Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Subjects
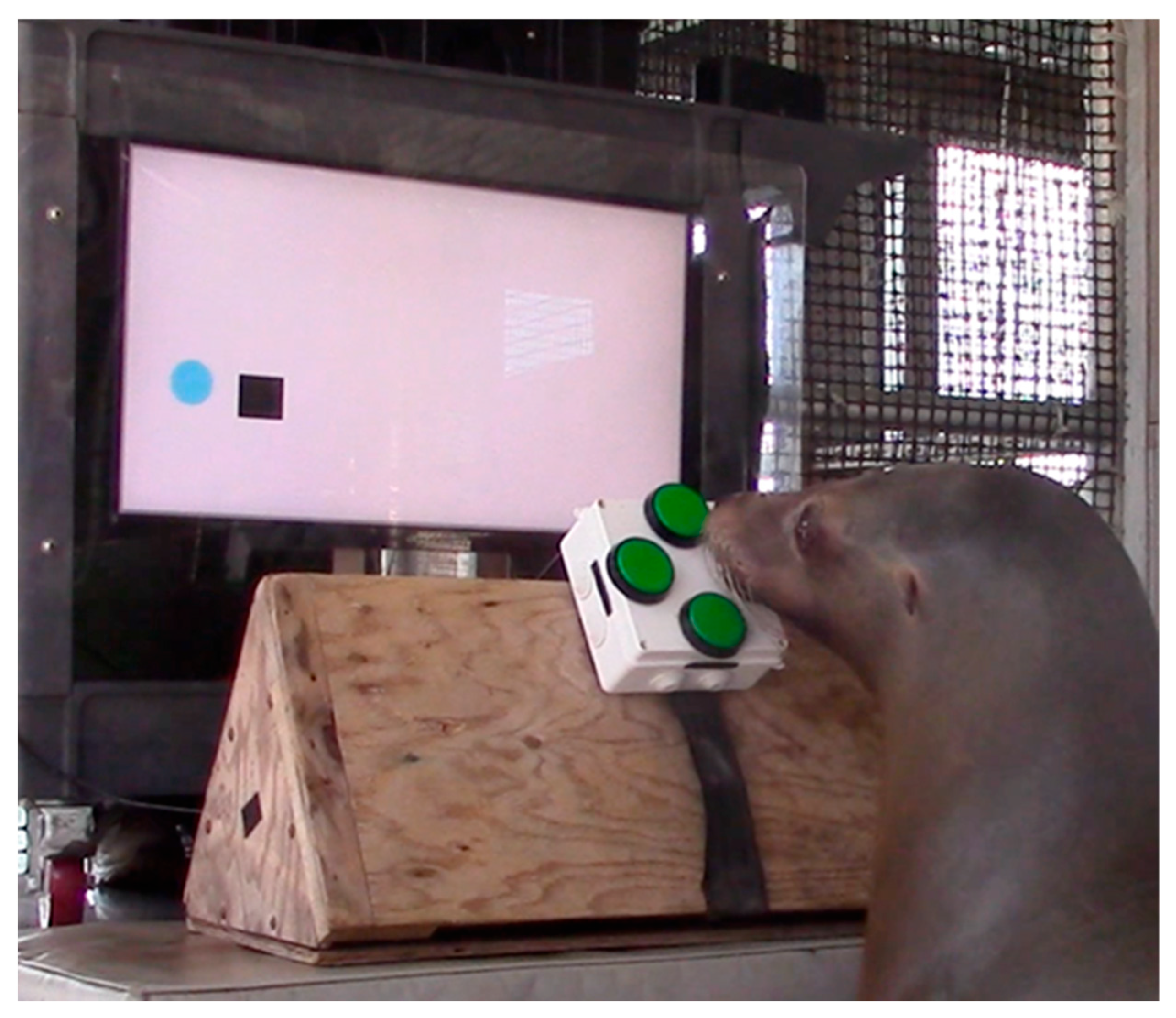
2.2. EVE Apparatus
2.3. Procedures
2.4. Data Analysis
2.5. Ethics Statement
3. Results
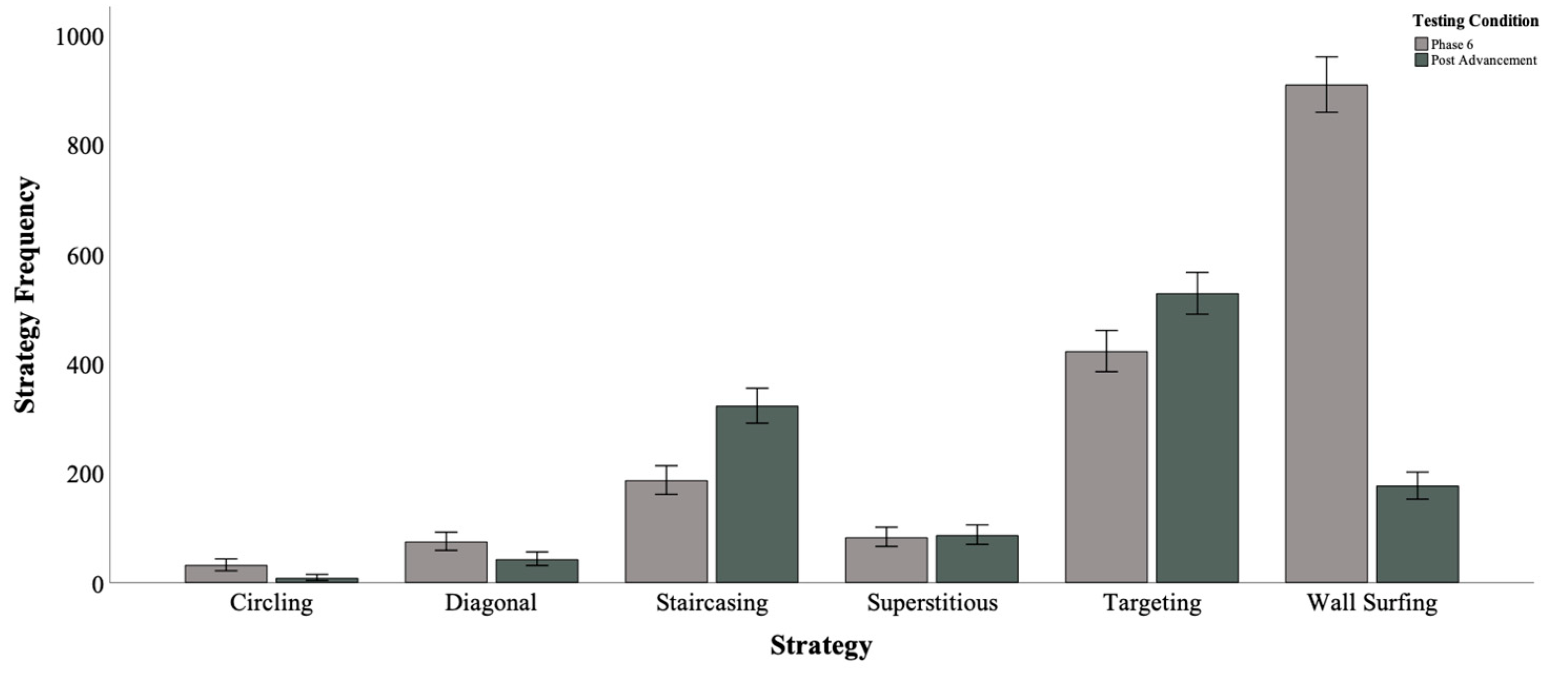
3.1. Frequency of Strategies by Testing Condition and Individual
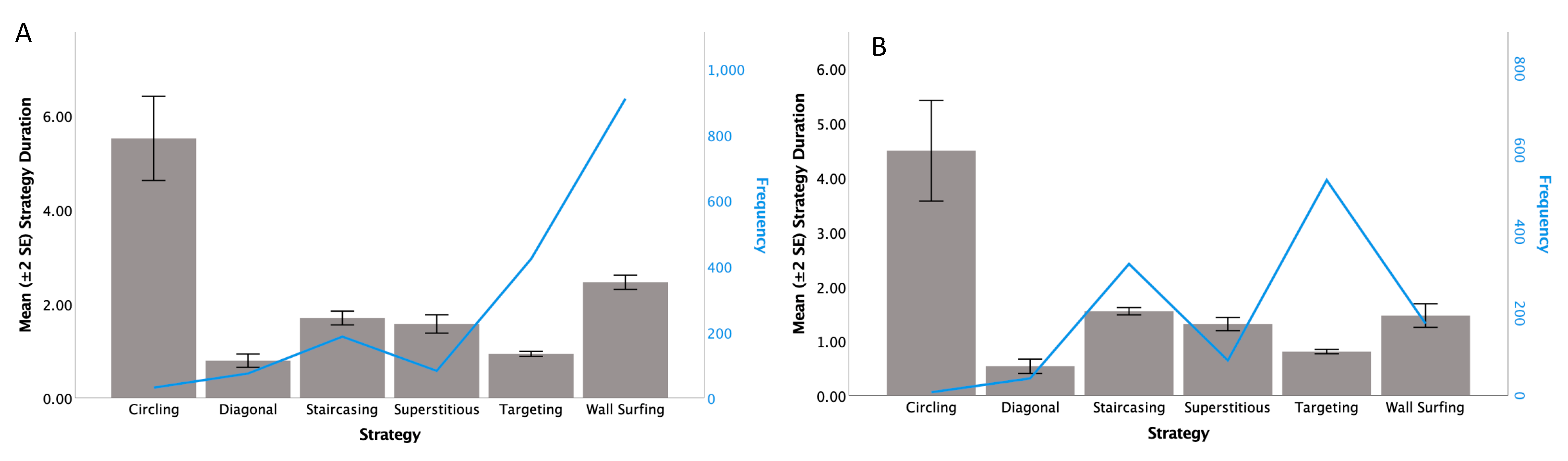
3.2. Strategy Duration and Button Pushes Compared between Conditions-Paired t-Test
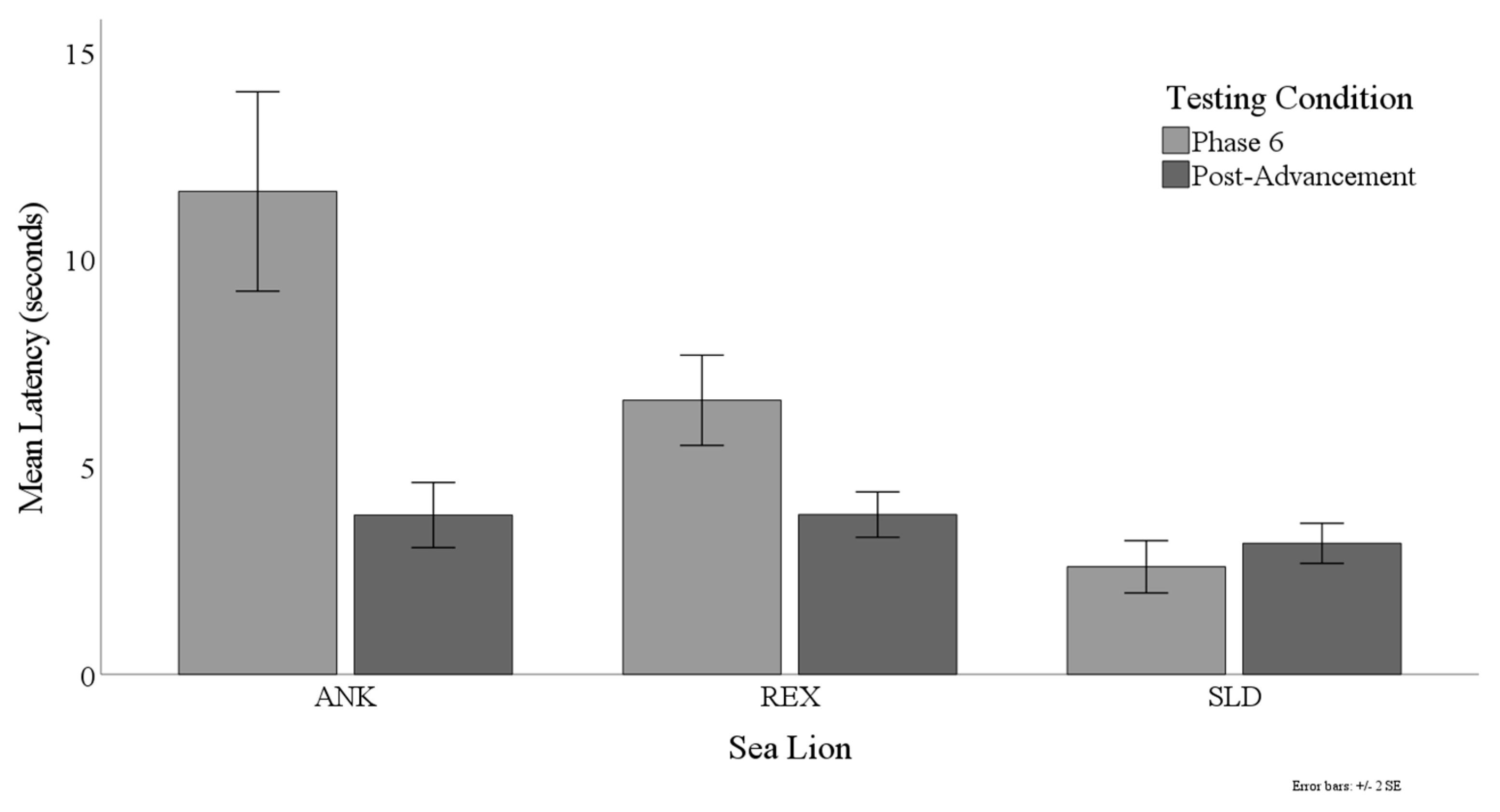
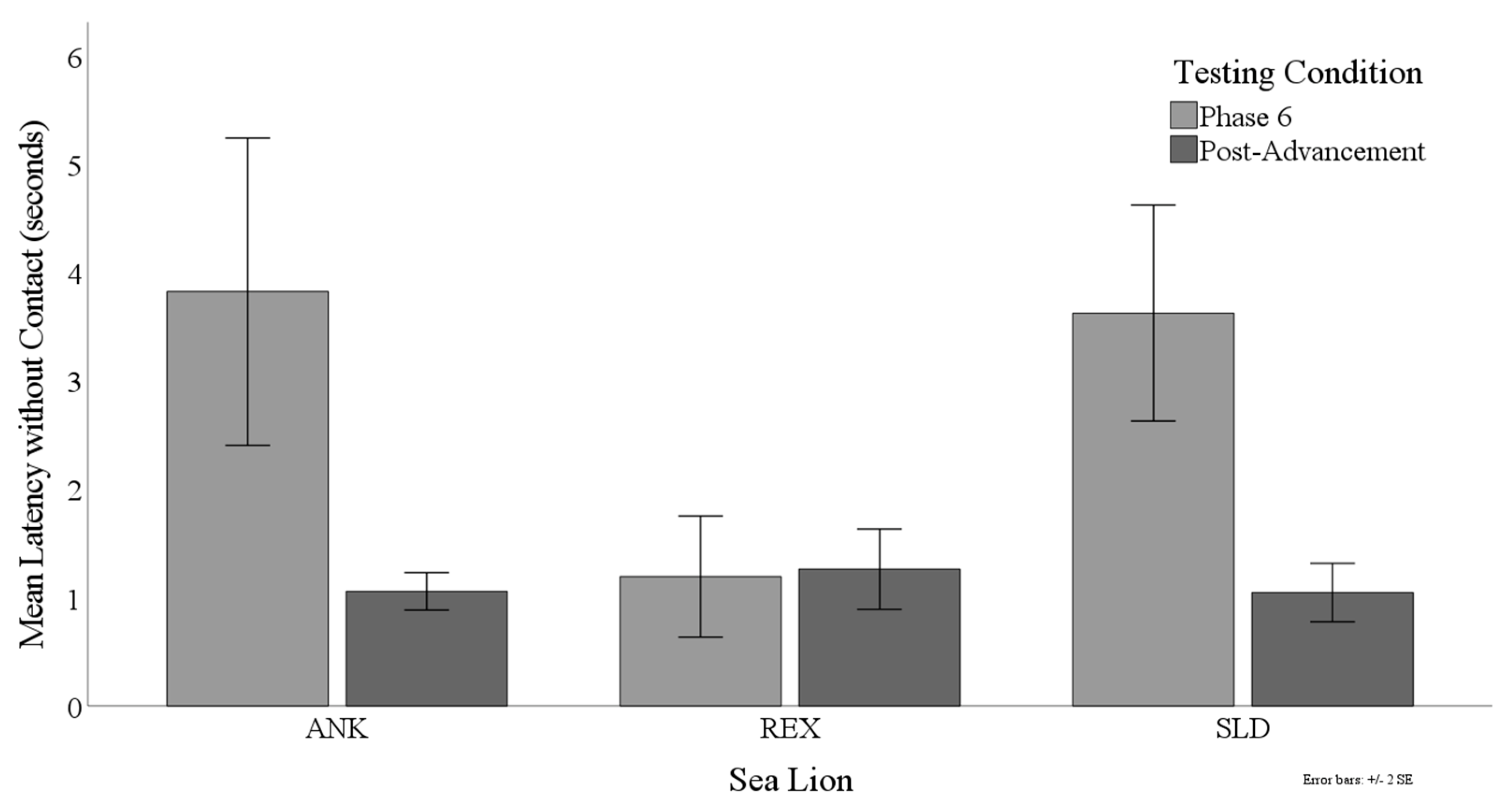
3.3. Latency to Target Contact
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egelkamp, C.L.; Ross, S.R. A review of zoo-based cognitive research using touchscreen interfaces. Zoo Biol. 2018, 38, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pelegrin, E.; Clark, F.; Miller, R. Increasing animal cognition research in zoos. Zoo Biol. 2022, 41, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hopper, L. Cognitive research in zoos. Behav. Sci. 2017, 16, 100–110. [Google Scholar] [CrossRef]
- Brydges, N.M.; Braithwaite, V.A. Measuring animal welfare: What can cognition contribute. Annu. Rev. Biomed Sci. 2008, 10, 91–103. [Google Scholar]
- Clark, F.E. Great ape cognition and captive care: Can cognitive challenges enhance well-being? Appl. Anim. Behav. Sci. 2011, 135, 1–12. [Google Scholar] [CrossRef]
- Moon, L.E.; Lodahl, T.M. The reinforcing effect of changes in illumination on lever-pressing in the monkey. Am. J. Psychol. 1956, 69, 288–290. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Clark, F.E. Cognitive enrichment and welfare: Current approaches and future directions. Anim. Behav. Cogn. 2017, 4, 52–71. [Google Scholar] [CrossRef]
- Clark, F.E. Marine mammal cognition and captive care: A proposal for cognitive enrichment in zoos and aquariums. J. Zoo Aquar. Res. 2013, 1, 1–6. [Google Scholar]
- Meehan, C.L.; Mench, J.A. The challenge of challenge: Can problem solving opportunities enhance animal welfare? Appl. Anim. Behav. Sci. 2007, 102, 246–261. [Google Scholar] [CrossRef]
- Burn, C.C. Bestial boredom: A biological perspective on animal boredom and suggestions for its scientific investigation. Anim. Behav. 2017, 130, 141–151. [Google Scholar] [CrossRef]
- Meehan, C.L.; Millam, J.R.; Mench, J.A. Foraging opportunity and increased physical complexity both prevent and reduce psychogenic feather picking by young Amazon parrots. Appl. Anim. Behav. Sci. 2003, 80, 71–85. [Google Scholar] [CrossRef]
- Runeson, E.P.; Lee, G.H.; Crockett, C.M.; Bellanca, R.U. Evaluating paint rollers as an intervention for alopecia in monkeys in the laboratory (Macaca nemestrina). J. Appl. Anim. Welf. Sci. 2011, 14, 138–149. [Google Scholar] [CrossRef]
- Dorey, N.R.; Rosales-Ruiz, J.; Smither, R.; Lovelace, D. Functional analysis and treatment of self-injury in a captive olive baboon. J. Appl. Behav. Anal. 2009, 42, 785–794. [Google Scholar] [CrossRef]
- Hosey, G.R.; Skyner, L.J. Self-injurious behavior in zoo primates. Int. J. Primatol. 2007, 28, 1431–1437. [Google Scholar] [CrossRef]
- Baker, K.C.; Easley, S.P. An analysis of regurgitation and reingestion in captive chimpanzees. Appl. Anim. Behav. Sci. 1996, 49, 403–415. [Google Scholar] [CrossRef]
- Hill, S.P. Regurgitation and reingestion (R/R) in great apes: A review of current knowledge. Int. Zoo Yearb. 2018, 52, 62–78. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies: A critical review. Anim. Behav. 1991, 41, 1015–1037. [Google Scholar] [CrossRef]
- Shyne, A. Meta-analytic review of the effects of enrichment on stereotypic behavior in zoo mammals. Zoo Biol. 2006, 25, 317–337. [Google Scholar] [CrossRef]
- Struck, K.S.; Videan, E.N.; Fritz, J.; Murphy, J. Attempting to reduce regurgitation and reingestion in a captive chimpanzee through increased feeding opportunities: A case study. Lab. Anim. 2007, 36, 35–38. [Google Scholar] [CrossRef]
- Fagot, J.; Gullstrand, J.; Kemp, C.; Defilles, C.; Mekaouche, M. Effects of freely accessible computerized test systems on the spontaneous behaviors and stress level of Guinea baboons (Papio papio). Am. J. Primatol. 2014, 6, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Washburn, D.A.; Rumbaugh, D.M. Investigations of rhesus monkey video-task performance: Evidence for enrichment. Contemp. Top. Lab. Anim. Sci. 1992, 31, 6–10. [Google Scholar] [PubMed]
- Washburn, D.A. The four Cs of psychological wellbeing: Lessons from three decades of computer-based environmental enrichment. Anim. Behav. Cogn. 2015, 2, 218–232. [Google Scholar] [CrossRef]
- Ruby, S.; Buchanan-Smith, H.M. The effects of individual cubicle research on the social interactions and individual behavior of brown capuchin monkeys (Sapajus apella). Am. J. Primatol. 2015, 77, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, J.; Micheletta, J.; Poewll, L.E.; Bordier, C.; Waller, B.M. The impact of cognitive testing on the welfare of group housed primates. PLoS ONE 2013, 8, e78308. [Google Scholar] [CrossRef]
- Schusterman, R.J.; Kellogg, W.N.; Rice, C.E. Underwater visual discrimination by the California sea lion. Science 1965, 147, 1594–1596. [Google Scholar] [CrossRef]
- Schusterman, R.J.; Krieger, K. Artificial language comprehension and size transposition by a California sea lion (Zalophus californianus). J. Comp. Psychol. 1986, 100, 348. [Google Scholar] [CrossRef]
- Rumbaugh, D.M.; Richardson, W.K.; Washburn, D.A.; Savage-Rumbaugh, E.S.; Hopkins, W.D. Rhesus monkeys (Macaca mulatta), video tasks, and implications for stimulus-response spatial contiguity. J. Comp. Psychol. 1989, 103, 32. [Google Scholar] [CrossRef]
- Hille, P.; Dehnhardt, G.; Mauck, B. An analysis of visual oddity concept learning in a California sea lion (Zalophus californianus). Learn. Behav. 2006, 34, 144–153. [Google Scholar] [CrossRef]
- Schusterman, R.J.; Kastak, D. A California sea lion (Zalophus californianus) is capable of forming equivalence relations. Psychol. Rec. 1993, 43, 823–839. [Google Scholar] [CrossRef]
- Washburn, D.A.; Whitham, W. The ‘shoulds’ and ‘coulds’ of meaningful failures: Introduction to the special issue. Anim. Behav. Cog. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Harlow, H.F. The formation of learning sets. Psychol. Rev. 1949, 56, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Schusterman, R.J. Serial discrimination-reversal learning with and without errors by the California sea lion 1. J. Exp. Anal. Behav. 1966, 9, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Pack, A.A.; Herman, L.M.; Roitblat, H.L. Generalization of visual matching and delayed matching by a California sea lion (Zalophus californianus). Anim. Learn. Behav. 1991, 19, 37–48. [Google Scholar] [CrossRef]
- Cook, P.; Reichmuth, C.; Hanke, F.D. The mind of a sea lion. In Ethology and Behavioral Ecology of Otariids and the Odobenid; Springer International Publishing: Cham, Switzerland, 2021; pp. 323–345. [Google Scholar]
- Frohoff, T.G.; Packard, J.M. Human interactions with free-ranging and captive bottlenose dolphins. Anthrozoös 1995, 8, 44–53. [Google Scholar] [CrossRef]
- Trone, M.; Kuczaj, S.; Solangi, M. Does participation in Dolphin–Human Interaction Programs affect bottlenose dolphin behaviour? Appl. Anim. Behav. Sci. 2005, 93, 363–374. [Google Scholar] [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Boivin, X.; Delfour, F. Looking forward to interacting with their caretakers: Dolphins’ anticipatory behaviour indicates motivation to participate in specific events. Appl. Anim. Behav. Sci. 2018, 202, 85–93. [Google Scholar] [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Mercera, B.; Van der Heul, S.; Schrijvers, T.; De Laender, P.; Gojceta, R.; Zimmitti, M.; Verhoeven, E.; Burger, J.; et al. Dolphins’ willingness to participate (WtP) in positive reinforcement training as a potential welfare indicator, where WtP predicts early changes in health status. Front. Psychol. 2019, 10, 2112. [Google Scholar] [CrossRef]
- Dawkins, M.S. From an animal’s point of view: Motivation, fitness, and animal welfare. Behav. Brain Sci. 1990, 13, 1–9. [Google Scholar] [CrossRef]
- Manteuffel, G.; Langbein, J.; Puppe, B. From operant learning to cognitive enrichment in farm animal housing: Bases and applicability. Anim. Welf. 2009, 18, 87–95. [Google Scholar] [CrossRef]
- Kirkden, R.D.; Pajor, E.A. Using preference, motivation and aversion tests to ask scientific questions about animals’ feelings. Appl. Anim. Behav. Sci. 2006, 100, 29–47. [Google Scholar] [CrossRef]
- Salamone, J.D.; Cousins, M.S.; McCullough, L.D.; Carriero, D.L.; Berkowitz, R.J. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 1994, 49, 25–31. [Google Scholar] [CrossRef]
- De Jonge, F.H.; Tilly, S.L.; Baars, A.M.; Spruijt, B.M. On the rewarding nature of appetitive feeding behaviour in pigs (Sus scrofa): Do domesticated pigs contrafreeload? Appl. Anim. Behav. Sci. 2008, 114, 359–372. [Google Scholar] [CrossRef]
- Jensen, G.D. Preference for bar pressing over “freeloading” as a function of number of rewarded presses. J. Exp. Psychol. 1963, 65, 451–454. [Google Scholar] [CrossRef]
- Inglis, I.R.; Forkman, B.; Lazarus, J. Free food or earned food? A review and fuzzy model of contrafreeloading. Anim. Behav. 1997, 53, 1171–1191. [Google Scholar] [CrossRef]
- Watson, S.L.; Shively, C.A.; Voytko, M.L. Can puzzle feeders be used as cognitive screening instruments? Differential performance of young and aged female monkeys on a puzzle feeder task. Am. J. Primatol. 1999, 49, 195–202. [Google Scholar] [CrossRef]
- Broadhurst, P.L. Emotionality and the Yerkes-Dodson law. J. Exp. Psychol. 1957, 54, 345. [Google Scholar] [CrossRef]
- Larson, S.J. Behavioral and motivational effects of immune-system activation. J. Gen. Psychol. 2002, 129, 401–414. [Google Scholar] [CrossRef]
- De La Garza, R. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neurosci. Biobehav. Rev. 2005, 29, 761–770. [Google Scholar] [CrossRef]
- Kleen, J.K.; Sitomer, M.T.; Killeen, P.R.; Conrad, C.D. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 2006, 120, 842–851. [Google Scholar] [CrossRef]
- Delfour, F.; Monreal-Pawlowsky, T.; Vaicekauskaite, R.; Pilenga, C.; Garcia-Parraga, D.; Rödel, H.G.; Caro, N.G.; Campos, E.P.; Mercera, B. Dolphin welfare assessment under professional care: ‘Willingness to Participate’, an indicator significantly associated with six potential ‘alerting factors’. J. Zool. Bot. Gard. 2020, 1, 42–60. [Google Scholar] [CrossRef]
- Samuelson, M.M.; Lauderdale, L.K.; Pulis, K.; Solangi, M.; Hoffland, T.; Lyn, H. Olfactory enrichment in California sea lions (Zalophus californianus): An effective tool for captive welfare? J. Appl. Anim. Welf. Sci. 2017, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.P.; Litchfield, C.A. An empirical case study examining effectiveness of environmental enrichment in two captive Australian sea lions (Neophoca cinerea). J. Appl. Anim. Welf. Sci. 2010, 13, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I.L.; Butterworth, A. Assessing the welfare of pinnipeds. In Marine Mammal Welfare; Springer: Cham, Switzerland, 2017; pp. 273–295. [Google Scholar]
- Hocking, D.P.; Salverson, M.; Evans, A.R. Foraging-based enrichment promotes More varied behaviour in captive Australian fur seals (Arctocephalus pusillus doriferus). PLoS ONE 2015, 10, e0124615. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, R.A.; Wiepkema, P.R. The significance of training for the behaviour of Steller sea lions (Eumetopias jubata) in human care. Aquat. Mamm. 1988, 14, 39–41. [Google Scholar]
- Schusterman, R.J.; Kastak, C.R.; Kastak, D. The Cognitive Sea Lion: Meaning and Memory in the Laboratory and in Nature; The MIT Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Kuczaj, S.A.; Xitco, M.J., Jr. It takes more than fish: The psychology of marine mammal training. Int. J. Comp. Psychol. 2002, 15, 186–200. [Google Scholar] [CrossRef]
- Winship, K.A.; Ramos, A.M.; Xitco, M.J. The introduction of a novel computerized apparatus to California sea lions (Zalophus californianus). Aquat. Mamm. 2023, 49, 73–86. [Google Scholar] [CrossRef]
- Goldblatt, A. Automatic feeder for marine mammals. Aquat. Mamm. 1992, 18, 82–84. [Google Scholar]
- Beran, M.J.; Parrish, A.E.; Futch, S.E.; Evans, T.A.; Perdue, B.M. Looking ahead? Computerized maze task performance by chimpanzees (Pan troglodytes), rhesus monkeys (Macaca mulatta), capuchin monkeys (Cebus apella), and human children (Homo sapiens). J. Comp. Psychol. 2015, 129, 160. [Google Scholar] [CrossRef]
- Unity Technologies. Unity Real-Time Development Platform (Version 2019.2.15f1). 2019. Available online: https://unity.com/releases/editor/whats-new/2019.2.15#release-notes (accessed on 15 January 2020).
- Péter, A. Solomon Coder: A Simple and Free Solution for Behavior Coding (Version Beta 19.08.02). 2019. Available online: https://solomon.andraspeter.com (accessed on 15 May 2021).
- Ghafurian, M.; Reitter, D. Gender Differences in the Effect of Impatience on Men and Women’s Timing Decisions. In Proceedings of the 38th Annual Meeting of the Cognitive Science Society, Philadelphia, PA, USA, 10–13 August 2016. [Google Scholar]
- Kohrs, C.; Hrabal, D.; Angenstein, N.; Brechmann, A. Delayed system response times affect immediate physiology and the dynamics of subsequent button press behavior. Psychophysiology 2014, 51, 1178–1184. [Google Scholar] [CrossRef]
- Mandler, G. The interruption of behavior. In Proceedings of the Nebraska Symposium on Motivation; Levine, D., Ed.; University of Nebraska Press: Lincoln, NE, USA, 1964. [Google Scholar]
- Paukner, A.; Suomi, S.J.; Visalberhi, E.; Ferrari, P.F. Capuchin monkeys display affiliation toward humans who imitate them. Science 2009, 325, 880–883. [Google Scholar] [CrossRef]
- Rochat, P. The Infant’s World; Harvard University Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Harley, H. Consciousness in dolphins? A review of recent evidence. J. Comp. Physciol. A 2013, 199, 565–582. [Google Scholar] [CrossRef]
- Perdue, B.M.; Evans, T.A.; Washburn, D.A.; Rumbaugh, D.M.; Beran, M.J. Do Monkeys Choose to Choose? Learn. Behav. 2014, 42, 164–175. [Google Scholar] [CrossRef]
- Clark, F.E.; Davies, S.L.; Madigan, A.W.; Warner, A.J.; Kuczaj, S.A. Cognitive enrichment for bottlenose dolphins (Tursiops truncatus): Evaluation of a novel underwater maze device. Zoo Biol. 2013, 32, 608–619. [Google Scholar] [CrossRef]
- Eskelinen, H.C.; Winship, K.A.; Borger Turner, J.L. Sex, age, and individual differences in bottlenose dolphins (Tursiops truncatus) in response to environmental enrichment. Anim. Behav. Cogn. 2015, 2, 241–253. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Jennings, N.; Postma, J. Feeding enrichment methods for Pacific walrus calves. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 2007, 26, 175–186. [Google Scholar] [CrossRef]
- Ciardelli, L.E.; Weiss, A.; Powell, D.M.; Reiss, D. Personality dimensions of the captive California sea lion (Zalophus californianus). J. Comp. Psychol. 2017, 131, 50. [Google Scholar] [CrossRef]
- De Vere, A.J.; Lilley, M.K.; Highfill, L. Do pinnipeds have personality? Broad dimensions and contextual consistency of behavior in harbor seals (Phoca vitulina) and California sea lions (Zalophus californianus). Int. J. Comp. Psychol. 2017, 30, 1–16. [Google Scholar] [CrossRef]
- Dweck, C.S.; Reppucci, N.D. Learned helplessness and reinforcement responsibility in children. J. Pers. Soc. Psychol. 1973, 25, 109. [Google Scholar] [CrossRef]
- Diener, C.I.; Dweck, C.S. An Analysis of learned helplessness: Continuous changes in performance, strategy, and achievement cognitions following failure. J. Pers. Soc. Psychol. 1978, 36, 451. [Google Scholar] [CrossRef]
- Dweck, C.S.; Leggett, E.L. A social-cognitive approach to motivation and personality. Psychol. Rev. 1988, 95, 256. [Google Scholar] [CrossRef]
- Perdue, B.M.; Beran, M.J.; Washburn, D.A. A computerized testing system for primates: Cognition, welfare, and the Rumbaughx. Behav. Process. 2018, 156, 37–50. [Google Scholar] [CrossRef] [PubMed]


| Strategy | Operational Definition | Button-Pushing Example | Visual Representation of Cursor Moves |
|---|---|---|---|
| Circling | Any set of five button presses where buttons are pressed in order either clockwise or counterclockwise in the same direction. Restrictions: limited to less than half of the screen (x < 0.8 s) | UP, RIGHT, DOWN, LEFT, UP | 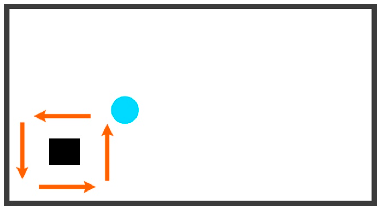 |
| Diagonal | Any movement that utilizes two buttons simultaneously | UP and RIGHT | 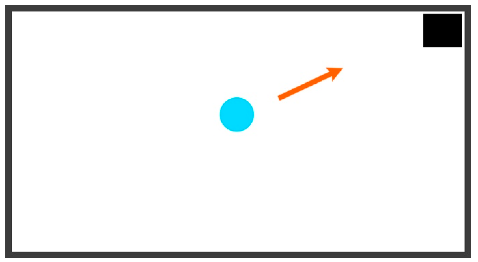 |
| Staircasing | An alternating series of at least three alternating vertical and horizontal movements | UP, RIGHT, UP, RIGHT | 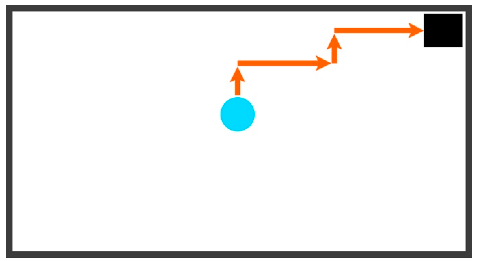 |
| Superstitious | A superstitious initial movement (e.g., UP), followed by a pause. Restrictions: Can only be considered for the first movement of the trial. | UP |  |
| Targeting | One or two direct movements to the target that result in acquiring the target. Restrictions: Can only be considered for the first two moves of the trial | UP, RIGHT |  |
| Wall Surfing | Continuous movements in the same direction along the wall. Considerations: the cursor starts in the center for all Phase 6 and Post-Advancement (time taken to move to wall is not considered in wall-surfing duration) | (UP), LEFT, DOWN, RIGHT, RIGHT, UP | 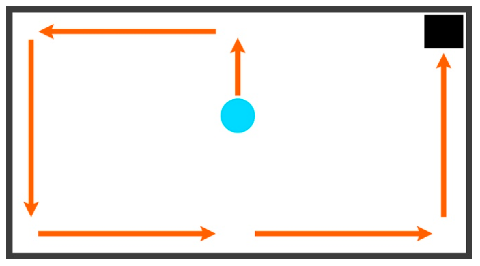 |
| No Explicit Strategy (NES) | Any movements that cannot be considered as any defined strategy. Considerations: may include unsuccessful presses of defined strategy criteria (ex. A: unsuccessful staircase) | Ex. A: UP, RIGHT, RIGHT, UP, RIGHT Ex. B: DOWN, RIGHT, UP | 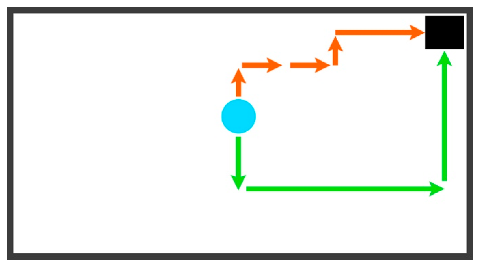 |
| Mean Strategy Duration (Seconds) by Testing Condition and Strategy—All Sea Lions Combined | ||||
|---|---|---|---|---|
| Strategy | Testing Condition | N | Mean | D |
| Circling | Phase 6 Post-Advancement | 31 8 | 5.52 4.49 | 2.49 1.31 |
| Diagonal | Phase 6 Post-Advancement | 74 42 | 0.79 0.54 | 0.60 0.43 |
| Staircasing | Phase 6 Post-Advancement | 186 322 | 1.70 1.55 | 1.00 0.59 |
| Superstitious | Phase 6 Post-Advancement | 82 86 | 1.57 1.31 | 0.88 0.56 |
| Targeting | Phase 6 Post-Advancement | 422 527 | 0.94 0.81 | 0.56 0.47 |
| Wall Surfing | Phase 6 Post-Advancement | 422 527 | 2.46 1.47 | 2.29 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, D.L.; Eskelinen, H.C.; Winship, K.A.; Ramos, A.M.; Xitco, M.J. Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds. J. Zool. Bot. Gard. 2023, 4, 240-255. https://doi.org/10.3390/jzbg4010021
Roberts DL, Eskelinen HC, Winship KA, Ramos AM, Xitco MJ. Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds. Journal of Zoological and Botanical Gardens. 2023; 4(1):240-255. https://doi.org/10.3390/jzbg4010021
Chicago/Turabian StyleRoberts, Danielle L., Holli C. Eskelinen, Kelley A. Winship, Amber M. Ramos, and Mark J. Xitco. 2023. "Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds" Journal of Zoological and Botanical Gardens 4, no. 1: 240-255. https://doi.org/10.3390/jzbg4010021
APA StyleRoberts, D. L., Eskelinen, H. C., Winship, K. A., Ramos, A. M., & Xitco, M. J. (2023). Effects of Failure on California Sea Lion (Zalophus californianus) Gameplay Strategies and Interest in a Cognitive Task: Implications for Cognitive Enrichment in Pinnipeds. Journal of Zoological and Botanical Gardens, 4(1), 240-255. https://doi.org/10.3390/jzbg4010021






