Effects of Background Color on Stress-Linked Behavior in the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ethical Approval
2.3. Data Collection
2.4. Statistical Analysis
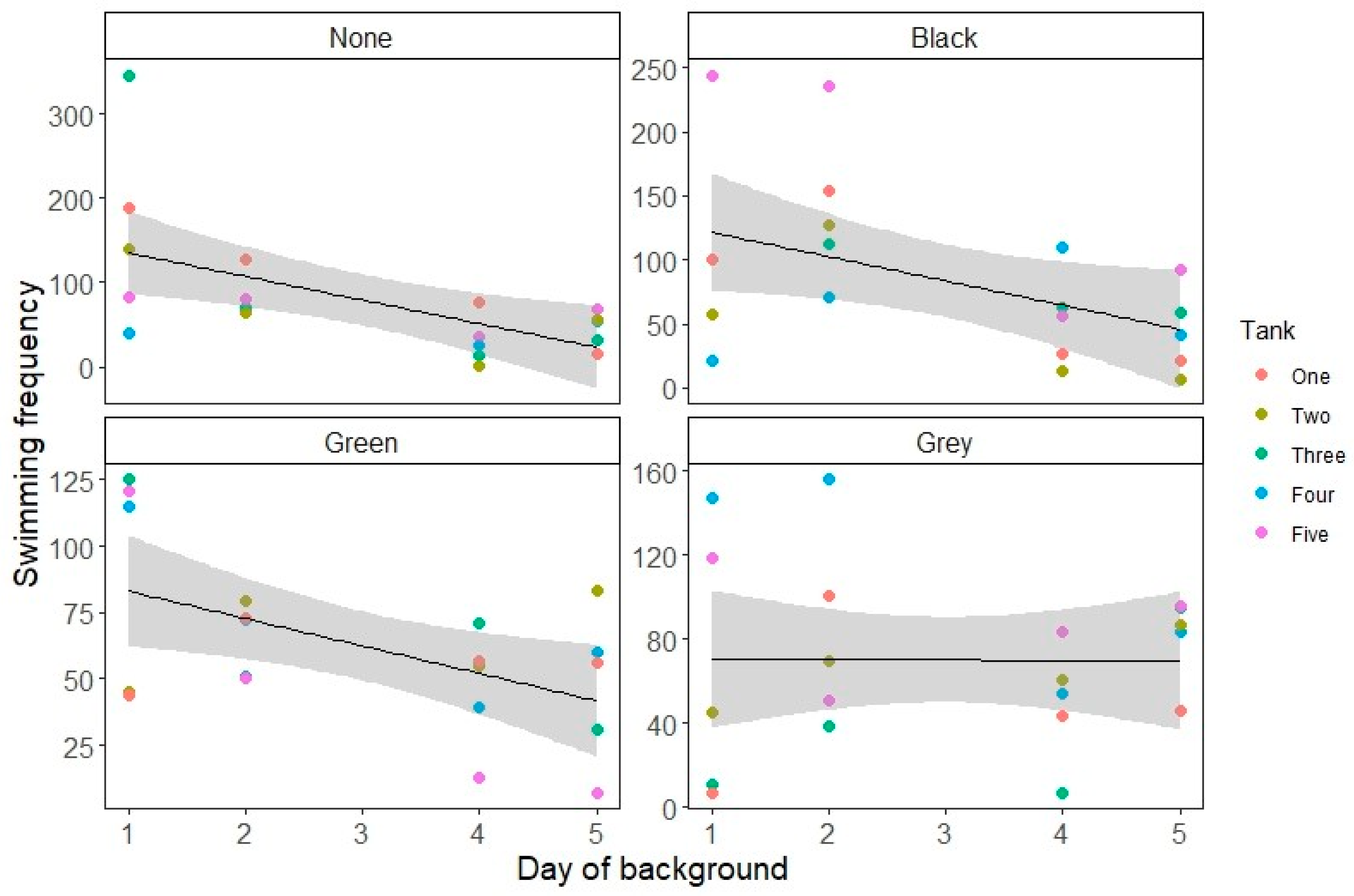
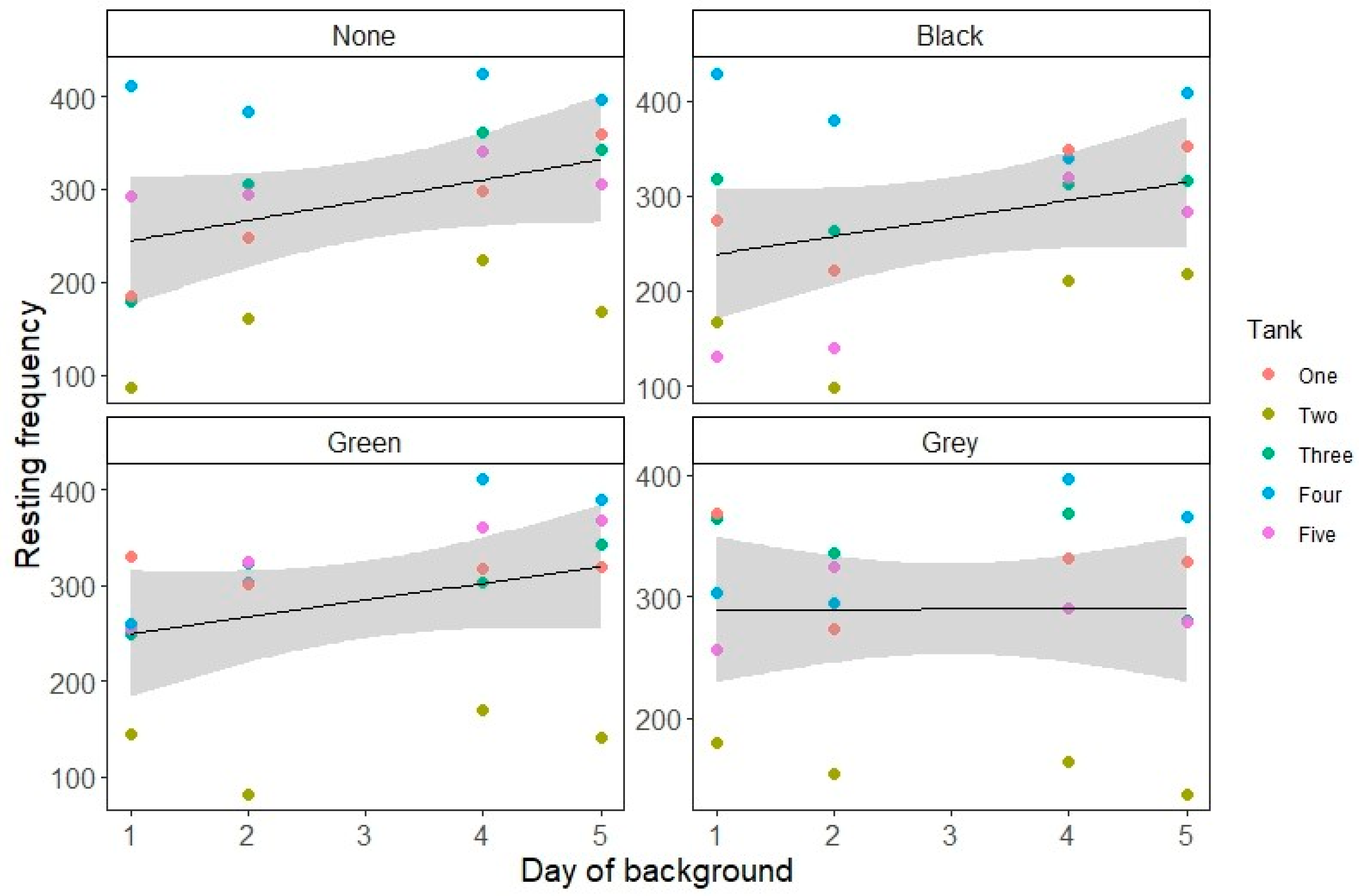
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrows, M. Welfare assessment in zoo animals. Vet. Rec. 2017, 181, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Brod, S.; Brookes, L.; Garner, T.W.J. Discussing the future of amphibians in research. Lab. Anim. 2019, 48, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Michaels, C.; Downie, R.; Campbell-Palmer, R. The importance of enrichment for advancing amphibian welfare and conservation goals: A review of a neglected topic. Amphib. Reptile Conserv. 2014, 8, 7–23. [Google Scholar]
- Maple, T.L. Toward a Science of Welfare for Animals in the Zoo. J. Appl. Anim. Welf. Sci. 2007, 10, 63–70. [Google Scholar] [CrossRef]
- Beebee, T.J.C.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Browne, R.K.; Wolfram, K.; García, G.; Bagaturov, M.F.; Pereboom, Z.J.J.M. Zoo-based amphibian research and conservation breeding programs. Amphib. Reptile Conserv. 2011, 5, 1–14. [Google Scholar]
- Tillbrook, A.J.; Ralph, C.R. Hormones, stress and the welfare of animals. Anim. Prod. Sci. 2017, 58, 408–415. [Google Scholar] [CrossRef]
- Holmes, A.M.; Emmans, C.J.; Coleman, R.; Smith, T.E.; Hosie, C.A. Effects of transportation, transport medium and re-housing on Xenopus laevis (Daudin). Gen. Comp. Endocrinol. 2018, 266, 21–28. [Google Scholar] [CrossRef]
- Michaels, C.J. Effects of aquatic and terrestrial habitats on the skin microbiome and growth rate of juvenile alpine newts Ichthyosaura alpestris. Herpetol. J. 2022, 2, 51–58. [Google Scholar] [CrossRef]
- Carter, K.C.; Fieschi-Méric, L.; Servini, F.; Wilkinson, M.; Gower, D.J.; Tapley, B.; Michaels, C.J. Investigating the Effect of Disturbance on Prey Consumption in Captive Congo Caecilians Herpele squalostoma. J. Zool. Bot. Gard. 2021, 2, 705–715. [Google Scholar] [CrossRef]
- Boultwood, J.; O’Brien, M.; Rose, P. Bold frogs or shy toads? How did the COVID-19 closure of zoological organisations affect amphibian activity? Animals 2021, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Antwis, R.E.; Preziosi, R.F.; Fidgett, A.L. The effect of different UV and calcium provisioning on health and fitness traits of red-eyed tree frogs (Agalychnis callidryas). J. Zoo Aquar. Res. 2014, 2, 69–76. [Google Scholar]
- Michaels, C.J.; Antwis, R.E.; Preziosi, R.F. Impact of plant cover on fitness and behavioural traits of captive red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 2014, 9, e95207. [Google Scholar] [CrossRef]
- Holmes, A.M.; Emmans, C.J.; Jones, N.; Coleman, R.; Smith, T.E.; Hosie, C.A. Impact of tank background on the welfare of the African clawed frog, Xenopus laevis (Daudin). Appl. Anim. Behav. Sci. 2016, 185, 131–136. [Google Scholar] [CrossRef]
- Hilken, G.; Willmann, F.; Dimigen, J.; Iglauer, F. Preference of Xenopus laevis for different housing conditions. Scand. J. Lab. Anim. Sci. 1994, 21, 71. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Xenopus longipes (amended version of 2017 assessment). In The IUCN Red List of Threatened Species 2020: E.T58176A177346697; IUCN: Gland, Switzerland, 2020; Available online: https://www.iucnredlist.org/species/58176/177346697 (accessed on 10 December 2022).
- Blackburn, D.C.; Evans, B.J.; Pessier, A.P.; Vredenburg, V.T. An enigmatic mortality event in the only population of the Critically Endangered Cameroonian frog Xenopus longipes. Afr. J. Herpetol. 2010, 59, 111–122. [Google Scholar] [CrossRef]
- Doherty-Bone, T.M.; Ndifon, R.K.; Nyingchia, O.N.; Landrie, F.E.; Yonghabi, F.T.; Duffus, A.L.J.; Price, S.; Perkins, M.; Bielby, J.; Kome, N.B.; et al. Morbidity and mortality of the Critically Endangered Lake Oku clawed frog Xenopus longipes. Endanger. Species Res. 2013, 21, 115–128. [Google Scholar] [CrossRef]
- Michaels, C.J.; Tapley, B.; Harding, L.; Bryant, Z.; Grant, S.; Sunter, G.; Gill, I.; Nyingchia, O.; Doherty-Bone, T. Breeding and rearing the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes Loumont and Kobel 1991). Amphib. Reptile Conserv. 2015, 9, 100–110. [Google Scholar]
- Gurdon, J.B.; Hopwood, N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2000, 44, 43–50. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Xenopus laevis. In The IUCN Red List of Threatened Species 2020: E.T110466172A3066881; Available online: https://www.iucnredlist.org/species/110466172/3066881 (accessed on 10 December 2022).
- Dias, J.E.; Ellis, C.; Smith, T.E.; Hosie, C.A.; Tapley, B.; Michaels, C.J. Baseline Behavioral Data and Behavioral Correlates of Disturbance for the Lake Oku Clawed Frog (Xenopus longipes). J. Zool. Bot. Gard. 2022, 3, 184–197. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Baird, T.A. Influence of social and predatory stimuli on the air-breathing behavior of the African clawed frog, Xenopus laevis. Copeia 1983, 2, 411–420. [Google Scholar] [CrossRef]
- Goode, K.; Rey, K. ggResidpanel Tutorial and User Manual. 2019. Available online: https://goodekat.github.io/ggResidpanel-tutorial/tutorial.html (accessed on 10 December 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 10 December 2022).
- Polo-Cavia, N.; Gomez-Mestre, I. Pigmentation plasticity enhances crypsis in larval newts: Associated metabolic cost and background choice behaviour. Sci. Rep. 2017, 7, 39739. [Google Scholar] [CrossRef] [PubMed]
- Tapley, B.; Michaels, C.; Harding, L.; Bryant, Z.; Gill, I.; Grant, S.; Chaney, N.; Dunker, F.; Freiermuth, B.; Willis, J.; et al. Amphibian Taxon Advisory Group Best Practice Guidelines for the Lake Oku frog Xenopus longipes, Version 1; EAZA: Amsterdam, The Netherlands, 2016; Available online: https://www.eaza.net/assets/Uploads/CCC/2016-Lake-Oku-frog-EAZA-Best-Practice-Guidelines-Approved.pdf (accessed on 10 December 2022).
- Whatley, C.; Tapley, B.; Michaels, C.J.; Gower, D.J.; Wilkinson, M. Substrate preference in the fossorial caecilian Microcaecila unicolor (Amphibia: Gymnophiona, Siphonopidae). Herpetol. Bull. 2020, 152, 18–20. [Google Scholar] [CrossRef]
- Guimarães, I.S.C.; Hemnani, M.; Kaefer, I.L.; da Silva Pires, T.H. Fear of the dark: Substrate preference in Amazonian tadpoles. Acta Ethol. 2021, 24, 177–183. [Google Scholar] [CrossRef]
- Ramos, J.; Ortiz-Díez, G. Evaluation of environmental enrichment for Xenopus laevis using a preference test. Lab. Anim. 2021, 55, 428–434. [Google Scholar] [CrossRef]
- Hilken, G.; Dimigen, J.; Iglauer, F. Growth of Xenopus laevis under different laboratory rearing conditions. Lab. Anim. 1995, 29, 152–162. [Google Scholar] [CrossRef]
| Tank Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Week of data collection | 1 | None | Black | Grey | Green | None |
| 2 | Black | Grey | Green | None | Black | |
| 3 | Grey | Green | None | Black | Gre | |
| 4 | Green | None | Black | Grey | Green | |
| Behavior | Variable | Wald Chi-Square (Degrees of Freedom) | p Value |
|---|---|---|---|
| Swimming | Background | 75.128 (3) | <0.0001 |
| Day | 488.226 (1) | <0.0001 | |
| Background: Day interaction | 248.609 (3) | <0.0001 | |
| Resting | Background | 8.152 (3) | 0.04300 |
| Day | 149.566 (1) | <0.0001 | |
| Background: Day interaction | 51.866 (3) | <0.0001 |
| Model | Parameter | Estimate (SD for Random Effect) | Standard Error of Estimate (Variance for Random Effect) | t Value | p Value |
|---|---|---|---|---|---|
| Swimming | Black background (relative to no background) | −0.29489 | 0.06577 | −4.484 | <0.001 |
| Green background (relative to no background) | −0.75298 | 0.07223 | −10.424 | <0.001 | |
| Grey background (relative to no background) | −1.09762 | 0.07354 | −14.925 | <0.001 | |
| Day | −0.38828 | 0.01837 | −21.141 | <0.001 | |
| Black background: Day interaction | 0.15365 | 0.02459 | 6.250 | <0.001 | |
| Green background: Day interaction | 0.21956 | 0.02602 | 8.439 | <0.001 | |
| Grey background: Day interaction | 0.38572 | 0.02494 | 15.464 | <0.001 | |
| R2 marginal | 0.8547157 | ||||
| R2 conditional | 0.9263873 | ||||
| Random effect | 0.1149 | ||||
| Resting | Black background (relative to no background) | −0.016659 | 0.042465 | −0.392 | 0.695 |
| Green background (relative to no background) | 0.036850 | 0.042024 | 0.877 | 0.381 | |
| Grey background (relative to no background) | 0.240268 | 0.041015 | 5.858 | <0.001 | |
| Day | 0.076545 | 0.008379 | 9.136 | <0.001 | |
| Black background: Day interaction | −0.007958 | 0.011968 | −0.665 | 0.506 | |
| Green background: Day interaction | −0.015026 | 0.011869 | −1.266 | 0.206 | |
| Grey background: Day interaction | −0.075923 | 0.011799 | −6.435 | <0.001 | |
| R2 marginal | 0.09660030 | ||||
| R2 conditional | 0.9643724 | ||||
| Random effect | 0.2917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graves, A.E.; Dias, J.E.; Michaels, C.J. Effects of Background Color on Stress-Linked Behavior in the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes). J. Zool. Bot. Gard. 2023, 4, 99-107. https://doi.org/10.3390/jzbg4010011
Graves AE, Dias JE, Michaels CJ. Effects of Background Color on Stress-Linked Behavior in the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes). Journal of Zoological and Botanical Gardens. 2023; 4(1):99-107. https://doi.org/10.3390/jzbg4010011
Chicago/Turabian StyleGraves, Arabella E., Jemma E. Dias, and Christopher J. Michaels. 2023. "Effects of Background Color on Stress-Linked Behavior in the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes)" Journal of Zoological and Botanical Gardens 4, no. 1: 99-107. https://doi.org/10.3390/jzbg4010011
APA StyleGraves, A. E., Dias, J. E., & Michaels, C. J. (2023). Effects of Background Color on Stress-Linked Behavior in the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes). Journal of Zoological and Botanical Gardens, 4(1), 99-107. https://doi.org/10.3390/jzbg4010011








