Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Sites
2.2. Behavioural Observations
2.3. Data Analysis
2.4. Ethical Review Statement
3. Results
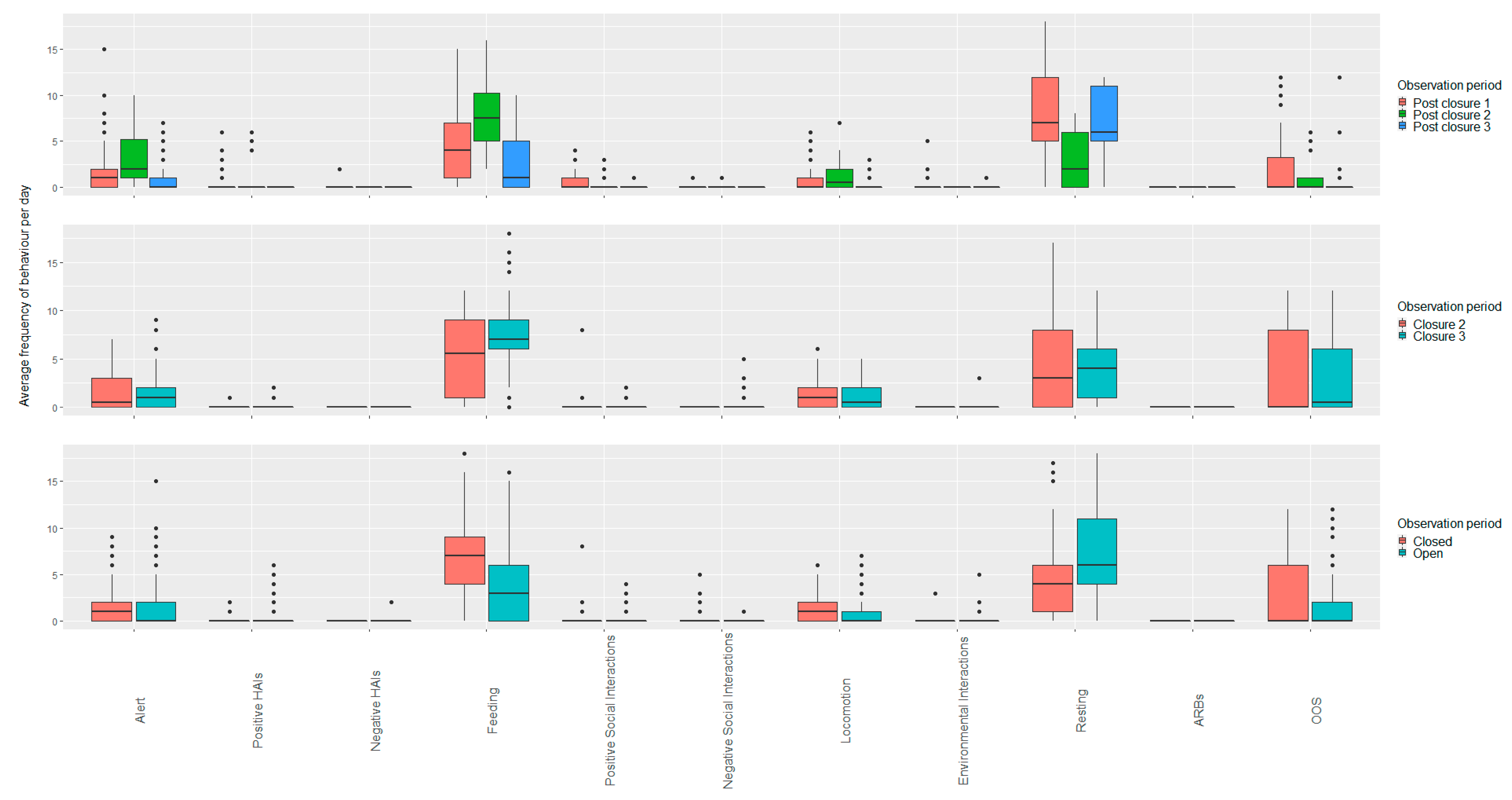
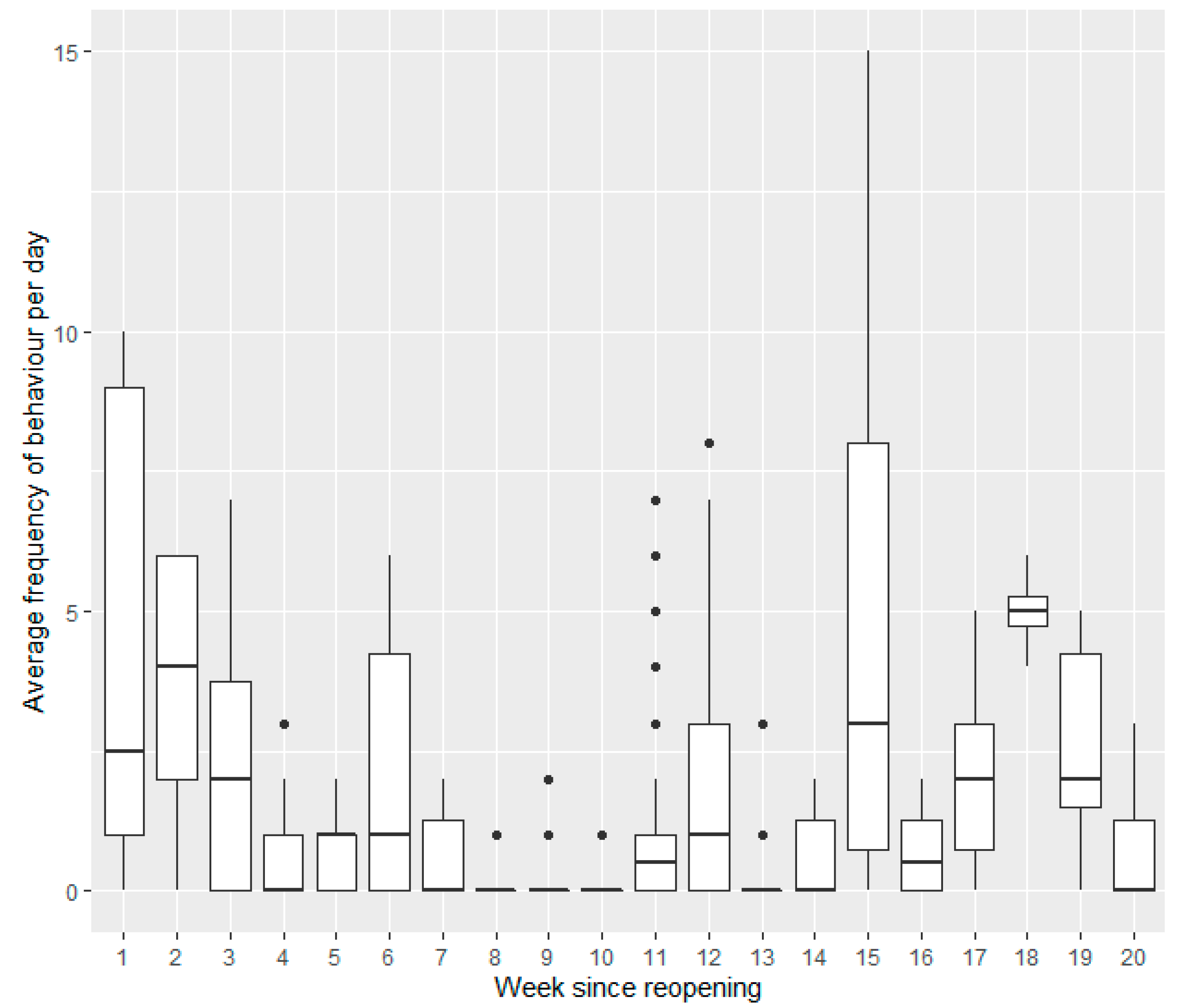
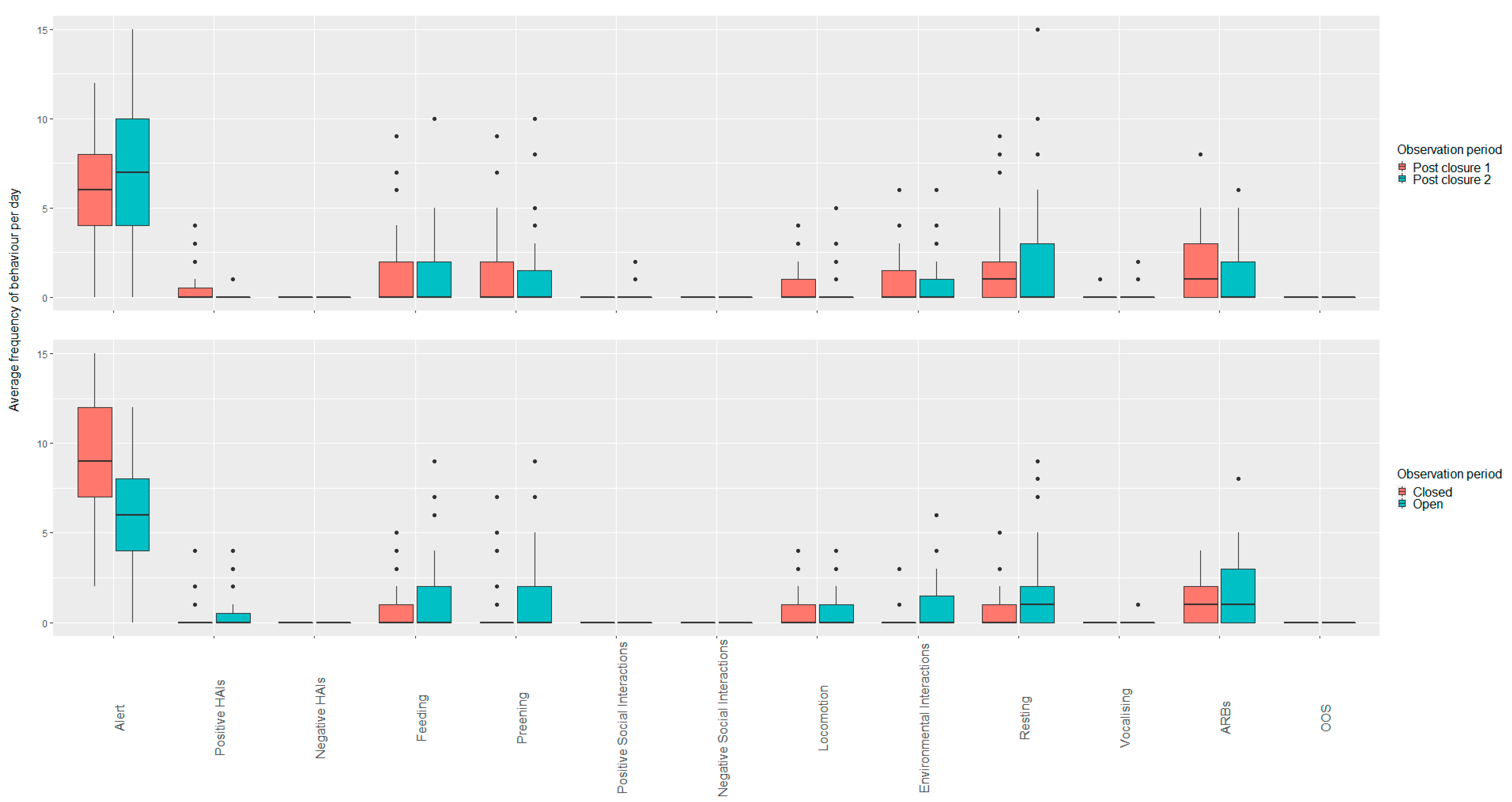
3.1. Wallabies
3.1.1. Open vs. Closed Periods (Closure Two and Three; Post Closure One, Two and Three)
3.1.2. Closed Period Comparisons (Closure Period Two and Three)
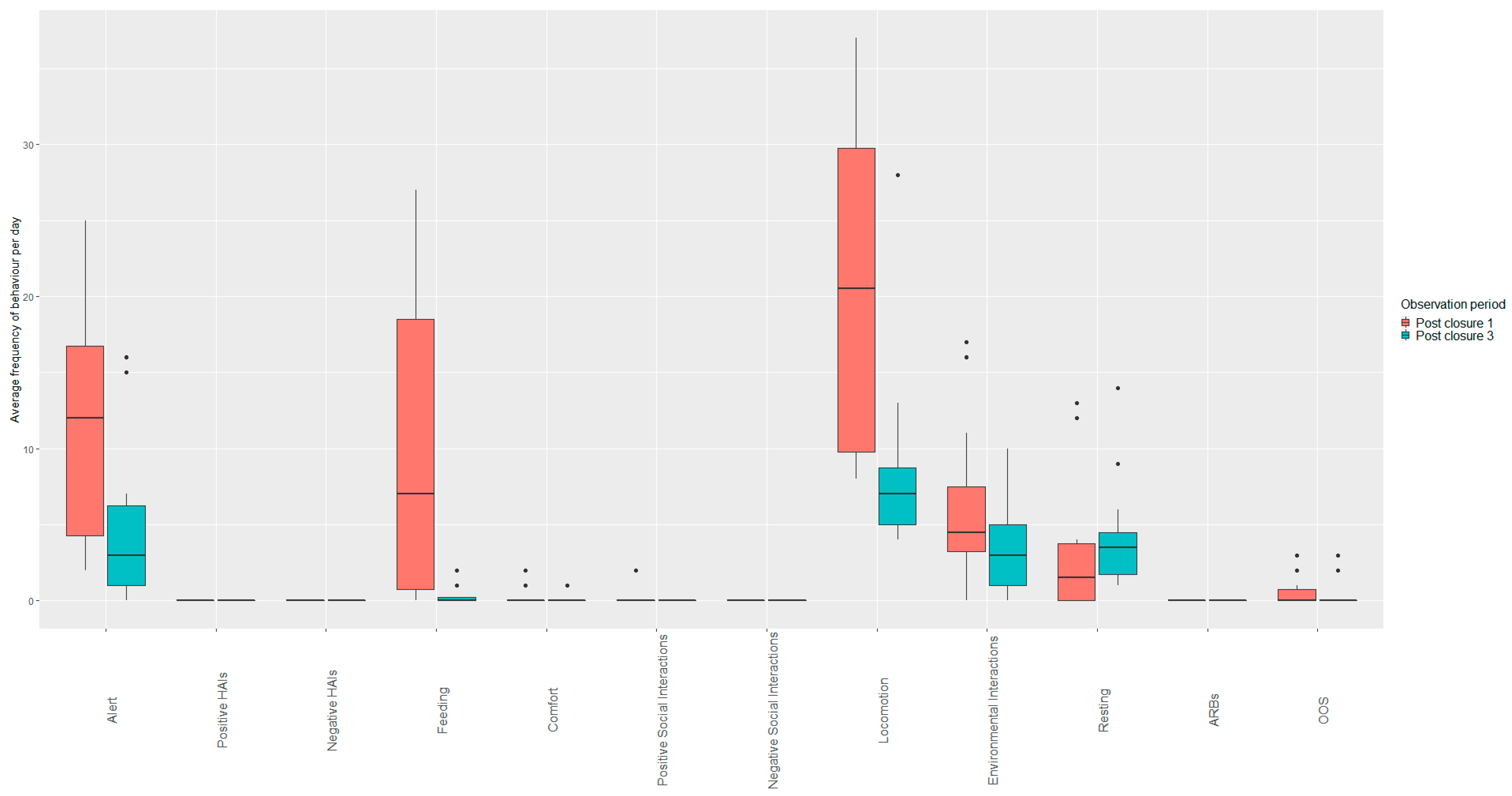
3.1.3. Open Period Comparisons (Post Closure One, Two and Three)
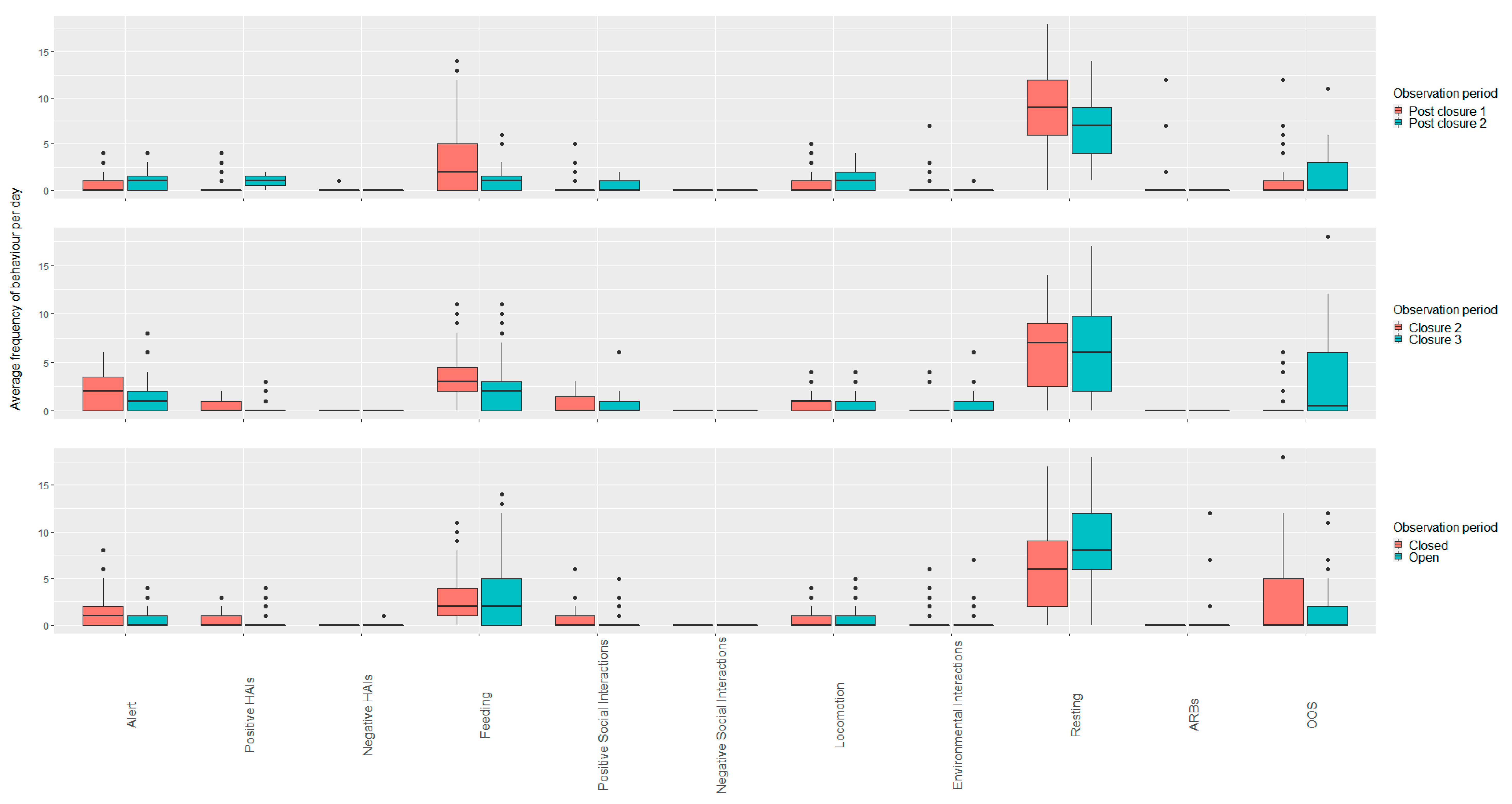
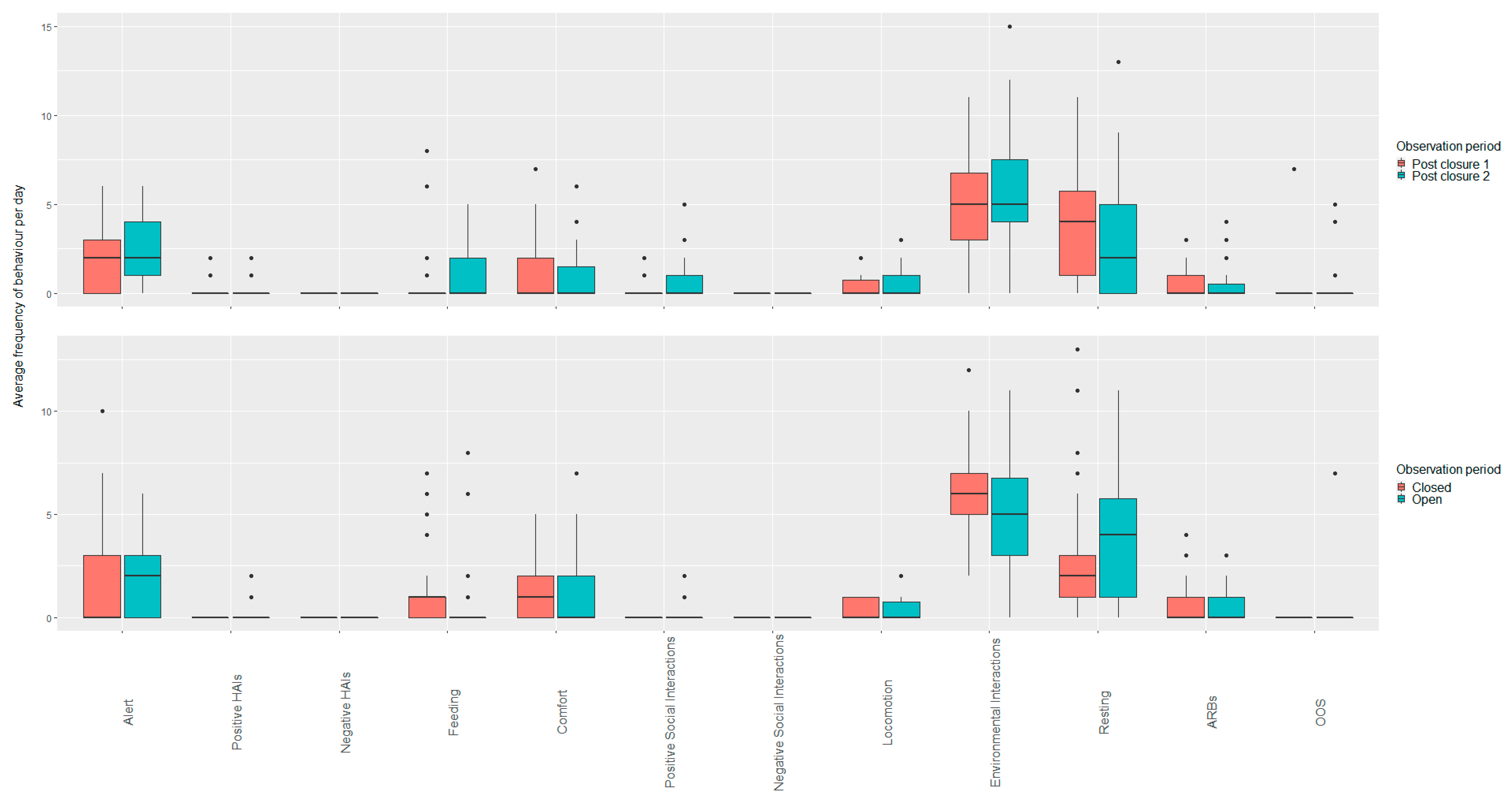
3.2. Rabbits
3.2.1. Open vs. Closed Periods (Closure Periods Two and Three; Post Closure One and Two)
3.2.2. Closed Period Comparisons (Closure Period Two and Three)
3.2.3. Open Period Comparisons (Post Closure One and Two)
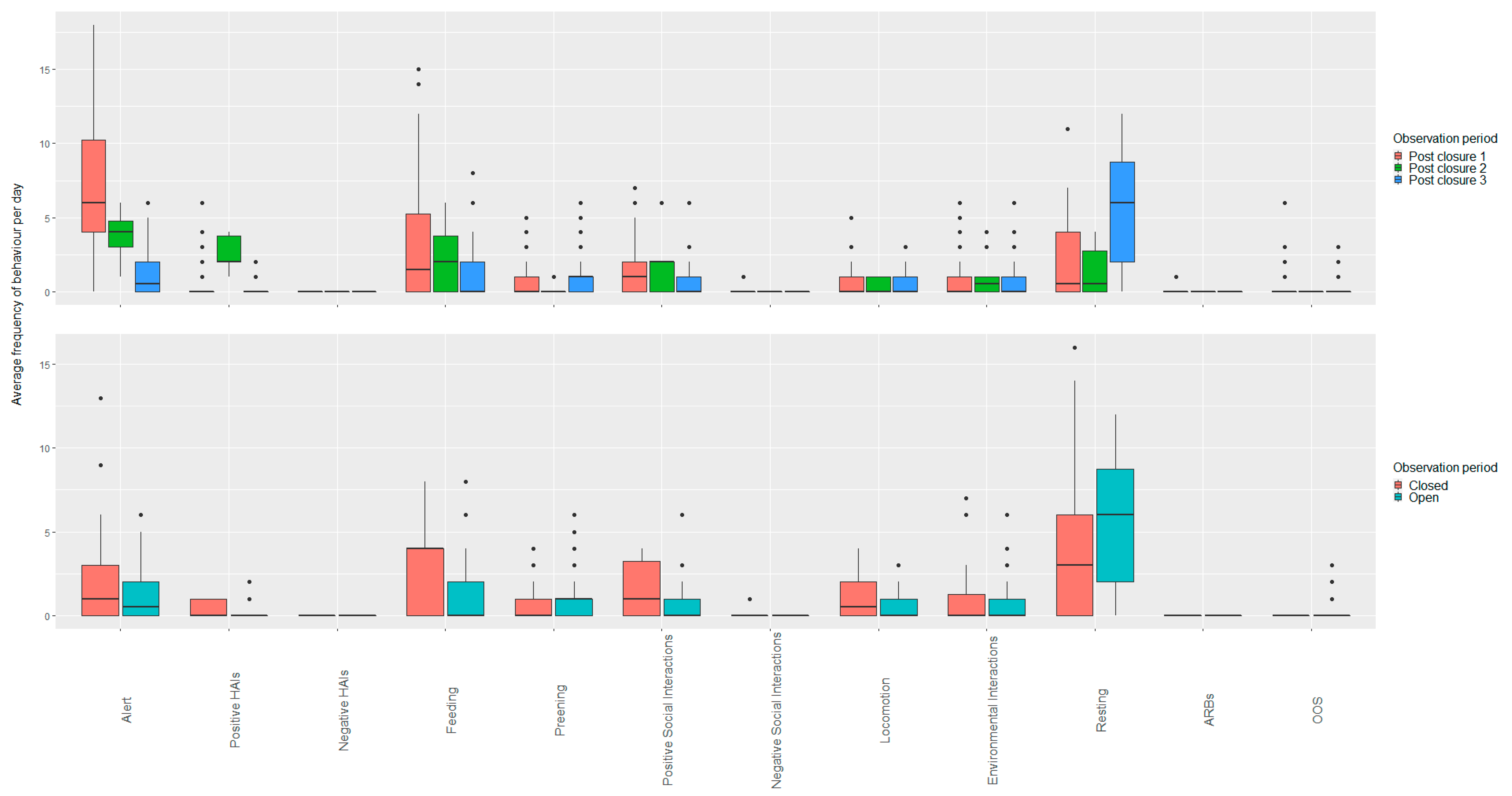
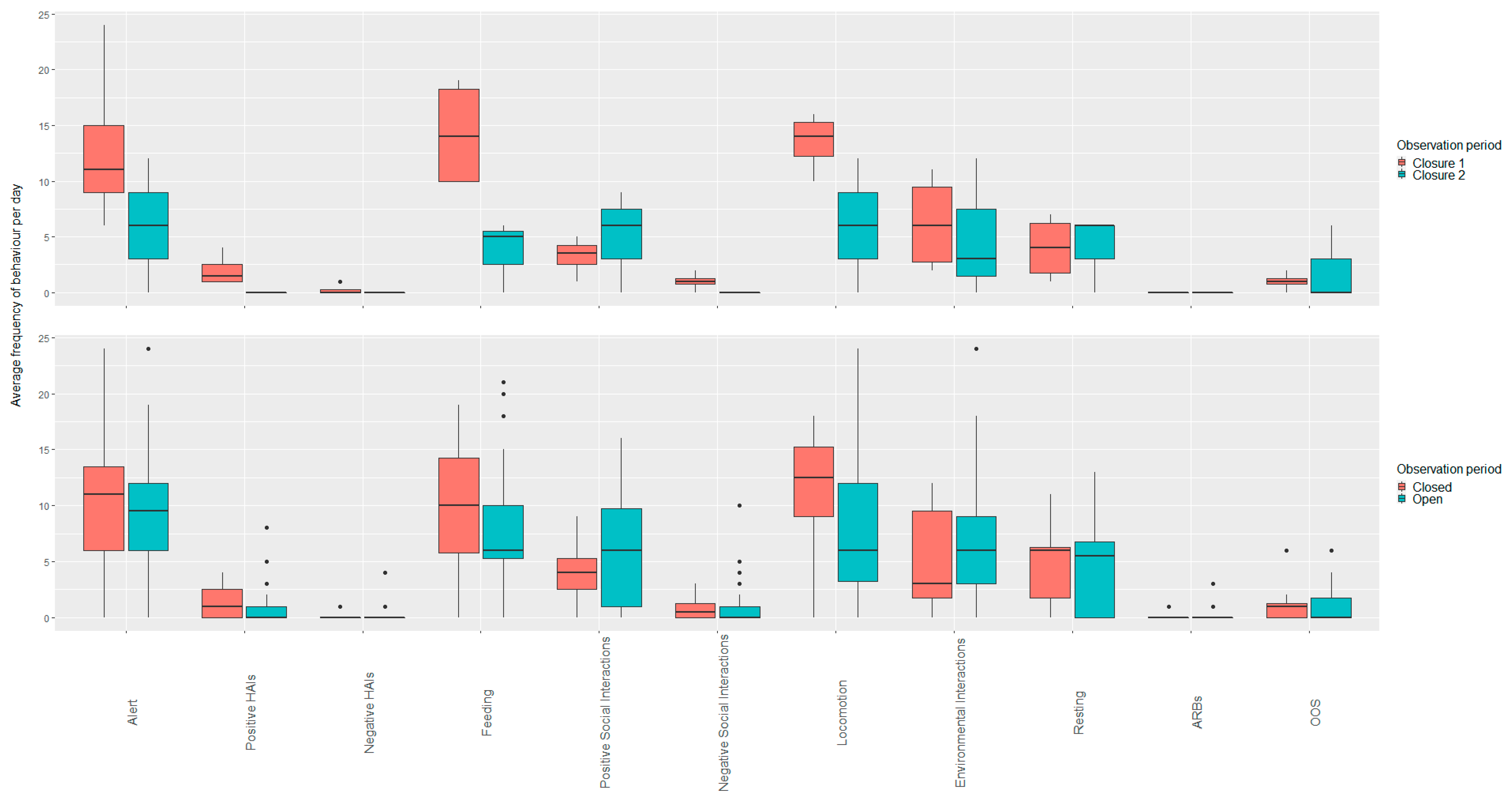
3.3. Macaws
3.3.1. White Post Farm
Open vs. Closed Periods (Closure Three)
Open Period Comparisons (Post Closure One, Two and Three)
3.3.2. Plantasia
Open vs. Closed Periods (Closure One)
Open Period Comparisons (Post Closure One and Two)
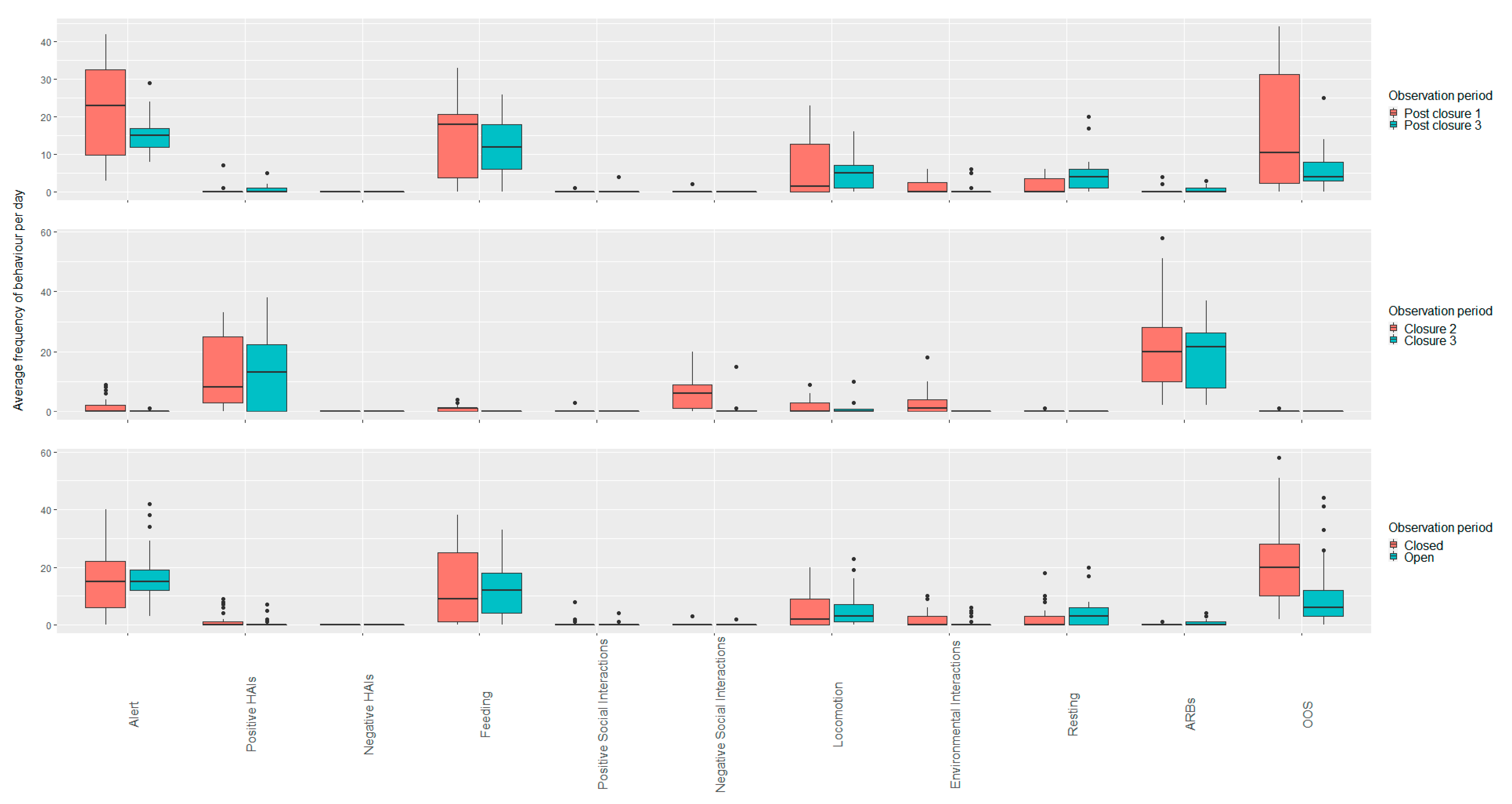
3.4. Meerkats
3.4.1. White Post Farm
Open vs. Closed Periods (All Open and Closure Periods)
Closure Period Comparisons (Closure Two and Three)
Open Period Comparisons (Post Closure One and Three)
3.4.2. Plantasia
Open vs. Closed Periods (Closure One)
Open Period Comparisons (Post Closure One and Two)
3.4.3. Knowsley Safari
Open vs. Closed Periods (Closure One and Two; Post Closure One)
Closure Period Comparisons (Closure One and Two)
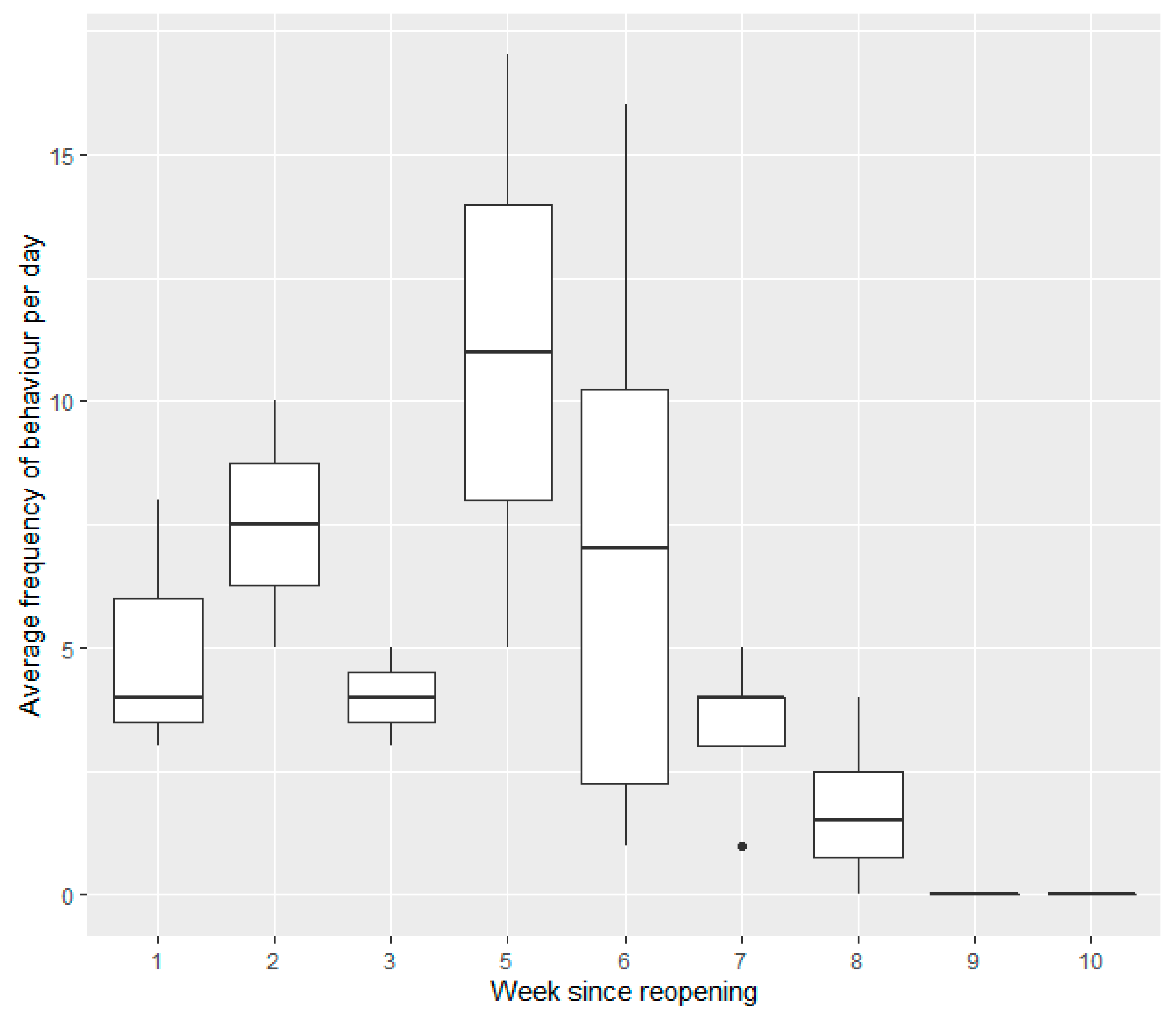
Weeks since Reopening (Post Closure One)
3.4.4. Dartmoor Zoo
Open Period Comparisons (Post Closure One and Three)
4. Discussion
4.1. Wallabies
4.2. Rabbits
4.3. Macaws
4.4. Meerkats
4.5. Impact of Prior Interactions with Humans
4.6. Limitations
4.6.1. Seasonal Impacts on Behaviour
4.6.2. Data Collection Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WAZA World’s Leading Zoos and Aquariums. Available online: https://www.waza.org/ (accessed on 2 April 2022).
- Miller, M.E.; Robinson, C.M.; Margulis, S.W. Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop. Animals 2021, 11, 1346. [Google Scholar] [CrossRef]
- Roth, A.M.; Cords, M. Zoo Visitors Affect Sleep, Displacement Activities, and Affiliative and Aggressive Behaviors in Captive Ebony Langurs (Trachypithecus Auratus). Acta Ethol. 2020, 23, 61–68. [Google Scholar] [CrossRef]
- Chiew, S.J.; Butler, K.L.; Sherwen, S.L.; Coleman, G.J.; Fanson, K.V.; Hemsworth, P.H. Effects of Regulating Visitor Viewing Proximity and the Intensity of Visitor Behaviour on Little Penguin (Eudyptula Minor) Behaviour and Welfare. Animals 2019, 9, 285. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.R.; Passos, L.; Vecci, M.A.M.; Young, R.J. Zoo Visitor Effect on Mammal Behaviour: Does Noise Matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Hosey, G.R. Zoo Animals and Their Human Audiences: What Is the Visitor Effect? Anim. Welf. 2000, 9, 343–357. [Google Scholar]
- Sherwen, S.; Harvey, T.; Magrath, M.; Butler, K.L.; Fanson, K.; Hemsworth, P.H. Effects of Visual Contact with Zoo Visitors on Black-Capped Capuchin Welfare. Appl. Anim. Behav. Sci. 2015, 167, 65–73. [Google Scholar] [CrossRef]
- Rajagopal, T.; Archunan, G.; Sekar, M. Impact of Zoo Visitors on the Fecal Cortisol Levels and Behavior of an Endangered Species: Indian Blackbuck (Antelope cervicapra L.). J. Appl. Anim. Welf. Sci. 2011, 14, 18–32. [Google Scholar] [CrossRef]
- de Vere, A.J. Visitor Effects on a Zoo Population of California Sea Lions (Zalophus Californianus) and Harbor Seals (Phoca Vitulina). Zoo Biol. 2018, 37, 162–170. [Google Scholar] [CrossRef]
- Rabat, A. Extra-Auditory Effects of Noise in Laboratory Animals: The Relationship between Noise and Sleep. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 35–41. [Google Scholar]
- Rabat, A.; Bouyer, J.J.; Aran, J.M.; Courtiere, A.; Mayo, W.; Le Moal, M. Deleterious Effects of an Environmental Noise on Sleep and Contribution of Its Physical Components in a Rat Model. Brain Res. 2004, 1009, 88–97. [Google Scholar] [CrossRef]
- Hunton, V.; Rendle, J.; Carter, A.; Williams, E. Communication from the Zoo: Reports from Zoological Facilities of the Impact of COVID-19 Closures on Animals. J. Zool. Bot. Gard. 2022, 3, 271–288. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Impacts of COVID-19 on Animals in Zoos: A Longitudinal Multi-Species Analysis. J. Zool. Bot. Gard. 2021, 2, 130–145. [Google Scholar] [CrossRef]
- Cairo-Evans, A.; Wierzal, N.K.; Wark, J.D.; Cronin, K.A. Do Zoo-housed Primates Retreat from Crowds? A Simple Study of Five Primate Species. Am. J. Primatol. 2022, 84, e23386. [Google Scholar] [CrossRef]
- Edes, A.N.; Liu, N.C.; Baskir, E.; Bauman, K.L.; Kozlowski, C.P.; Clawitter, H.L.; Powell, D.M. Comparing Space Use and Fecal Glucocorticoid Concentrations during and after the COVID-19 Closure to Investigate Visitor Effects in Multiple Species. J. Zool. Bot. Gard. 2022, 3, 328–348. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Fontani, S.; Walsh, N.D.; Armstrong, S.; Hickman, S.; Vaglio, S.; Ward, S.J. The Impact of COVID-19 Zoo Closures on Behavioural and Physiological Parameters of Welfare in Primates. Animals 2022, 12, 1622. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Understanding Impacts of Zoo Visitors: Quantifying Behavioural Changes of Two Popular Zoo Species during COVID-19 Closures. Appl. Anim. Behav. Sci. 2021, 236, 105253. [Google Scholar] [CrossRef]
- Huskisson, S.M.; Doelling, C.R.; Ross, S.R.; Hopper, L.M. Assessing the Potential Impact of Zoo Visitors on the Welfare and Cognitive Performance of Japanese Macaques. Appl. Anim. Behav. Sci. 2021, 243, 105453. [Google Scholar] [CrossRef]
- Finch, K.; Leary, M.; Holmes, L.; Williams, L.J. Zoo Closure Does Not Affect Behavior and Activity Patterns of Palawan Binturong (Arctictis Binturong Whitei). J. Zool. Bot. Gard. 2022, 3, 398–408. [Google Scholar] [CrossRef]
- Masman, M.; Scarpace, C.; Liriano, A.; Margulis, S.W. Does the Absence of Zoo Visitors during the COVID-19 Pandemic Impact Gorilla Behavior? J. Zool. Bot. Gard. 2022, 3, 349–356. [Google Scholar] [CrossRef]
- Kidd, P.; Ford, S.; Rose, P.E. Exploring the Effect of the COVID-19 Zoo Closure Period on Flamingo Behaviour and Enclosure Use at Two Institutions. Birds 2022, 3, 117–137. [Google Scholar] [CrossRef]
- Podturkin, A.A. Behavioral Changes of Brown Bears (Ursus Arctos) during COVID-19 Zoo Closures and Further Reopening to the Public. J. Zool. Bot. Gard. 2022, 3, 256–270. [Google Scholar] [CrossRef]
- Salak, R.E.; Cloutier Barbour, C. Is Chimpanzee (Pan Troglodytes) Wounding Frequency Affected by the Presence Versus Absence of Visitors? A Multi-Institutional Study. J. Zool. Bot. Gard. 2022, 3, 316–327. [Google Scholar] [CrossRef]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.C.; Soltis, J.; Leeds, A. Evaluating the Effect of Visitor Presence on Nile Crocodile (Crocodylus Niloticus) Behavior. J. Zool. Bot. Gard. 2021, 2, 115–129. [Google Scholar] [CrossRef]
- Carter, K.C.; Keane, I.A.; Clifforde, L.M.; Rowden, L.J.; Fieschi-Méric, L.; Michaels, C.J. The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. J. Zool. Bot. Gard. 2021, 2, 664–676. [Google Scholar] [CrossRef]
- Hamilton, J.; Gartland, K.N.; Jones, M.; Fuller, G. Behavioral Assessment of Six Reptile Species during a Temporary Zoo Closure and Reopening. Animals 2022, 12, 1034. [Google Scholar] [CrossRef]
- Jones, M.; Gartland, K.; Fuller, G. Effects of Visitor Presence and Crowd Size on Zoo-Housed Red Kangaroos (Macropus Rufus) during and after a COVID-19 Closure. Anim. Behav. Cogn. 2021, 8, 521–537. [Google Scholar] [CrossRef]
- Boultwood, J.; O’Brien, M.; Rose, P. Bold Frogs or Shy Toads? How Did the COVID-19 Closure of Zoological Organisations Affect Amphibian Activity? Animals 2021, 11, 1982. [Google Scholar] [CrossRef]
- RStudio: Integrated Development for R; R Studio Team: Boston, MA, USA, 2020.
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots R package version 0.4.0; 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 29 August 2022).
- Sherwen, S.L.; Hemsworth, P.H.; Butler, K.L.; Fanson, K.V.; Magrath, M.J.L. Impacts of Visitor Number on Kangaroos Housed in Free-Range Exhibits. Zoo Biol. 2015, 34, 287–295. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Van Bemmel, E.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals 2019, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Krebs, B.L.; Eschmann, C.L.; Watters, J.V. Dither: A Unifying Model of the Effects of Visitor Numbers on Zoo Animal Behavior. Zoo Biol. 2022. [Google Scholar] [CrossRef]
- Rizzo, C. A Japanese Aquarium Is Urging People to Have Video Calls with Its Eels to Make Them Feel Less Alone. Available online: https://www.insider.com/facetime-an-eel-social-anxiety-japan-aquarium-2020-5 (accessed on 24 September 2022).
- Wright, R. Some Zoos, and Some of Their Animals, May Not Survive the Pandemic. The New Yorker, 18 May 2020. Available online: https://www.newyorker.com/news/our-columnists/some-zoos-and-some-of-their-animals-may-not-survive-the-pandemic (accessed on 8 October 2022).
- Bittel, J. Zoos Are Closed Because of Coronavirus, but the Animals Still Need Care. The Washington Post, 27 March 2020. Available online: https://www.washingtonpost.com/science/2020/03/27/zoos-are-closed-due-coronavirus-animals-still-need-care/ (accessed on 1 October 2022).
- Hosey, G.; Melfi, V. Human-Animal Interactions, Relationships and Bonds: A Review and Analysis of the Literature. Int. J. Comp. Psychol. 2014, 27, 117–142. [Google Scholar] [CrossRef]
- Godinez, A.M.; Fernandez, E.J. What Is the Zoo Experience? How Zoos Impact a Visitor’s Behaviors, Perceptions, and Conservation Efforts. Front. Psychol. 2019, 10, 1746. [Google Scholar] [CrossRef]
- Perlow, B. Life under COVID-19 for the Animals and Zookeepers at the Maryland Zoo. Available online: https://abcnews.go.com/US/life-covid-19-animals-zookeepers-maryland-zoo/story?id=70422788 (accessed on 24 September 2022).
- Williams, I.; Hoppitt, W.; Grant, R. The Effect of Auditory Enrichment, Rearing Method and Social Environment on the Behavior of Zoo-Housed Psittacines (Aves: Psittaciformes); Implications for Welfare. Appl. Anim. Behav. Sci. 2017, 186, 85–92. [Google Scholar] [CrossRef]
- Woods, J.M.; Eyer, A.; Miller, L.J. Bird Welfare in Zoos and Aquariums: General Insights across Industries. J. Zool. Bot. Gard. 2022, 3, 198–222. [Google Scholar] [CrossRef]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of Animal Welfare Research in Zoos and Aquariums: Where Are We, Where to Next? J. Zoo Aquar. Res. 2020, 8, 166–174. [Google Scholar] [CrossRef]
- Zhang, J.; Quirke, T.; Wu, S.; Li, S.; Butler, F. Impact of Weather Changes and Human Visitation on the Behavior and Activity Level of Captive Humboldt Penguins. Pak. J. Zool. 2021, 53, 591–602. [Google Scholar] [CrossRef]
- Eckert, N. Animals Notice Something Missing at the Zoo—People. Available online: https://www.wsj.com/articles/animals-zoo-covid-closures-lockdown-pandemic-los-angeles-phoenix-11596827024 (accessed on 24 September 2022).
- Sherwen, S.L.; Magrath, M.J.L.; Butler, K.L.; Phillips, C.J.C.; Hemsworth, P.H. A Multi-Enclosure Study Investigating the Behavioural Response of Meerkats to Zoo Visitors. Appl. Anim. Behav. Sci. 2014, 156, 70–77. [Google Scholar] [CrossRef]
- Scott, K.; Heistermann, M.; Cant, M.A.; Vitikainen, E.I.K. Group Size and Visitor Numbers Predict Faecal Glucocorticoid Concentrations in Zoo Meerkats. R. Soc. Open Sci. 2017, 4, 161017. [Google Scholar] [CrossRef] [PubMed]
| Study Site | Species (Number of Individuals) | Period of Data Collection | Data Points | Number of Days | Number of Observation Periods |
|---|---|---|---|---|---|
| White Post Farm | Bennet’s wallabies (n = 4, 4 M) | 31 August 2020–1 June 2021 | Open vs. closed (all data combined) | Open: 71 Closed: 29 | Open: 184 Closed: 86 |
| Open period comparisons | Post closure 1: 38 Post closure 2: 6 Post closure 3: 27 | Post closure 1: 113 Post closure 2: 15 Post closure 3: 53 | |||
| Closed period comparisons | Closure 2: 9 Closure 3: 20 | Closure 2: 27 Closure 3: 59 | |||
| Rabbits (n = 3, 1 M, 2 F) | 24 August 2020–20 February 2021 | Open vs. closed | Open: 43 Closed: 27 | Open: 121 Closed: 78 | |
| Closed period comparisons | Closure 2: 9 Closure 3: 18 | Closure 2: 27 Closure 3: 51 | |||
| Open period comparisons | Post closure 1: 38 Post closure 2: 5 | Post closure 1: 107 Post closure 2: 14 | |||
| Military macaw (n = 1, 1 M) Blue and yellow macaw (n = 1, 1 F) | 24 August 2020–22 December 2021 | Open vs. closed (Closure period 3) | Open: 34 Closed: 14 | Open: 46 Closed: 33 | |
| Open period comparisons | Post closure 1: 34 Post closure 2: 10 Post closure 3: 50 | Post closure 1: 97 Post closure 2: 22 Post closure 3: 92 | |||
| Meerkat (n = 4, 4 M) | 24 August 2020–24 June 2021 | Open vs. closed | Open: 37 Closed: 29 | Open: 79 Closed: 73 | |
| Closed period comparisons | Closure 2: 21 Closure 3: 8 | Closure 2: 59 Closure 3: 14 | |||
| Open period comparisons | Post closure 1: 10 Post closure 3: 25 | Post closure 1: 28 Post closure 3: 48 | |||
| Plantasia | Red and green macaw (n = 1, 1 F) | 8 June 2020–31 August 2020 | Open vs. closed | Open: 47 Closed: 29 | Open: 135 Closed: 86 |
| Open period comparisons | Post closure 1: 47 Post closure 2: 63 | Post closure 1: 135 Post closure 2: 180 | |||
| Meerkat (n = 2, 1 M, 1 F) | 8 June 2020–31 August 2020 | Open vs. closed (Closure 1) | Open: 46 Closed: 29 | Open: 131 Closed: 86 | |
| Open period comparisons | Post closure 1: 46 Post closure 2: 63 | Post closure 1: 131 Post closure 2: 183 | |||
| Dartmoor Zoo | Meerkat (n = 3, 2 M, 1 F) * | 24 August 2020–15 June 2021 | Open period comparisons | Post closure 1: 14 Post closure 3: 12 | Post closure 1: 123 Post closure 3: 46 |
| Knowsley Safari | Meerkat (n = 7, 4 M, 3 F) | 11 June 2020–26 November 2020 | Open vs. closed | Open: 51 Closed: 7 | Open: 107 Closed: 17 |
| Closure period comparisons | Closure period 1: 4 Closure period 2: 3 | Closure period 1: 12 Closure period 2: 5 |
| Species | Approximate Enclosure Size | Description of Enrichment Practices | HAI Opportunities Pre-COVID | HAI Opportunities Post-COVID | |
|---|---|---|---|---|---|
| White Post Farm | Meerkats | 36 m2 | Scatter fed at each of the three mealtimes. Food also sometimes hidden in objects | Visitors scatter feed in the same way keepers do | No visitor interactions during COVID-19 closures |
| Bennet’s wallabies | 900 m2 | Browse and vegetables scattered, moveable objects filled with food and placed around the enclosure | Walkthrough enclosure but no physical interactions are permitted | Walkthrough enclosure but no physical interactions are permitted | |
| Rabbits | 16 m2 | Food placed in objects to facilitate foraging. Browse and substrates to allow digging | None | None | |
| Military and Blue and yellow macaws | 240 m3 | Seeds placed in alternate feeders, browse provided. Food hidden in objects | None | None | |
| Plantasia | Meerkats | 25 m2 | Daily scatter feeds, treats hidden around the enclosure and in puzzle feeders. Occasional changes to enclosure furnishings | None | None |
| Red and green macaw | 80 m3 | Randomised provision of parrot toys, treats frozen in ice blocks and tactile/puzzle feeders | Zoo keeper experience and birthday parties feed the macaw from outside the enclosure | Zoo keeper experience and birthday parties feed the macaw from outside the enclosure | |
| Knowsley Safari | Meerkats | 258 m2 | Food hidden in objects, new enclosure furniture, olfactory enrichment occasionally provided | Public talks and encounters | Encounters but no public talks |
| Dartmoor Zoo | Meerkats | 36 m2 | Food hidden in objects to facilitate foraging | Public talks and encounters (meet the meerkat and feed the meerkat experiences) | Between the first and third closures public talks were exchanged for virtual talks (accessible via QR codes). Public talks commenced after closure 3. Feed the meerkat expe-riences a |
| Behaviour | Description |
|---|---|
| Alert | Showing an awareness of/interest in their environment (including looking around/looking at visitors) |
| Positive human–animal interactions (HAIs) | Moving towards or seeking interaction from humans |
| Negative human–animal interactions (HAIs) | Avoiding, moving away from, or showing fear of humans. Behaviour performed in response to the presence of humans. |
| Foraging/feeding | Locating and consuming foodstuffs |
| Comfort a | Any self-maintenance or self-grooming behaviour |
| Social (positive) | Engaging in positive social behaviours (e.g., social play, grooming) |
| Social (negative) | Engaging in negative social behaviour (e.g., fighting, displaying) |
| Locomotion | Moving around the enclosure in a non-repetitive pattern. For birds this included climbing around the enclosure/up enclosure bars. |
| Interaction with the environment | Investigating or interacting with things in the environment (other than food). For meerkats this also included digging behaviour. |
| Resting | Animal is inactive. Sitting/perching or lying motionless with eyes open or closed. No other behaviour is being performed. |
| Preening b | Using beak to peck, stroke, or comb feathers in any region of the body |
| Abnormal repetitive behaviour (ARBs) | Repetitive behaviour with no obvious function or purpose |
| Other | Any other behaviour not detailed in the ethogram |
| Out of sight (OOS) c | Animal out of sight of observer |
| Species | Zoo | Observation Periods * | Behaviours Modelled | Behaviours Not Modelled Due to Low or No Occurrence in Some or All Observation Conditions |
|---|---|---|---|---|
| Bennet’s wallabies | White Post Farm | Open vs. closed (closure periods two and three; post closure periods one, two and three) | Alert, comfort, feeding, locomotion, resting, positive HAIs and time spent OOS modelled using negative binomial GLMMs Positive social interactions modelled using a gaussian GLMM | Environmental interactions, negative HAIs and ARBs |
| Closed period comparisons (closure periods two and three) | Alert, feeding, comfort, resting, locomotion and time spent OOS modelled using negative binomial GLMMs | Environmental interactions, positive and negative social interactions, positive and negative HAIs, ARBs | ||
| Open period comparisons (post closure one, two and three) | Alert, feeding, resting, locomotion, time spent OOS modelled using negative binomial GLMMs Comfort modelled using a gaussian GLMM | ARBs, environmental interactions, positive and negative social interactions and positive and negative HAIs | ||
| Weeks since reopening (post closure one, two and three) | Alert, feeding, resting and time spent OOS modelled using NB GLMMs Comfort and locomotion modelled using gaussian GLMMs | |||
| Rabbits | White Post Farm | Open vs. closed periods (closure periods two and three; post closure one and two) | Alert, feeding, resting, positive HAIs, comfort, positive social interactions, environmental interactions, locomotion and time spent OOS modelled using negative binomial GLMMs | Negative social interactions, negative HAIs and ARBs |
| Closed period comparisons (closure period two and three) | Alert, positive HAIs, feeding, comfort, positive social interactions, locomotion, resting and time spent OOS modelled using negative binomial GLMMs Environmental interactions modelled using a gaussian GLMM | ARBs, negative social interactions and negative HAIs | ||
| Open period comparisons (post closure one and two) | Positive HAIs, feeding, comfort, resting and time spent OOS modelled using negative binomial GLMMs Positive social interactions, locomotion and Alert modelled using gaussian GLMMs | Negative social, ARBs, environmental interactions and negative HAIs | ||
| Weeks since reopening (post closure one and two) | ||||
| Macaws | White Post Farm | Open vs. closed (closure three; post closure three) | Alert, environmental interactions, positive HAIs, feeding, preening, positive social interactions and resting modelled using negative binomial GLMMs Locomotion modelled using a gaussian GLMM Environmental interactions had one influential outlier removed + | Time spent OOS, ARBs, negative social interactions and negative HAIs |
| Open period comparisons (post closure one, two and three) | Alert, feeding, comfort, positive social interactions, locomotion, environmental interactions and resting modelled using negative binomial GLMMs Positive HAIs modelled using a gaussian GLMM Environmental interactions had one influential outlier removed + | |||
| Weeks since reopening (post closure one, two and three) | ||||
| Plantasia | Open vs. closed periods (closure one; post closure one) | Alert, positive HAIs, environmental interactions, feeding, preening, locomotion, resting and ARBs modelled using negative binomial GLMMs | Positive and negative social interactions, vocalising and time spent OOS | |
| Open period comparisons (post closure one and two) | Alert, positive HAIs, environmental interactions, feeding, preening, vocalising, locomotion, resting and ARBs modelled using negative binomial GLMMs | Positive and negative social interactions and time spent OOS | ||
| Weeks since reopening (post closure one and two) | Alert, positive HAIs, environmental interactions, feeding, preening, locomotion, resting and ARBs modelled using negative binomial GLMMs Vocalisation assessed using a gaussian GLMM | |||
| Meerkats | White Post Farm | Open vs. closed periods (closure two and three; post closure one and three) | Alert, positive HAIs, feeding, comfort, locomotion, environmental interactions, resting and time spent OOS modelled using negative binomial GLMs | ARBs, positive and negative social interactions and negative HAIs |
| Closure period comparisons (closure two and closure three) | Alert, positive HAIs positive, feeding, locomotion, environmental interactions, resting and time spent OOS modelled using negative binomial GLMs Comfort was modelled using a poisson GLMs Locomotion had one influential outlier removed + | |||
| Open period comparisons (post closure one and three) | Alert, positive HAIs, feeding, comfort, locomotion, environmental interactions, resting and time spent OOS modelled using negative binomial GLMs ARB was modelled using a poisson GLM | Positive and negative social interactions and negative HAIs | ||
| Weeks since reopening (post closure one and three) | ||||
| Dartmoor | Open period comparisons (post closure one and three) | Alert, feeding, locomotion, resting and environmental interactions were modelled using negative binomial GLMs Time spent OOS was modelled using a poisson GLM | Positive and negative HAIs, positive and negative social interactions, comfort and ARBs | |
| Weeks since reopening (post closure one and three) | Alert, locomotion, resting, environmental interactions modelled using negative binomial GLMs Locomotion had one influential outlier removed + | Positive and negative social interactions, positive and negative HAIs, ARBs, comfort and feeding | ||
| Plantasia | Open vs. closed (closure one; post closure one) | Alert, feeding, comfort, locomotion, resting, ARBs and environmental interactions modelled using negative binomial GLMs | Positive and negative HAIs, positive and negative social interactions and time spent OOS | |
| Open period comparisons (post closure one and two) | Alert, feeding, comfort, locomotion, environmental interactions, resting, ARBs, positive social interactions modelled using negative binomial GLMs | Positive and negative HAIs, negative social interactions and time spent OOS | ||
| Weeks since reopening (post closure one and two) | ||||
| Knowsley Safari | Open vs. closed (closure one and two; post closure one) | Alert, positive HAIs, feeding, comfort, positive and negative social interactions, locomotion, environmental interactions, resting and time spent OOS were modelled using negative binomial GLMs | Negative HAIs and ARBs | |
| Closure periods (closure one and two) | Alert, feeding, comfort, positive social interactions, locomotion, environmental interactions, resting and time spent OOS modelled using negative binomial GLMs | Positive and negative HAIs, negative social interactions and ARBs | ||
| Weeks since reopening (post closure one) | Alert, positive HAIs positive, feeding, comfort, positive social interactions, locomotion, environmental interactions and resting modelled using negative binomial GLMs | ARBs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frost, N.; Carter, A.; Vernon, M.; Armstrong, S.; Walsh, N.D.; Colwill, M.; Turner-Jepson, L.; Ward, S.J.; Williams, E. Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison. J. Zool. Bot. Gard. 2022, 3, 586-615. https://doi.org/10.3390/jzbg3040044
Frost N, Carter A, Vernon M, Armstrong S, Walsh ND, Colwill M, Turner-Jepson L, Ward SJ, Williams E. Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison. Journal of Zoological and Botanical Gardens. 2022; 3(4):586-615. https://doi.org/10.3390/jzbg3040044
Chicago/Turabian StyleFrost, Naomi, Anne Carter, Martin Vernon, Sarah Armstrong, Naomi Davies Walsh, Michael Colwill, Lorna Turner-Jepson, Samantha J. Ward, and Ellen Williams. 2022. "Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison" Journal of Zoological and Botanical Gardens 3, no. 4: 586-615. https://doi.org/10.3390/jzbg3040044
APA StyleFrost, N., Carter, A., Vernon, M., Armstrong, S., Walsh, N. D., Colwill, M., Turner-Jepson, L., Ward, S. J., & Williams, E. (2022). Behavioural Changes in Zoo Animals during the COVID-19 Pandemic: A Long-Term, Multi Species Comparison. Journal of Zoological and Botanical Gardens, 3(4), 586-615. https://doi.org/10.3390/jzbg3040044








