Abstract
Ex situ amphibian populations are a key component of global amphibian conservation strategy, and optimal husbandry is vital to ex situ conservation success. Animal behavior can be used to inform captive welfare and improve husbandry practices. However, it has been little used for amphibians compared with mammals and birds. The goal of this study was to explore the effect of different colored tank backgrounds on the behavior of the critically endangered Lake Oku clawed frog (Xenopus longipes) in captivity. This was conducted by studying the behavior of a group of 24 captive frogs in 5 groups using established behavioral indicators of presumed stress. Resting and swimming behaviors, established in the literature as linked to acute stress, were recorded under conditions of three background colors and a standard husbandry control of no background. Frog groups were exposed to each background for five days with behavioral frequencies recorded daily from 11:00 until 13:00. Using generalized linear mixed models, we identified a significant effect of background days after the background was changed and the interaction between the two variables on both swimming and resting behavior. The results of this study suggest an initial response of stress to altering the background, modulated by the color of the background, followed by the extinction of the stress response such that by five days after the background change, behaviors were similar to the baseline and indistinguishable between treatments. Overall, this study suggests that frog stress behavior was not differentially directly affected by green, grey, black, or transparent backgrounds but that green and grey backgrounds were associated with the smallest stress response to background change. These colors may therefore be recommended to reduce the impact of stress from disturbance.
1. Introduction
Ensuring optimal welfare standards for captive species is imperative [1]. Inadequate welfare standards can result in chronically stressed individuals and can affect species’ health [2], as well as pose ethical implications for housing individuals in captivity. Amphibian welfare is poorly studied and subject to negative bias in research [3,4], but species-specific welfare understanding needs to be developed for amphibians to inform the often complex and problematic husbandry needs of this group [5]. Developing this foundation of research can improve the way captive animals are cared for [6], and given the threats many amphibians face [7], enhancing amphibian care should be a priority to advance ex situ conservation goals. Ex situ conservation is an essential component of conservation by enabling research, breeding, and education programs for a species [8].
Stress can be expressed in behavioral and/or physiological aspects [9]. Behavior can be used to indicate welfare state and to quantify the impact of husbandry interventions, non-invasively using skilled interpretation [10]. There are many aspects of enclosure design that can affect amphibian welfare [5,11,12,13,14,15]. One aspect of this is the color of the tank background, which has been seen to influence behavior and welfare [16,17] due to ecological relevance, such as the perceived predation risk of species that rely on cryptic camouflage, perception of the internal architecture of the captive environment, and visual ability.
The Lake Oku clawed frog (Xenopus longipes) is endemic to Lake Oku in Cameroon and is classified as Critically Endangered with a declining population [18]. This species has experienced recurring mortality events, further decreasing the population [19,20]. A population of X. longipes is kept at ZSL London Zoo to facilitate ongoing conservation research [21]. X. longipes is a close relative of the African clawed frog (Xenopus laevis), a species often used for research, such as developmental biology [22,23]; therefore, X. longipes is deemed an appropriate candidate for applying husbandry research in a zoo context. However, research by Dias et al. (2022) [24] revealed that the behavioral repertoire and potential behavioral indicators of stress differ greatly between the two species, despite being closely related, with X. longipes not exhibiting the walling behavior deemed an indicator of stress in X.laevis [16] but instead performing a significant increase in swimming behavior [24]. Therefore, the stress indicator identified by Dias et al. (2022) [24] of increased rates of swimming should be used to assess the enclosure design effect on welfare to improve husbandry practices for X. longipes in order to provide species-specific evidence to guide husbandry for this species.
This study expanded on a proxy of stress previously identified by Dias et al. (2022) [24] and investigates changes in this behavior in response to the presence of an environmental change in order to identify welfare implications of enclosure design which has been previously suggested to influence behavior and welfare [16,17]. The outcomes of this research will be used to inform husbandry practices for the species. This study also explored the use of low-cost dashboard car cameras in X. longipes tanks to record frog behavior.
2. Materials and Methods
2.1. Study Subjects
The study population consisted of 24 wild-caught adult X. longipes, including 7 males and 17 females, housed at ZSL London Zoo since being obtained legally and ethically from the wild in 2008 [21]. Frogs were housed across five tanks in mixed-sex groups. Tanks 1, 2, and 5 contained five frogs, tank 3 contained six frogs, and tank 4 contained three frogs. While the variation in the number and sex ratio of frogs in each tank is recognized by the authors, alteration of tank grouping was deemed to be an unnecessary cause of stress in this study. Each tank contained several terracotta tunnels, large stones, and a section of plastic square-holed mesh, with very similar enclosure layouts across all tanks. Husbandry was otherwise as described by Dias et al. (2022) [24]. The data were collected by a lone observer who had received prior training and practice in this species by members of staff at ZSL London Zoo in order to accurately identify each X. longipes behavior.
2.2. Ethical Approval
Ethical approval was not needed for this study because ZSL London Zoo deems the activities conducted within usual husbandry practices. The Royal Veterinary College, therefore, deemed that explicit ethical approval was not necessary following the conclusion from ZSL London Zoo. The project was registered and approved by ZSL (project registration number ZDR462).
2.3. Data Collection
Five dashboard car cameras (Orskey 1080P resolution with a 6-glass lens with 170 Degree wide angle lens; Orskey, Shenzhen, China) were mounted on tripods, equipped with a 32 GB SD card each, and placed in front of each tank. Electrical tape was used to mark the three points where the tripod legs stood to ensure that the tripod and camera were in the same place for all recordings. Before data collection began, all tanks were cleaned to ensure the background color was visible. The dashboard cameras were plugged individually into timers which were programmed to turn the cameras on and off for observation periods.
The X. longipes were recorded for four weeks in total. Each tank was exposed to four treatments, the order of which were randomly allocated: none (no background; clear glass only as per historic husbandry), green (RGB:31CE36), grey (RGB: 3B3F3F), or black (RGB: 090909). The codes relate to the ratio of red, green, and blue, as well as other colors that create the overall color. The colors used, according to the manufacturer, Simply Plastics (Colchester, UK), were black, grey 9981, and green 650. These colors were selected based on how they reflect the colors found in the natural habitat of the X. longipes and the conclusion from previous research that a black background resulted in a reduced stress response compared to a white background [16]. The acrylic sheets were fixed to the back of the tank using 3 cm strips of black adhesive Velcro strips on all four corners of the acrylic sheets. Backgrounds were 3 mm thick and measured 300 mm in width by 350 mm in length, completely covering the backs of the aquaria. Backgrounds were affixed to the outside of the tank on the back face at 09:00 on the Monday of each week using Velcro tape, and animals were given 2 h to settle into the new condition after the disturbance caused by the background being added. This period was based on experience with the species. The backgrounds remained in place for one week at a time.
Each treatment lasted seven days. The cameras began recording at 11:00 for two hours. Recording took place at the same time each day on Monday, Tuesday, Thursday, and Friday. The SD cards were changed on Wednesday, and no observations took place on this day. The rotation of tank backgrounds is shown in Table 1.

Table 1.
The rotation of colored acrylic sheets acting as backgrounds for the tanks over the data collection period. None refers to the lack of background, which also acted as a control measurement.
Only swimming and resting behavior were observed because they were identified by Dias et al. (2022) [24] to be indicators of stress. Instantaneous scan sampling was used to record swimming and resting behavior at a frequency of one-minute intervals; i.e., the total count of frogs engaged in each behavior was counted at each minute interval. All frogs were in sight at all times. Behavioral definitions followed the ethogram developed by Dias et al. (2022) [24]. Swimming was defined as the subject moving from one location to another through the water, exercising front limbs, back limbs, or both to travel. This may be horizontally or vertically. Resting was defined as the subject being stationary. None of the subject’s limbs are being exercised to actively travel in any direction. This may be in the water or resting on a substrate.
2.4. Statistical Analysis
All analyses were run using R version 4.1.1 using RStudio Version 1.4.17 (RStudio Team, Boston, MA, USA).
Models included four variables. Measured behavior (Swimming or Resting) was the behavior count described above. Background was the color of the background of the aquarium. Day was the day after the background change (1–5, with 1 as the day of the change), treated as a continuous variable. Tank was the aquarium ID.
Generalized Linear Mixed Models, using lme4 [25] and lmertest packages [26] and assuming a Poisson distribution of residuals to account for count data, were used to model the data. Each model used the measured behavior as the response variable, Background as a fixed effect (as we were interested in the specific effects of these colors to inform management protocols), and Day as a fixed covariate. Models were built with and without an interaction between Day and Background. We also included Tank as a random effect allowing variation in random intercept for each level in each model. The random effect controlled for repeated measurements from the same tank and the variation in frog count between tanks. The tank, rather than each individual, was used as the experimental unit, as behaviors were counted for each tank (see above). Within-tank frog count was confounded with tank number, and rearrangement of social groups was not possible due to predicted negative impacts on behavior and welfare [27], so the number of frogs could not be included as an additional predictor [24].
The Akaike Information Criterion (AIC) was used to find the best structure for the model by comparing models with and without interaction; the model with the lowest AIC was chosen for analysis. Temporal autocorrelation was assessed using the nlme package to plot the autocorrelation function (ACF). ACF was always <0.2 and was stable over time; we, therefore, did not correct models for temporal autocorrelation. Model assumptions of distribution of residuals against fitted values were confirmed visually using the ggresid package [28]. Lastly, the ANOVA function of the car package [29] was used, with Type 2 Wald chi-squared tests, to test the effect of each factor (time, background, and interaction) through analysis of deviance with Wald chi-square tests.
3. Results
The AIC was lowest for the models that included the day, background color, and their interaction for both swimming and resting. Therefore, the interaction model was used for resting and swimming analysis. The swim interaction AIC was 2518.443, and the degree of freedom was 9. The resting interaction AIC was 1270.353, and the degree of freedom was 9.
Swimming decreased over time, with a significant interaction between Background and Day (see Figure 1 and Table 2 and Table 3). All backgrounds showed a significant decrease in swimming behavior over time relative to no background (Table 3). Resting increased over time after background change overall, with the slope varying significantly between backgrounds (see Table 2 and Table 3; Figure 2).
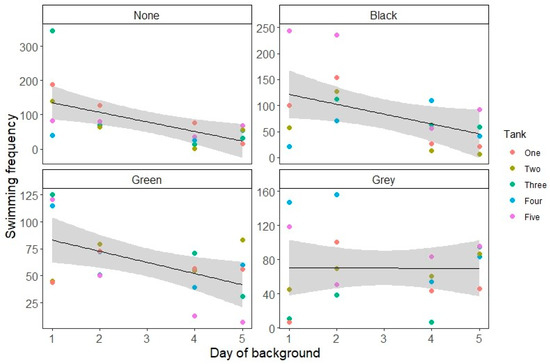
Figure 1.
Swimming count over time for each tank background, presented as swimming count for each tank on each day of the observation period. The grey area represents the standard error of the estimate.

Table 2.
The results of the analysis of deviance analysis for both swimming and resting models.

Table 3.
Model summary table of generalized linear mixed models swimming and resting behavior.
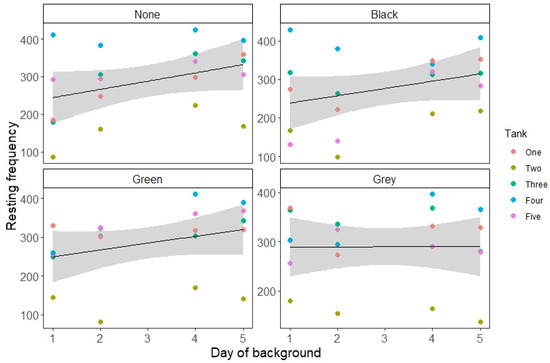
Figure 2.
Resting count over time for each tank background, presented as swimming count for each tank on each day of the observation period. The grey area represents the standard error of the estimate.
4. Discussion
Our models detected significant effects of background, days post-background change, and the interaction between variables on both swimming and resting behaviors. All background colors elicited, to varying degrees, an increase in swimming and a decrease in resting behavior immediately after installation. After this point, swimming behavior decreased, and resting behavior increased over the five days post-change, at different rates until, at five days post-change, both behaviors were exhibited at similar levels under all background conditions. Given the association between these behaviors and likely stress response [24], these results suggest an initial stress response to changing background, modulated by background color, followed by habituation over five days. Marginal R squared values indicate a relatively good model fit for swimming behavior but a poorer fit for resting, while conditional to marginal R squared ratios and random effect standard deviations indicate an important contribution of tank for resting and a relatively unimportant contribution for tank on swimming behavior. Swimming is the behavior positively associated with stressors in this species and is consequently the more important indicator of the two, while resting is simply the behavior most traded off against an increase in swimming behavior [24]. It is, therefore, more important to have a good model fit for this behavior, which is the case.
The initial increase in swimming and a decrease in resting frequency were observed in all five tanks immediately following the change to each of the four background options, which varied with the background color. This indicates a stress response as a result of environmental change (both the change in background and the vibrations associated with making the change), which was mediated by background color. The green and grey backgrounds resulted in the smallest initial increase in swimming and decrease in resting, suggesting that they mitigated some of the stress response to change. Predation pressure has resulted in high importance for camouflage in many amphibian species [30] and has likely driven the cryptic coloration of X. longipes. Green and grey background colors may enable the camouflage of frogs most effectively because the grey is most similar to the color of the X. longipes and the green background imitates the aquatic plant life of Lake Oku [31]. Similar results were found in X. laevis, which is known to rely on cryptic camouflage, with increases in corticosterone release and in stress-associated behaviors and reduction in body mass when housed with non-ecologically relevant background colors [16]. Green or grey background colors may, therefore, have a dampening effect on acute stress and be useful in the husbandry of X. longipes to mitigate against the effects of short-term stressors such as disturbance by humans.
Across all tanks, during the five-day observation period following background change, swimming decreased and resting increased until, at five days, both behaviors were the same under all treatments and were similar to baseline behaviors for this species [24]. This suggests that the frogs habituate to the change in background by 5 days post-change. In X. laevis, the proportion of walling behavior (a stress-elicited behavior absent in X. longipes, [24] indicated potential habituation to the tank background within 30 min [16]. In X. longipes, swimming behavior appears to be subject to much slower habituation than walling behavior in X. laevis, potentially because it may be a less acute stress-specific behavior and so may be less subject to threshold effects.
This study represents an initial investigation into background color for this species using a newly developed behavioral indicator, and several questions remain unaddressed. Effects of the specific order of backgrounds could not be explored due to limitations of experimental design constrained by timeframe and sample size.
Choice chambers have been used historically to inform amphibian husbandry and further understand welfare [32,33,34]. Preference tests to augment behavioral observations were outside of the scope of this study, but future work could further investigate background color using this approach. Similarly, Hilken et al. (1995) [35] showed the effects of background color on the growth rates of juvenile X. laevis, indicating more long-term physiological effects on this species. Should X. longipes be reproduced in captivity in suitable numbers, this experimental design could be used for this species. Physiological measures of welfare are necessary to validate the potential behavioral indicators of welfare. Currently, behavioral indicators of stress in X. longipes are founded on associations between behavioral changes and presumed acute stressors (capture and restraint of animals) [24]. Validation of this behavioral measure via corticosterone, as has been demonstrated in X. laevis [16], is a key next step in improving the robustness of stress indicators in X. longipes.
5. Conclusions
Our data suggest that background color may act as a modulator of stress response in X. longipes, as stress-linked behaviors were less pronounced with green and grey backgrounds than with black or no background following the disturbance associated with changing treatments. We detected evidence for habituation to new background colors, or the extinction of a stress response, as stress-linked behaviors adjusted and converged over five days to levels similar to baseline behavior in this species, suggesting that there is no long-term direct effect of background color on stress in this species. Our results, therefore, recommend the use of green or grey backgrounds for this species, not due to their direct effects but their apparent dampening effects on acute stress.
Author Contributions
Conceptualization, A.E.G., J.E.D. and C.J.M.; Methodology, A.E.G., J.E.D. and C.J.M.; Software, A.E.G. and C.J.M.; Validation, A.E.G., J.E.D. and C.J.M.; Formal Analysis, A.E.G. and C.J.M.; Investigation, A.E.G.; Resources, A.E.G., J.E.D. and C.J.M.; Data Curation, A.E.G. and C.J.M.; Writing—Original Draft Preparation, A.E.G. and C.J.M.; Writing—Review and Editing, A.E.G., J.E.D. and C.J.M.; Visualization, A.E.G. and C.J.M.; Supervision, C.J.M.; Project Administration, A.E.G. and C.J.M.; Funding Acquisition, A.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a small student research project grant provided by the Royal Veterinary College covering equipment.
Institutional Review Board Statement
Ethical review and approval were not necessary for this study because there was no deviation from standard husbandry practice. Further details are in the Methods, which covers details of institution review and approval.
Data Availability Statement
Data are available at https://github.com/CJMichaels/Graves-et-al-Xenopus-longipes-background (accessed on 20 January 2023).
Acknowledgments
This study was conducted as part of the MSci Wild Animal Biology course with the Royal Veterinary College. This study was made possible by the supportive contributions of Francesca Servini, Unnar Aevarsson, and Daniel Kane, who all carried out husbandry on the X. longipes and supported practical data collection. The captive population of X. longipes was first exported in 2008 and 2012 under a permit from the Cameroon Ministry of Forestry and Wildlife (0928/PRBS/MINFOF/SG/DFAP/SDVEF/SC and 0193/CO/MINFOF/SG/DFAP/SDVEF/SC), after prior consultation with the community of Oku.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barrows, M. Welfare assessment in zoo animals. Vet. Rec. 2017, 181, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Brod, S.; Brookes, L.; Garner, T.W.J. Discussing the future of amphibians in research. Lab. Anim. 2019, 48, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Michaels, C.; Downie, R.; Campbell-Palmer, R. The importance of enrichment for advancing amphibian welfare and conservation goals: A review of a neglected topic. Amphib. Reptile Conserv. 2014, 8, 7–23. [Google Scholar]
- Maple, T.L. Toward a Science of Welfare for Animals in the Zoo. J. Appl. Anim. Welf. Sci. 2007, 10, 63–70. [Google Scholar] [CrossRef]
- Beebee, T.J.C.; Griffiths, R.A. The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 2005, 125, 271–285. [Google Scholar] [CrossRef]
- Browne, R.K.; Wolfram, K.; García, G.; Bagaturov, M.F.; Pereboom, Z.J.J.M. Zoo-based amphibian research and conservation breeding programs. Amphib. Reptile Conserv. 2011, 5, 1–14. [Google Scholar]
- Tillbrook, A.J.; Ralph, C.R. Hormones, stress and the welfare of animals. Anim. Prod. Sci. 2017, 58, 408–415. [Google Scholar] [CrossRef]
- Holmes, A.M.; Emmans, C.J.; Coleman, R.; Smith, T.E.; Hosie, C.A. Effects of transportation, transport medium and re-housing on Xenopus laevis (Daudin). Gen. Comp. Endocrinol. 2018, 266, 21–28. [Google Scholar] [CrossRef]
- Michaels, C.J. Effects of aquatic and terrestrial habitats on the skin microbiome and growth rate of juvenile alpine newts Ichthyosaura alpestris. Herpetol. J. 2022, 2, 51–58. [Google Scholar] [CrossRef]
- Carter, K.C.; Fieschi-Méric, L.; Servini, F.; Wilkinson, M.; Gower, D.J.; Tapley, B.; Michaels, C.J. Investigating the Effect of Disturbance on Prey Consumption in Captive Congo Caecilians Herpele squalostoma. J. Zool. Bot. Gard. 2021, 2, 705–715. [Google Scholar] [CrossRef]
- Boultwood, J.; O’Brien, M.; Rose, P. Bold frogs or shy toads? How did the COVID-19 closure of zoological organisations affect amphibian activity? Animals 2021, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Antwis, R.E.; Preziosi, R.F.; Fidgett, A.L. The effect of different UV and calcium provisioning on health and fitness traits of red-eyed tree frogs (Agalychnis callidryas). J. Zoo Aquar. Res. 2014, 2, 69–76. [Google Scholar]
- Michaels, C.J.; Antwis, R.E.; Preziosi, R.F. Impact of plant cover on fitness and behavioural traits of captive red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 2014, 9, e95207. [Google Scholar] [CrossRef]
- Holmes, A.M.; Emmans, C.J.; Jones, N.; Coleman, R.; Smith, T.E.; Hosie, C.A. Impact of tank background on the welfare of the African clawed frog, Xenopus laevis (Daudin). Appl. Anim. Behav. Sci. 2016, 185, 131–136. [Google Scholar] [CrossRef]
- Hilken, G.; Willmann, F.; Dimigen, J.; Iglauer, F. Preference of Xenopus laevis for different housing conditions. Scand. J. Lab. Anim. Sci. 1994, 21, 71. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Xenopus longipes (amended version of 2017 assessment). In The IUCN Red List of Threatened Species 2020: E.T58176A177346697; IUCN: Gland, Switzerland, 2020; Available online: https://www.iucnredlist.org/species/58176/177346697 (accessed on 10 December 2022).
- Blackburn, D.C.; Evans, B.J.; Pessier, A.P.; Vredenburg, V.T. An enigmatic mortality event in the only population of the Critically Endangered Cameroonian frog Xenopus longipes. Afr. J. Herpetol. 2010, 59, 111–122. [Google Scholar] [CrossRef]
- Doherty-Bone, T.M.; Ndifon, R.K.; Nyingchia, O.N.; Landrie, F.E.; Yonghabi, F.T.; Duffus, A.L.J.; Price, S.; Perkins, M.; Bielby, J.; Kome, N.B.; et al. Morbidity and mortality of the Critically Endangered Lake Oku clawed frog Xenopus longipes. Endanger. Species Res. 2013, 21, 115–128. [Google Scholar] [CrossRef]
- Michaels, C.J.; Tapley, B.; Harding, L.; Bryant, Z.; Grant, S.; Sunter, G.; Gill, I.; Nyingchia, O.; Doherty-Bone, T. Breeding and rearing the Critically Endangered Lake Oku Clawed Frog (Xenopus longipes Loumont and Kobel 1991). Amphib. Reptile Conserv. 2015, 9, 100–110. [Google Scholar]
- Gurdon, J.B.; Hopwood, N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2000, 44, 43–50. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Xenopus laevis. In The IUCN Red List of Threatened Species 2020: E.T110466172A3066881; Available online: https://www.iucnredlist.org/species/110466172/3066881 (accessed on 10 December 2022).
- Dias, J.E.; Ellis, C.; Smith, T.E.; Hosie, C.A.; Tapley, B.; Michaels, C.J. Baseline Behavioral Data and Behavioral Correlates of Disturbance for the Lake Oku Clawed Frog (Xenopus longipes). J. Zool. Bot. Gard. 2022, 3, 184–197. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Baird, T.A. Influence of social and predatory stimuli on the air-breathing behavior of the African clawed frog, Xenopus laevis. Copeia 1983, 2, 411–420. [Google Scholar] [CrossRef]
- Goode, K.; Rey, K. ggResidpanel Tutorial and User Manual. 2019. Available online: https://goodekat.github.io/ggResidpanel-tutorial/tutorial.html (accessed on 10 December 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 10 December 2022).
- Polo-Cavia, N.; Gomez-Mestre, I. Pigmentation plasticity enhances crypsis in larval newts: Associated metabolic cost and background choice behaviour. Sci. Rep. 2017, 7, 39739. [Google Scholar] [CrossRef] [PubMed]
- Tapley, B.; Michaels, C.; Harding, L.; Bryant, Z.; Gill, I.; Grant, S.; Chaney, N.; Dunker, F.; Freiermuth, B.; Willis, J.; et al. Amphibian Taxon Advisory Group Best Practice Guidelines for the Lake Oku frog Xenopus longipes, Version 1; EAZA: Amsterdam, The Netherlands, 2016; Available online: https://www.eaza.net/assets/Uploads/CCC/2016-Lake-Oku-frog-EAZA-Best-Practice-Guidelines-Approved.pdf (accessed on 10 December 2022).
- Whatley, C.; Tapley, B.; Michaels, C.J.; Gower, D.J.; Wilkinson, M. Substrate preference in the fossorial caecilian Microcaecila unicolor (Amphibia: Gymnophiona, Siphonopidae). Herpetol. Bull. 2020, 152, 18–20. [Google Scholar] [CrossRef]
- Guimarães, I.S.C.; Hemnani, M.; Kaefer, I.L.; da Silva Pires, T.H. Fear of the dark: Substrate preference in Amazonian tadpoles. Acta Ethol. 2021, 24, 177–183. [Google Scholar] [CrossRef]
- Ramos, J.; Ortiz-Díez, G. Evaluation of environmental enrichment for Xenopus laevis using a preference test. Lab. Anim. 2021, 55, 428–434. [Google Scholar] [CrossRef]
- Hilken, G.; Dimigen, J.; Iglauer, F. Growth of Xenopus laevis under different laboratory rearing conditions. Lab. Anim. 1995, 29, 152–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).