoid lit and open areas of the stream. The best chances to observe tadpole development and behavior are early in the morning, within 30 min of the enclosure’s lights coming on and at night with a flashlight, where foraging activity is very high. With the lights on, the tadpoles will hide underneath the rocks in the stream or among the plants in the streams, foraging if they can. After both the hindlimbs and forelimbs have developed (Figure 9D), the toadlets will do trial runs, preparing to leave the water onto leaves, branches, bedding, etc. These tadpoles should not be pulled out until they have lost their tail and can freely move on land. Do not force the tadpoles to leave the water, as it may lead to certain deficiencies and stress in the neonate.
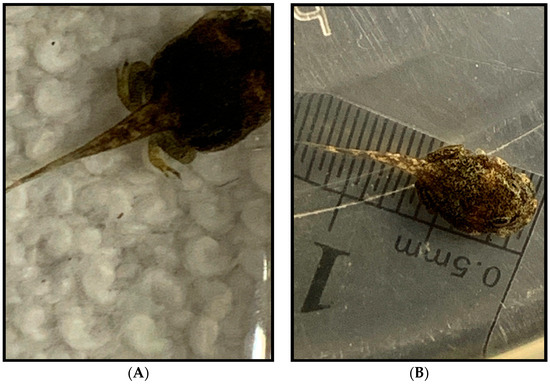
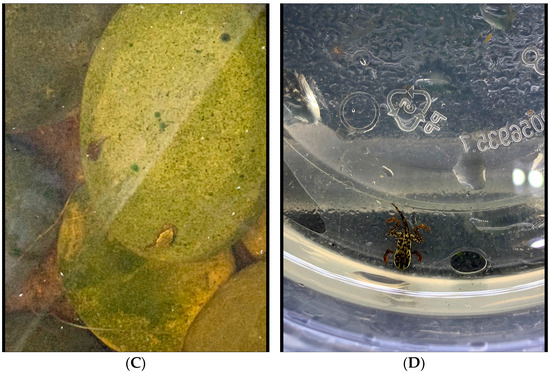
Figure 9.
Tadpole development stages. (A) One hundred days after emergence at stage 38 showing back limbs are highly developed with all digits present. Base pigmentation has remained unchanged from Figure 8D. (B) One hundred five days after emergence at stage 41. A trend toward lighter base color with dark splotches is more evident, and bulges from forelimbs are evident. (C) One hundred fifteen days after emergence from the egg at Stage 42. Color shift is apparent with the naked eye, and emergence from the water is likely less than a week away at this point in development. (D) This individual was found on land in the small auxiliary tank at Stage 45. This is the coloration of the toadlets, which is a more mottled mix of light and dark pigmentation instead of the neat patterning of the adults.
2.2.5. Tadpole Care
Larvae of this species thrive at DO levels above 90% with high levels of water movement and rock crevices to hide in. If DO% is low, the tadpoles will actively move toward the most turbulent part of the stream to access higher levels. From clutches raised at DWA, we have noticed that tadpoles refuse tadpole foods and prepared powdered fish diets, with a preference for naturally cultivated algae, wood, and vegetable matter. Supplemental food items may still be offered to the tadpoles, but caution must be taken to avoid adding too much nitrogenous byproduct to the enclosure. Tadpoles appear sensitive to light and spend most of the day on the hidden margins of the rocks or wood within the stream foraging. We have come to develop great faith in the ability of the tadpoles to exist near drains and outflows without disappearing into these apparatuses due to their stocky bodies and suctioning mouths and bodies.
2.2.6. Toadlet Development and Care
After 110–170 days, metamorphosed toadlets emerge from the water (Figure 10). Upon leaving the water, toadlets measure consistently 5.5 ± 0.2 mm in snout–vent length (SVL) and have a mass between 0.010 and 0.013 g. This diminutive size makes them particularly difficult to care for even after you create an environment with favorable growing conditions. At DWA, our toadlets are divided into groups of 20, sequential to when they emerged from the water. Separating into groups of 20 allows for the allocation of the same amounts of food and makes keeping track of individuals who might need focused care easier. As the toadlets come onto land, they generally begin to ascend the sides of the tank or the nearest vegetation once they emerge. As they emerge, they are collected and placed in 96 oz. clear plastic circular containers (9.75″ diameter 3.5″ depth) (Superior Shipping Supplies). These containers have leaf litter and rinsed charcoal substrate to maintain humidity and provide an environment suitable to culture microfauna as a food source for developing toadlets. Collembola cultures used to seed the adult tanks again come into play, as these grout tubs should all be seeded with enough adult springtails to create reproducing cultures. Humidity should be kept at ~100%, with temperatures ranging from 23 to 27 °C. Toadlets grow very quickly, adding nearly 50% of their SVL in the first two weeks and tripling their mass at emergence in those same two weeks. Such extreme growth rates mean that springtail populations are greatly depleted and are in need of supplementation at least once a week. Once the toadlets grow to about 9 mm, they will be ready to move on to D. melanogaster fruit flies.
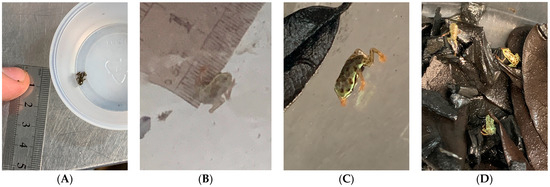
Figure 10.
Toadlet development. (A) Toadlet just out of the water (OOW) measuring ~5.5 mm long. (B,C) Toadlet about 2 weeks OOW, measuring around 7.5 mm in SVL; the same toadlet foraging the grow-out enclosure. (D) Group of siblings around 1 month OOW foraging for food in the growout enclosure. The shift of the color pattern from a mottled black/olive to a more definite adult spotting pattern is evident in only 2 weeks’ time OOW.
3. Observations at The Dallas World Aquarium
Clutch 1: This clutch occurred in the large stream enclosure, likely around mid-April. Deposition of this clutch was not witnessed, and tadpoles were not noticed until 15 May 2019, approximately one month after emergence from the egg (GSD 25). The date of emergence was regressively estimated based on knowledge of the development stage, pigmentation, and general size gained through observation of later clutches. Tadpoles began sprouting back leg buds on 10 June at 8 weeks of development (GSD 26–30) and began to develop hindlimb digits in early July after 11 weeks. On 22 July 2019, the first metamorphosed toadlets left the water.
Clutch 2: The second clutch of around 150 eggs was laid in the smaller auxiliary enclosure on top of the filtration material during the night of 22 June 2019 (Figure 11). The auxiliary habitat comprised three amplectic pairs, with all females being gravid. The deposition of the clutch was not witnessed, but the parents of the clutch were easily determined by witnessing the other two pairs still in amplexus and females bearing eggs. The night the egg strings were deposited, the parents were observed out of amplexus, with the female following the pattern of total submergence prior to releasing the eggs and the male on a branch mid-level in the enclosure. The next morning, when the eggs were observed, both parents were separated, which led us to believe the eggs were unfertilized. These eggs were also laid under direct sunlight just below the water’s surface. The clutch was indeed fertilized, but we believe that due to the combination of constant direct light and partial fertilization, only around 15% of the eggs produced free-swimming tadpoles. Upon emergence, tadpoles were white and measured around 3.75 mm in total length (TL). One month after emergence, these tadpoles had gained their dark color and more developed mouth parts and respiratory apparatuses.

Figure 11.
Clutch 2 was deposited near the surface of the small auxiliary tank behind the circulation pump. We hypothesize that due to light exposure and lack of water flow, this clutch rapidly developed fungal growth.
Clutch 3: This clutch was laid on 5 July 2019 in the large stream enclosure and was the most well-documented of the clutches laid here at DWA (Figure 12). Because deposition of the clutch was observed, the parents are known. This clutch provided the best evidence of total emergence underwater in preparation for egg deposition. The deposition process occurred over 6 h under the large rocks on the floor of the stream. The female appeared to search the bottom of the stream for a preferable site to attach the eggs. Around 250 eggs were laid, fertilized, and positioned on the underside of a small pocket created by the stones in the stream After the clutch was secured, the pair exited the water, and the amplexus was broken (Figure 13). In this location, eggs were hidden from direct light and had high water flow. After eight days, almost all tadpoles emerged from their eggs and swam into the rock bottom of the enclosure. About two weeks later, the tadpoles, now with darker pigmentation, became observable, venturing onto the tops of rocks and the front of the enclosure to take advantage of the high algal growth.

Figure 12.
Clutch 3, consisting of about 250 eggs, after being deposited underneath river rocks in the stream. This clutch had an extremely high hatch rate of about 95%.

Figure 13.
Pair emerging after depositing eggs. After more than 6 h underwater, this pair emerged and separated about 10 min after emergence.
Clutch 4: The 4th clutch was laid on 12 July 2019 in the small auxiliary enclosure in the rocks underneath a log. The parents of this clutch are known and have been the only pair to stay in amplexus following a reproductive event. Although most of the clutch was laid under the log, out of the light, the parts hidden from the light suffered from low levels of water circulation, and some parts were directly exposed to the light, which led to fungal growth. Only around 10% of the tadpole emerged from the eggs on 18 July 2019.
Clutch 5: This clutch was laid on the night of 24 July 2019 in the smaller auxiliary tank. The clutch of 150–200 eggs was laid spread out near the water’s surface and in an area of low water flow. Because clutches laid in these conditions have had high levels of fungal growth and low hatch rates, the clutch was harvested and placed in the larger enclosure with cover from the light and high water flow.
4. Conclusions
The successful captive management and breeding of Atelopus balios has become an important banner for amphibian conservation at DWA and represents an opportunity to build momentum to improve husbandry regimes, conservation practices, and public awareness, which are valuable parts of our conservation goals. Captive care of neotropical amphibians functions best with high attention to detail on diet and environment and low direct interaction or disturbance with the animals. With global amphibian populations continuing to decline as a whole, information on captive management of threatened and endangered species that can be formed into an easy-to-follow guide can serve to expedite the efforts to conserve the species in question.
Coordinating with zoological institutions and in-situ conservation centers with news and updates on breeding success and possibilities for participation in existing conservation programs is key to accomplishing the final goal of conserving the species in question. As stated at the beginning of this manual, zoos are only able to focus on a certain number of amphibian species [35], and so, participation from private facilities and caretakers can fill in as viable conservation options for endangered amphibians as long as certain conservation standards are upheld. DWA will continue to provide manuals such as this one to help highlight key factors in the captive management and reproduction of endangered amphibians.
Author Contributions
Development and daily management of the species in this work by C.B. and L.S. Writing, photos, and extrapolation by C.B. Translation of related works and Introduction by L.D.N.G. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data concerning the subject specimens for this publication are available upon request via the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lötters, S.; Van der Meijden, A.; Coloma, L.A.; Boistel, R.; Cloetens, P.; Ernst, R.; Lehr, E.; Veith, M. Assessing the molecular phylogeny of a near extinct group of vertebrates: The Neotropical harlequin frogs (Bufonidae; Atelopus). Syst. Biodivers. 2011, 9, 45–57. [Google Scholar] [CrossRef]
- Guayasamin, J.M.; Bonaccorso, E.; Duellman, W.E.; Coloma, L.A. Genetic differentiation in the nearly extinct harlequin frogs (Bufonidae: Atelopus), with emphasis on the Andean Atelopus ignescens and A. bomolochos species complexes. Zootaxa 2010, 2574, 55–68. [Google Scholar] [CrossRef]
- Noonan, B.P.; Gaucher, P. Phylogeography and demography of Guianan harlequin toads (Atelopus): Diversification within a refuge. Mol. Ecol. 2005, 14, 3017–3031. [Google Scholar] [CrossRef]
- Lötters, S. On the systematics of the harlequin frogs (Amphibia: Bufonidae: Atelopus) from Amazonia. II: Redescription of Atelopus pulcher (BOULENGER, 1882) from the eastern Andean versant in Peru. Salamandra 2002, 38, 165–184. [Google Scholar]
- La Marca, E.; Lips, K.R.; Lötters, S.; Puschendorf, R.; Ibáñez, R.; Rueda-Almonacid, J.V.; Schulte, R.; Marty, C.; Castro, F.; Manzanilla-Puppo, J.; et al. Catastrophic population declines and extinctions in Neotropical harlequin frogs (Bufonidae: Atelopus) 1. Biotrop. J. Biol. Conserv. 2005, 37, 190–201. [Google Scholar] [CrossRef]
- Cohen, J.M.; Civitello, D.J.; Venesky, M.D.; McMahon, T.A.; Rohr, J.R. An interaction between climate change and infectious disease drove widespread amphibian declines. Glob. Change Biol. 2019, 25, 927–937. [Google Scholar] [CrossRef]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R., III; Sears, M.W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161. [Google Scholar] [CrossRef]
- Whitfield, S.M.; Lips, K.R.; Donnelly, M.A. Amphibian Decline and Conservation in Central America. Copeia 2016, 104, 351–379. [Google Scholar]
- Lötters, S.; Schulte, R.; Córdova, J.H.; Veith, M. Conservation priorities for harlequin frogs (Atelopus spp.) of Peru. Oryx 2005, 39, 343–346. [Google Scholar] [CrossRef]
- Young, B.E.; Lips, K.R.; Reaser, J.K.; Ibáñez, R.; Salas, A.W.; Cedeño, J.R.; Coloma, L.A.; Ron, S.; La Marca, E.; Meyer, J.R.; et al. Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 2001, 15, 1213–1223. [Google Scholar] [CrossRef]
- Peters, J.A. The frog genus Atelopus in Ecuador (Anura: Bufonidae). Smithson. Contrib. Zool. 1973, 145, 1–49. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.0. Electronic Database. American Museum of Natural History: New York, NY, USA, June 2015. Available online: http://research.amnh.org/herpetology/amphibia/index.html (accessed on 16 April 2019).
- Ron, S.R.; Merino, A. Amphibian declines in Ecuador: Overview and first report of chytridiomycosis from South America. Froglog 2000, 42, 2–3. [Google Scholar]
- McCaffery, R.; Richards-Zawacki, C.L.; Lips, K.R. The demography of Atelopus decline: Harlequin frog survival and abundance in central Panama prior to and during a disease outbreak. Glob. Ecol. Conserv. 2015, 4, 232–242. [Google Scholar] [CrossRef]
- Poole, V. Panamanian Golden Frog Husbandry Manual; National Aquarium in Baltimore: Baltimore, MD, USA, 2006. [Google Scholar]
- Gratwicke, B.; Murphy, J.B. Amphibian Conservation Efforts at the Smithsonian’s National Zoological Park and Conservation Biology Institute. Herpetol. Rev. 2016, 47, 711–718. [Google Scholar]
- Gratwicke, B.; Ross, H.; Batista, A.; Chaves, G.; Crawford, A.J.; Elizondo, L.; Estrada, A.; Evans, M.; Garelle, D.; Guerrel, J.; et al. Evaluating the probability of avoiding disease-related extinctions of Panamanian amphibians through captive breeding programs. Anim. Conserv. 2016, 19, 324–336. [Google Scholar] [CrossRef]
- Coloma, L.A.; Lötters, S. The tadpole of Atelopus balios (Anura: Bufonidae) from the Pacific lowlands of Ecuador. Herpetologica 1996, 52, 66–70. [Google Scholar]
- World Weather Information Service—Guayaquil. 2017. Available online: www.worldweather.wmo.int/en/city.html?cityId=2048 (accessed on 19 May 2019).
- Gawor, A.; Rauhaus, A.; Karbe, D.; VanDerStraeten, K.; Lötters, S.; Ziegler, T. Is there a chance for conservation breeding? Ex situ management, reproduction, and early life stages of the harlequin toad Atelopus flavescens Duméril & Bibron, 1841 (Amphibia: Anura: Bufonidae). Amphib. Reptile Conserv. 2012, 5, 29–44. [Google Scholar]
- Ferrie, G.M.; Alford, V.C.; Atkinson, J.; Baitchman, E.; Barber, D.; Blaner, W.S.; Crawshaw, G.; Daneault, A.; Dierenfeld, E.; Finke, M.; et al. Nutrition and health in amphibian husbandry. Zoo Biol. 2014, 33, 485–501. [Google Scholar] [CrossRef]
- Pessier, A.P.; Baitchman, E.J.; Crump, P.; Wilson, B.; Griffith, E.; Ross, H. Causes of mortality in anuran amphibians from an ex situ survival assurance colony in Panama. Zoo Biol. 2014, 33, 516–526. [Google Scholar] [CrossRef]
- Browne, R.; Vercammen, F.; Verschooren, E.; Antwis, R.E. UV-B, Vitamin D3, and Amphibian Health and Behavior. 2009. Available online: https://www.amphibianark.org/wp-content/uploads/2018/08/Amphibian-UV-B-and-vitamin-D3.pdf (accessed on 2 July 2019).
- Densmore, C.L.; Green, D.E. Diseases of Amphibians. ILAR J. 2007, 48, 235–254. [Google Scholar] [CrossRef]
- Donoghue, S. Nutrition of pet amphibians and reptiles. In Seminars in Avian and Exotic Pet Medicine; WB Saunders: Philadelphia, PA, USA; Nutrition Support Services Inc, Walkabout Farms: Pembroke, VA, USA, 1998; Volume 7, pp. 148–153. [Google Scholar]
- McWilliams, D.A. Nutrition recommendations for some captive amphibian species (Anura and Caudata). Can. Assoc. Zoo Aquar. Nutr. Advis Res. Group 2008, 8–9, 15–18. [Google Scholar]
- Lynch, J.D. Notes on the Reproductive Biology of Atelopus subornatus. J. Herpetol. 1986, 20, 126. [Google Scholar] [CrossRef]
- Rocha Usuga, A.A.; Vargas-Salinas, F.; Rueda Solano, L.A. Not every drought is bad: Quantifying reproductive effort in the harlequin frog Atelopus laetissimus (Anura: Bufonidae). J. Nat. Hist. 2017, 51, 1913–1928. [Google Scholar] [CrossRef]
- General, E.; de Patilla, E.D.C. Reproductive ecology of Atelopus zeteki and comparisons to other members of the genus. Herpetol. Rev. 2006, 37, 284–288. [Google Scholar]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Newman, R.A. Adaptive Plasticity in Development of Scaphiopus couchii Tadpoles in Desert Ponds. Evolution 1988, 42, 774–783. [Google Scholar] [CrossRef]
- Lötters, S. Tadpole of Atelopus mindoensis Peters (Anura, Bufonidae) from Northwestern Ecuador. Copeia 2001, 2001, 276–278. [Google Scholar] [CrossRef]
- Lindquist, E.D.; Hetherington, T.E. Tadpoles and juveniles of the Panamanian golden frog, Atelopus zeteki (Bufonidae), with information on development of coloration and patterning. Herpetologica 1998, 54, 370–376. [Google Scholar]
- Gratwicke, B.; Lovejoy, T.E.; Wildt, D.E. Will Amphibians Croak under the Endangered Species Act? BioScience 2012, 62, 197–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).