Abstract
To explore the potential macroscopic tissue effects of select remote biopsy tools to common bottlenose dolphins (Tursiops truncatus), carcasses were darted and their traumatic effects on the anatomy in target and non-target areas of the body were described. In total, 87 samples were collected (target area, n = 19; non-target area, n = 68) within standardized grid partitions from five carcasses of sub-adult to adult age classes with a range of body condition scores. We broadly classified impacts penetrating completely through the blubber into muscle or deeper internal tissues as over-penetrations (n = 51/87, 59%). For samples collected in the defined target area, there was a low number of over-penetrations (n = 5/51; 10%). However, for samples collected in the defined, non-target areas, a much higher number of over-penetrations occurred (n = 45/51 88%). A visual examination of some samples indicated that sample length and appearance may not be reliable guides to assess the penetration depth of the wounds. These preliminary results suggest samples collected in non-targeted areas could pose much higher risk to the individual. We encourage other researchers considering the use of remote biopsy tools to conduct similar assessments prior to field sampling to better understand the potential consequences of misplaced samples with a view towards continually improving remote biopsy tools and techniques for the benefit of cetacean welfare.
1. Introduction
Remote biopsy darting has been an important data collection tool in the repertoire of marine mammal scientists [1]. Samples collected with this technique offer potential insights into the complicated group dynamics of cetacean populations, including those of endangered or infrequently encountered species. Remote biopsy data has also been used in stock management decisions in the United States (USA) for well-known species such as the common bottlenose dolphin (Tursiops truncatus) and a wide array of other marine mammal species in USA waters [2]. Samples collected using stable isotopes may provide insights into animal foraging ranges and prey selection [3], while blubber samples, collected for toxicology and hormone chemistry, could provide insight into the cetacean’s physiological response to environmental pollutants [4,5].
Remote biopsy of marine mammals is often performed using a crossbow (or modified air rifle), and dart-delivered biopsy punch (reviewed in [1]). The majority of published remote biopsy methods describe the use of a dart-mounted biopsy punch to remove a conical plug of skin and blubber (e.g., see illustration in [6]), ideally excised at the level of the subdermal fascia at the moment of impact. The dimensions of the biopsy punch used is chosen to suit the blubber thickness of the target species. For example, for common bottlenose dolphins the biopsy punch size may range in width from 5 to 10 mm and in length from 9 to 40 mm (reviewed in [1]). To prevent penetration into the muscle tissue underlying the subdermal fascia, each remote biopsy dart is designed with a rigid backstop that assists with dart rebound on impact, at which point the obtained sample can be collected for processing. For small cetaceans such as common bottlenose dolphins, the target area on the body described by some researchers for sample collection is high on the lateral side of the animal, just below the dorsal fin [7,8,9,10] and above the transverse processes of the vertebrae, where substantial epaxial muscles lie underneath the subdermal fascial plane [11].
Concerns for the potentially invasive aspect of this biopsy technique on smaller odontocetes like common bottlenose dolphins (hereinafter referred to as “bottlenose dolphins” or “dolphins”), have been met with published observations of post-biopsy behavior and biopsy site wound healing. While a significant number of reports suggest remote biopsy sampling has a limited behavioral and physical effect on sampled subjects [8,12,13,14,15,16,17,18,19], Noren and Mocklin [1] found a general absence of data for short and long-term effects on cetacean behavior and physiology and suggested researchers may be unable to report negative consequences. Krutzen et al. [14] noted a significant positive correlation between the size of the biopsy obtained and the strength of the dolphin’s reaction and postulated the stronger response may be due to impacts with greater penetrating force. Some strong behavioral reactions correlating to deeper penetration may indicate a hidden injury that cannot be assessed by external visual examination alone. Additionally, it is proposed that due to a variety of factors including environmental conditions and unpredictable animal behavior, samples may be inadvertently collected from non-target areas of the body.
In truth, practical and ethical challenges prevent researchers from physically examining biopsy impact sites as they are not likely to capture and immediately necropsy (or perform imaging analyses) on animals with fresh biopsy wounds. To this point some researchers have suggested that in the interest of successful sample collection and cetacean safety, all biopsy projectile systems be tested on carcasses of the desired species before sampling in the field [20]. Such testing should be done to ensure the sampling device is of appropriate power to avoid over-penetration to the target species while collecting samples of a “specified, predictable and repeatable tissue depth” [20]. Some researchers have experimented with different collection devices to compare surface wounds to the epidermis and blubber of dead animals, and to test the efficacy of their equipment [12,14,21]; however, at this time a review of the literature found no experiments evaluating the impact of a remote biopsy dart to the subdermal anatomy of any cetacean. Here, in a pilot study to explore the macroscopic tissue effects of select remote biopsy tools to common bottlenose dolphins, carcasses were darted and their traumatic effects on the anatomy in target and non-target areas of the body were described. Due to the wide variety of remote biopsy techniques and gear in use, we did not attempt to replicate all the methods researchers employ, or claim these methods represent the techniques for any specific group of researchers; we describe the results for a particular subset of remote biopsy tools and suggest improvements for future assessments of remote biopsy sampling techniques.
2. Materials and Methods
2.1. Stranded Dolphin Carcasses
Stranded common bottlenose dolphins in a state of decomposition code 2 or early code 3 [22] of adult and sub-adult age-classes [23] were collected between fall and spring seasons during 2013–2014 from Texas, USA, Gulf of Mexico beaches by the Texas Marine Mammal Stranding Network (Table 1). The carcasses were assessed for a body condition score following Mazzoil et al. [24]. All sub-adult carcasses (GA1794, GA1817, PA1031) were frozen in a −20 °C freezer and thawed prior to sampling, while the two adult carcasses (GA1838, GA1846) were chilled on ice in an air-conditioned laboratory (ambient air temperature ~20 °C) until sampling commenced. This work was conducted in coordination with the Texas Marine Mammal Stranding Network under a Stranding Agreement per Section 112c of the Marine Mammal Protection Act.

Table 1.
Stranding data and sampling timeline for carcasses examined in this study. Decomposition codes followed Geraci and Lounsbury [22] and body scores followed Mazzoil et al. [24].
2.2. Carcass Sampling
Remote biopsy samples were collected from the dolphin carcasses using two different crossbows: the Barnett Panzer V (Barnett Outdoors, Tarpon Springs, FL, USA) and the MK-150A2 (Man Kung, Taichung City, Taiwan, Republic of China). The Barnett Panzer V [10,19,25], and MK-150A2 (pers. obs., Pensacola Bay August 2016; Timbalier/Terrebonne Bay June 2016) have been used to collect remote biopsy samples from common bottlenose dolphins in the field. The darts, biopsy punches, and crossbows were similar to the equipment used in the field by others [8,9,10,15,16,18,25,26] and evaluated by Ronje [27]. The darts were manufactured by CETA-DART (Aarhus, Denmark). The crossbow dart (length ~60 cm) consisted of a carbon dart shaft, a plastic nock, a 12.0 cm plastic fletching, and a threaded stainless steel flanged post enclosed in a 10.0 cm × 3.0 cm cone-shaped high-density polyurethane cast foam float (Figure 1). Each cylindrical biopsy punch (10 mm diameter × 25 mm length), threaded onto the end of the biopsy dart, featured three equidistant cylindrical barbs (approximately 4 mm in length × 2 mm diameter) brazed onto the inner surface of the punch that served to retain the sample once it was disarticulated from the target animal. In this study several of the collection darts were new while others were previously used in field work. Consequently, the foam back-stop on the previously used darts may have been compressed in comparison to new darts, which can result in variable penetration depths. Remote biopsy darts are often reused (after sterilizing) during remote biopsy sample collection projects; the darts used here were consistent with applications in the field. However, in an attempt to ensure that the length of exposed biopsy punch extending beyond the backstop was consistent, the biopsy punch was adjusted with snug-fitting rubber spacers (≤1 cm thick), so that no more than 25 mm of the biopsy punch was exposed. This measurement was verified before each sampling attempt.

Figure 1.
Sampling ends of some darts in this study (biopsy punch not shown). A cross-section was taken of a used dart (upper), the lower dart had not yet been used for sampling. Note apparent compression of the yellow foam in the upper (previously used) dart.
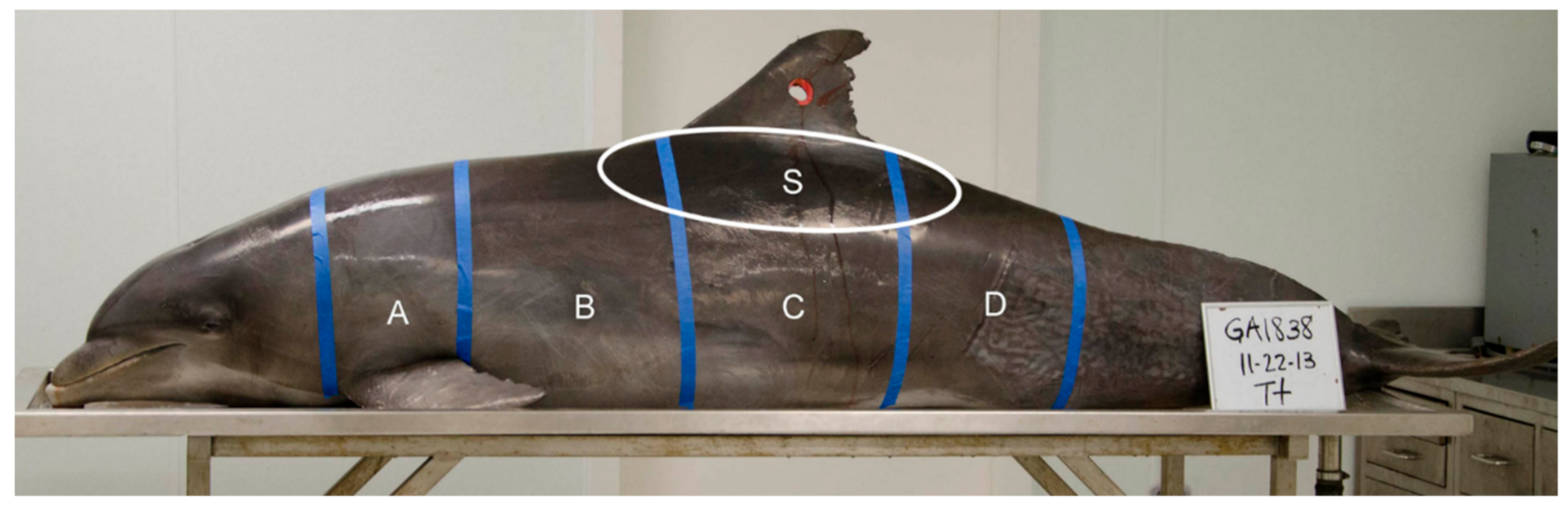
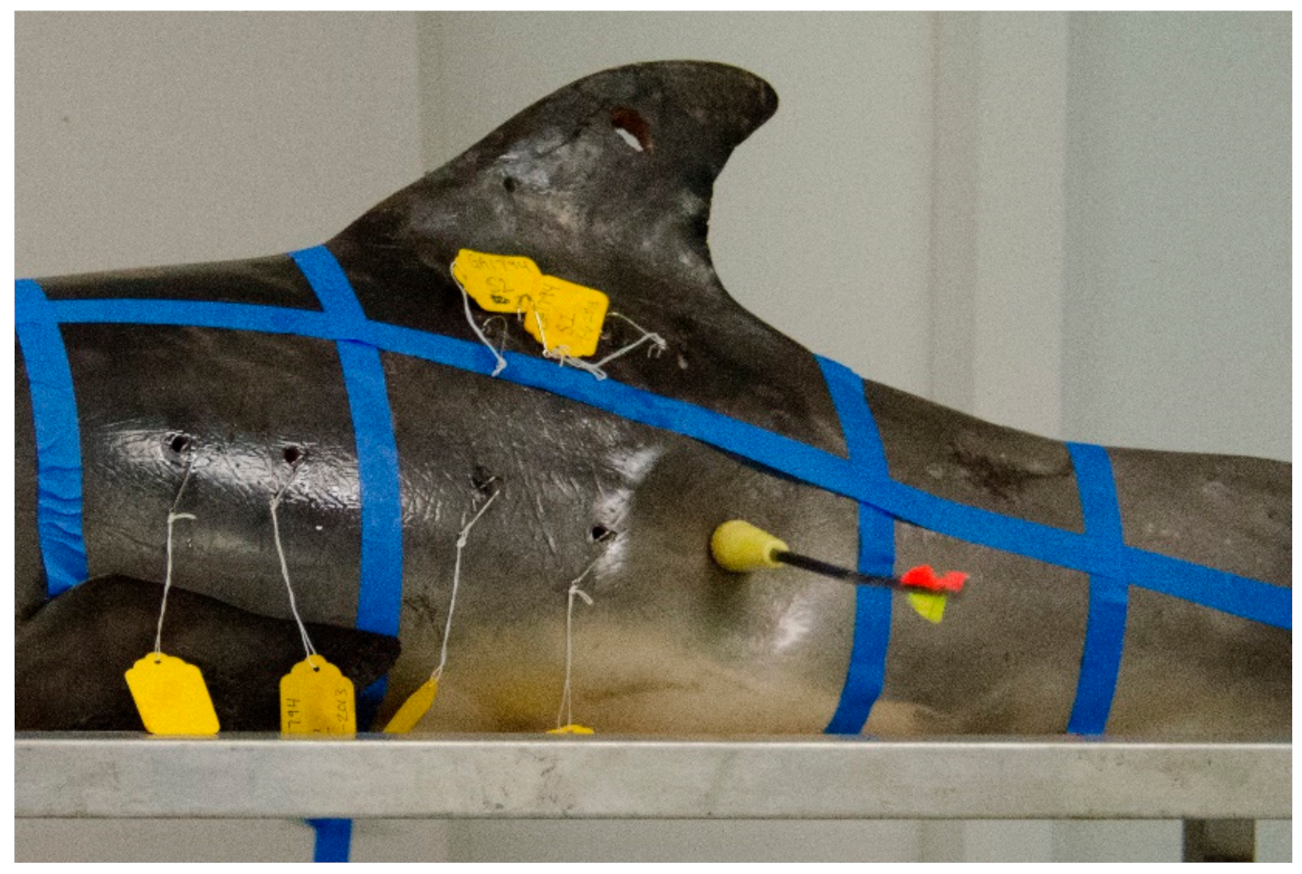
Prior to sampling, all carcasses were positioned perpendicular to the sampler at table height (ventral recumbency), except for carcass (PA1031), which was placed on the floor to better simulate the angle of sampling in the field. A grid constructed of masking tape was applied to each carcass and each grid partition was assigned an alphabetical identifier (A, B, C, D, and S, Figure 2). Grid partition “S” was intended to approximate the area on the carcass targeted by other researchers in the field [7,8,9]. The shooting range in the laboratory was marked at 3 to 5 m from the sampler to the carcass. Biopsy collection darts were fired beginning at 5 m within each grid partition marked on the carcass, and in most cases each grid partition was sampled again at 3 or 4 m (except for PA1031, sampled only at 5 m distant). The range of 3 to 5 m for sampling was chosen because other researchers report sampling common bottlenose dolphins within a similar range and changes in dart speed and energy are negligible in that range [27]. Paper identification tags corresponding to the sample label (e.g., GA1794-A1) in each grid partition were affixed to the wound using a fishhook (Figure 3). Carcasses GA1794, GA1817, GA1838, and GA1846 were sampled on their left sides with the Barnett Panzer V (draw weight 68 kg, power stroke 31.8 cm), while carcass PA1031 was sampled on the right side with the MK-150A2 crossbow (draw-weight 68 kg, power stroke 26.7 cm), reported as slightly less powerful than the Panzer V crossbow [27]. After each event, photographs were taken of the resulting external wound, the sample collected, and the sampling end of the dart.
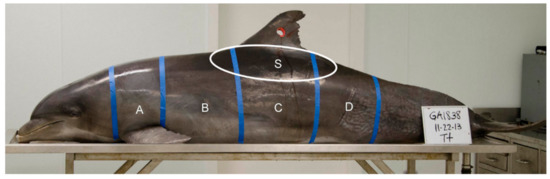
Figure 2.
Grid partitions A, B, C, D, and S marked on an adult common bottlenose dolphin (GA1838) placed on a necropsy table. The hole in the dorsal fin was cut to use as a lifting point to hoist the dolphin onto the table with a crane.
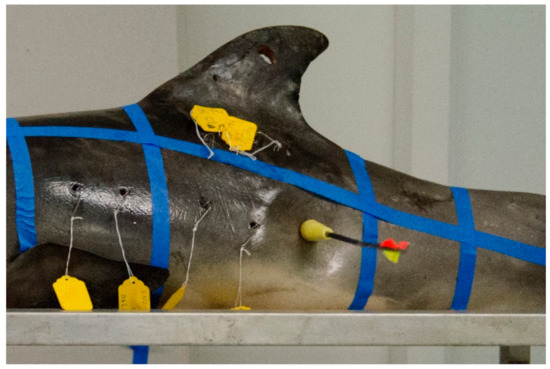
Figure 3.
Remote biopsy dart impacts carcass GA1794 in grid partition C.
2.3. Lesion Examination
After sampling was completed, the blubber thickness (including skin) of each sample was measured and the examination of each carcass began with a reflection of the blubber along the subdermal fascial plane, followed by a gross examination of each wound site and a detailed description of the wound. We broadly classified impacts penetrating completely through the blubber and subdermal fascia into the muscle or other internal tissues (e.g., bone) as over-penetrations (OP). Other impacts not penetrating beyond the subdermal fascia were designated as having no visible subdermal effect (NSE) for the purpose of this study. We attempted to measure the penetration depths of each wound with a depth gauge but some of our efforts were confounded by the difficulty of precisely identifying the deepest extent of the wound. For example, if the dart did not over-penetrate beyond the firm subdermal fascia, depth measurements were relatively straightforward; however, if the biopsy dart over-penetrated into soft tissue, it was sometimes difficult to know the full extent of penetration due to the irregular surface or tearing of the tissue. In addition, samples were not always obtained at right angles due to the natural curvature of the carcass and sampling angle, resulting in some samples with uneven sides. Therefore, a statistical analysis of penetration depth measurements (and corresponding sample lengths) is not presented here.
3. Results
Carcass Sampling and Lesion Examination
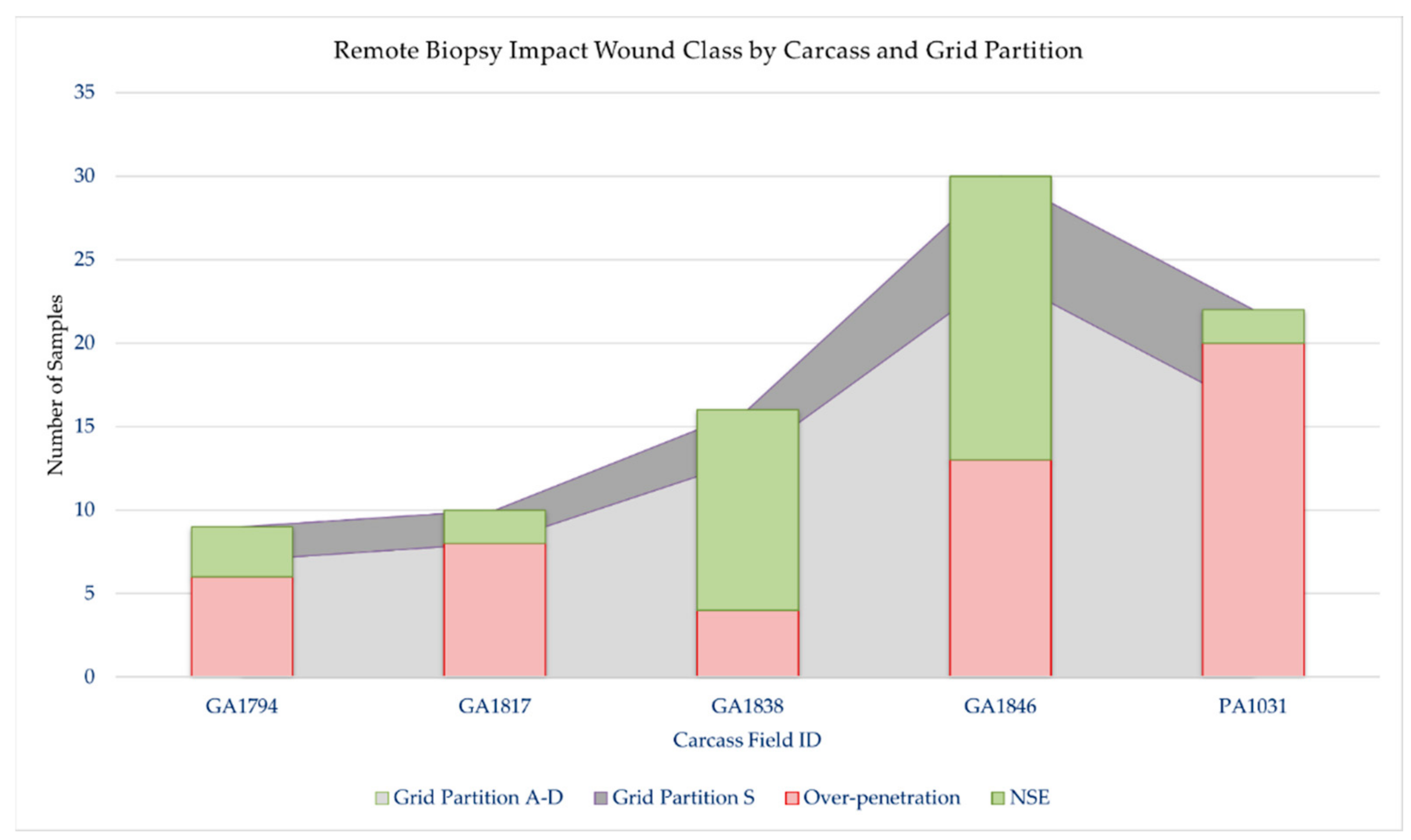
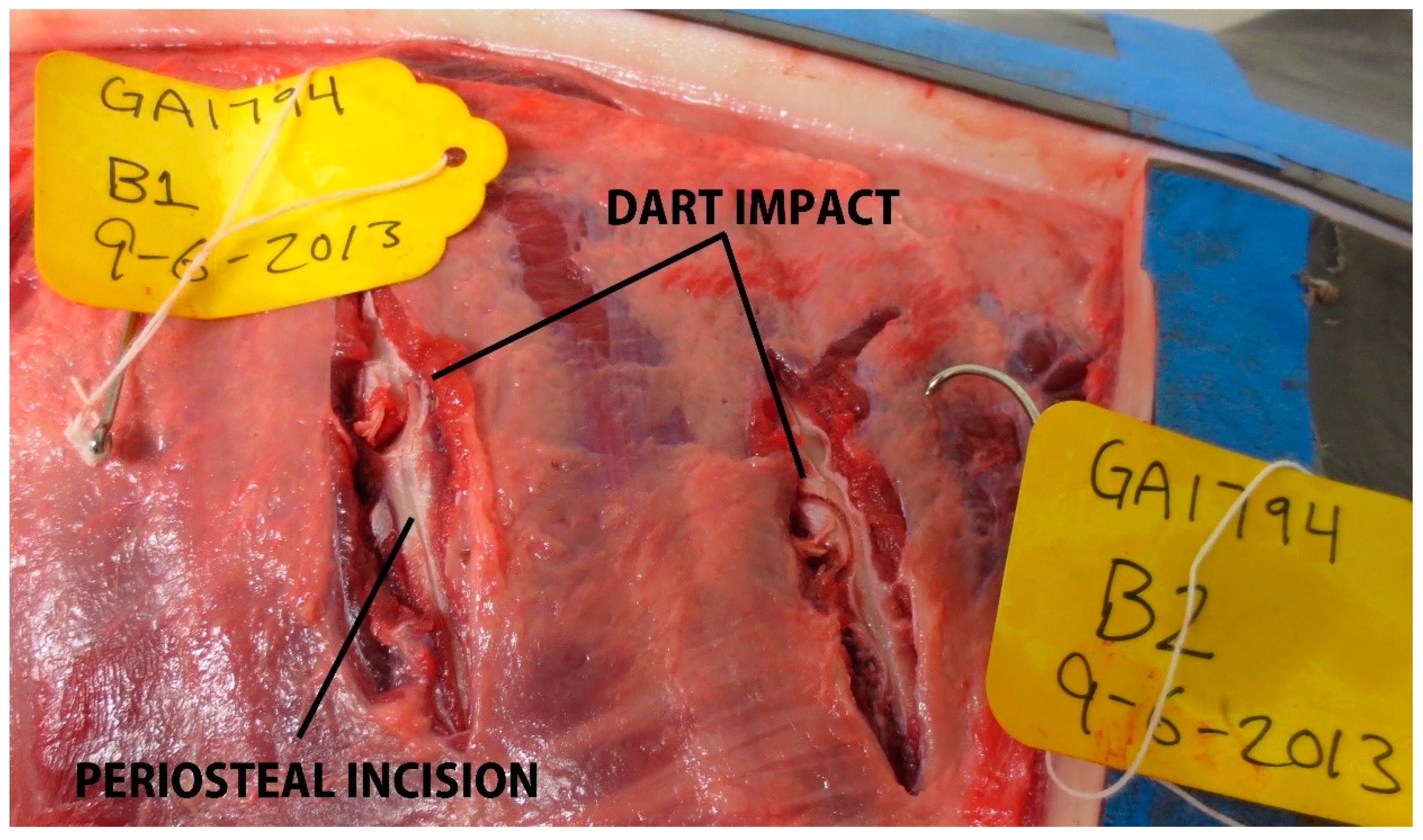
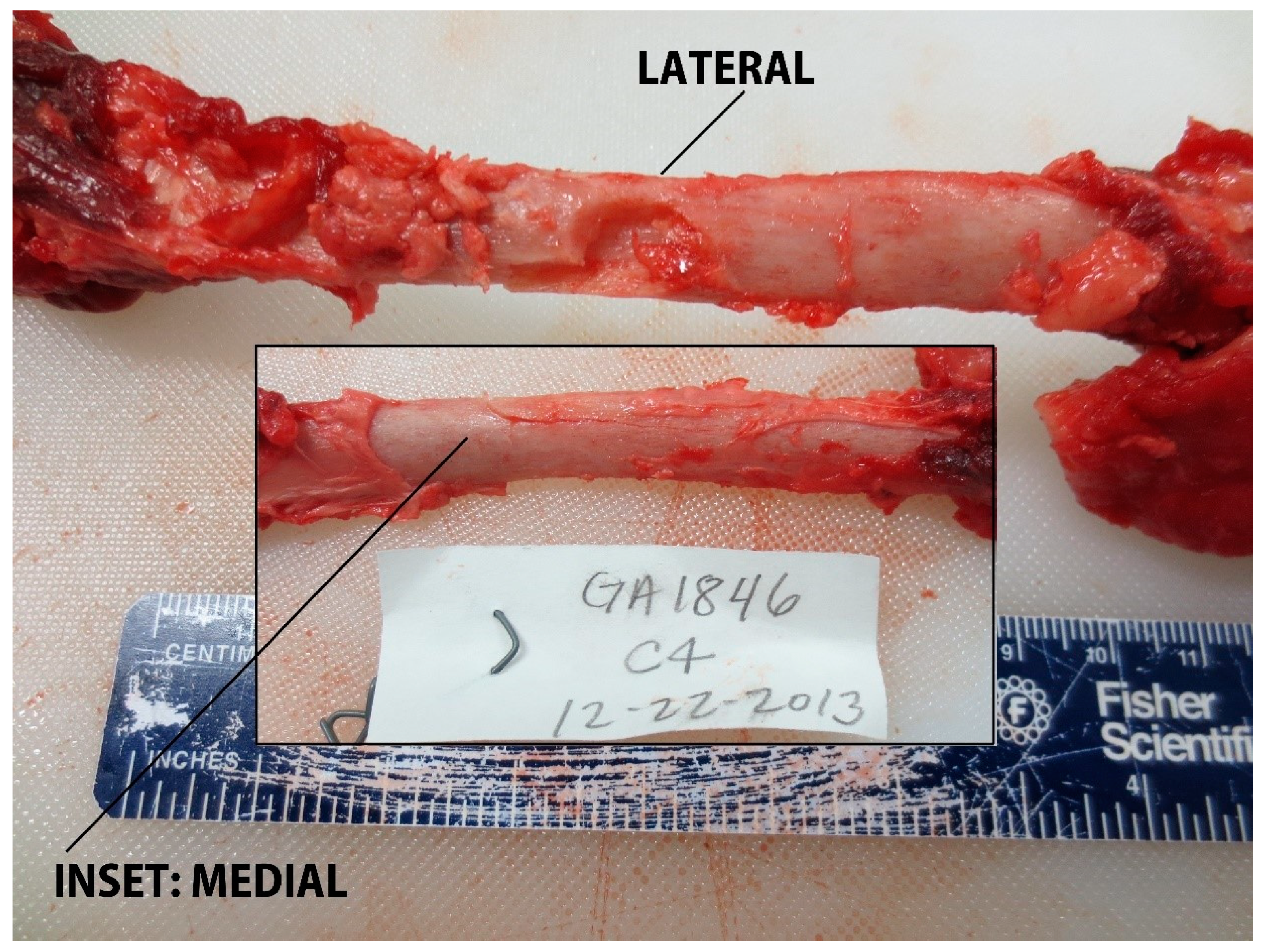
In total, 87 samples were collected (target area (S), n = 19; non-target area (A–D), n = 68, Table 2, Figure 4). Overall, 59% (n = 51/87) of samples resulted in over-penetrations, but only 10% (n = 5/51) of over-penetrations were observed in the target area (S), and only in the underweight sub-adult dolphin, PA1031 (Figure 4). Only 7% (n = 6/87) of the samples had a blubber thickness layer ≥ 25 mm. For those samples with a blubber thickness of ≤25 mm, 39% were NSE. Over-penetrations were visible in 67% (n = 6/9) of impacts to GA1794 in grid partitions A–C. Over-penetrations were visible in 80% (n = 8/10) of impacts to GA1817 in grid partitions A–D, out of which three were found to impact skeletal anatomy. Over-penetrations were visible in 25% (n = 4/16) of impacts to GA1838 in grid partition B–D, with no visible skeletal impacts. Over-penetrations were observed in 43% (n = 13/30) of impacts to GA1846 in grid partitions A–D, out of which three were found to impact skeletal anatomy. Over-penetrations were observed in 91% (n = 20/22) of impacts to PA1031 in all grid partitions (A–S), out of which five were found to impact skeletal anatomy. For PA1031, five out of six wounds in grid partition S were classified as over-penetrations. Notable wounds in grid partitions A–D in all age-classes included biopsies and splintering of scapula and ribs. For example, impact GA1794-B1 resulted in a complete rib fracture (Figure 5). Impact GA1846-C4 resulted in a bone biopsy and fracture from the lateral and medial sides of the rib, respectively (Figure 6). An examination of the samples collected indicated their appearance was not always consistent with what would be expected based on the gross description of the wounds (Table 2). For example, samples from carcass GA1794 (A1, A2, B1, and B2), were associated with impacts to the skeleton (scapula or ribs), yet only sample A2 visually appears to have over-penetrated, and there was no visual evidence of bone fragments on the samples (Figure 7). Samples GA1794-C2 and GA1794-C3 over-penetrated into the muscle 15–20 mm beyond the subdermal fascia underlying the blubber, yet corresponding muscle tissue was not retained with the samples that would provide a visual indication of over-penetration.

Table 2.
Impact label, wound classification (NSE = no visible subdermal effect, OP = over-penetration), sample blubber thickness, and gross description of wound sites. Unless otherwise stated, the biopsy impact created a well circumscribed full-thickness biopsy of the skin and blubber layer. Penetration depths mentioned in the gross description were measured from the dissection plane (inner surface of blubbler layer), not from the external surface of the wound, and do not include measurements of the full thickness penetration of the skin/blubber. Measurements are approximate due to the irregular tearing of soft tissue.
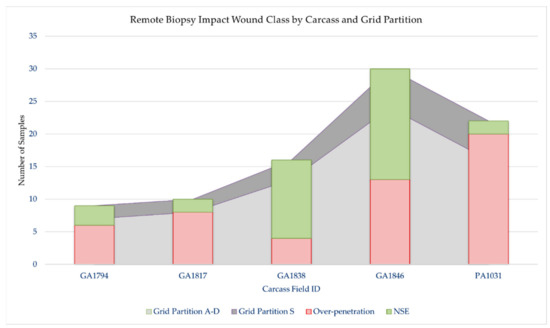
Figure 4.
Remote biopsy wound classification for each carcass by grid partition. Grid partitions on each carcass were A, B, C, D, and S. Grid partitions A–D were defined as non-target areas and S was defined as the target area of each carcass. OP = over-penetration, defined as a wound penetrating through the subdermal fascia into muscle tissue; NSE = no visible subdermal effect, defined as a wound with no over-penetration.
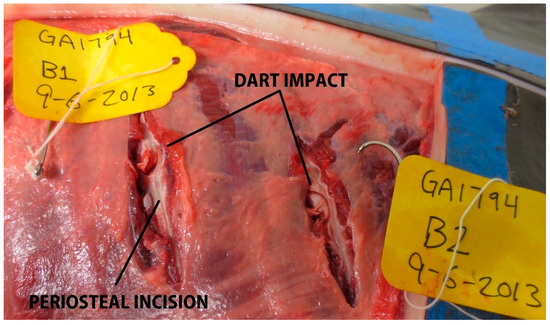
Figure 5.
Rib impacts to GA1794 (subadult), with a complete rib fracture for impact GA1794-B1.
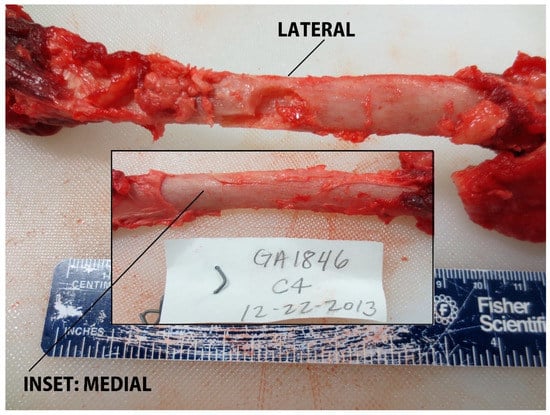
Figure 6.
Rib impact in grid partition C on GA1846 (adult) with fragmentation and linear scoring of cortex on the lateral side of the rib, and a fracture apparent on the medial side of the rib.

Figure 7.
Example of samples obtained from each grid partition from carcass GA1794. Compare samples to the gross description of wounds in Table 2. Samples A1, A2, B1, and B2 were associated with skeletal impacts. Samples C2 and C3 over-penetrated into muscle 15 and 20 mm beyond the blubber layer, respectively. Samples D1, S1, and S3 were described as having minimal or no visible subdermal effect on the carcass.
4. Discussion
Researchers in the field must prepare for many variables influencing the trajectory and impact location of a remote biopsy dart (e.g., sea state, wind, animal behavior, platform movement, equipment malfunction, or human error). It seems feasible that out of the thousands of remote biopsy samples collected from free-ranging dolphins [1], some dart impacts have inadvertently impacted areas of the body not intended by the sampler. However, very little is reported about the potential negative effects of improperly placed dart impacts [1] if they occur. Some researchers cite examples of uncomplicated wound healing and recovery from injuries caused by intraspecific or interspecific aggression or human interactions as another reason to conclude that well-placed biopsy wounds are easily managed by the animal (e.g., [28,29,30,31,32]. For example, it has been suggested that wounds introduced by cookie cutter sharks (Isistius spp.), which typically penetrate to the subdermal fascia or underlying musculature [33] in marine mammals, may be a close approximation of wounds incurred by a remote biopsy dart [13]. It is generally accepted that marine mammals manage cookie cutter shark wounds well [33,34], whose sequela are often nothing more than a slight depression or scar on the epidermis. However, the observation of uncomplicated wound-healing in the prey of cookie-cutter sharks may not consider the differences between the energy by which a cookie cutter shark propels itself, and the presumably significantly greater energy carried by the remote biopsy darts tested here [27].
One notable exception to the innocuous observations in support of remote biopsy was that by the Tethys Research Institute where darting resulted in the death of a common dolphin (Delphinus delphis) in the Eastern Ionian Sea [35]. This accidental death was thought to be the result of a complex series of factors. The dolphin in question was a 162 cm sub-adult with an unusually thin blubber layer (7 mm). This dolphin was sampled from a typical distance of 6 m with a dart calculated to carry significantly less energy than that used in this study [27,36], however the dart did not rebound and was found to penetrate deeply into the dorsal musculature, causing suspected vertebral trauma [35]. No darts were found to impact the ends of the transverse processes of the spinal vertebrae in this study, though this may be a possible scenario for bottlenose dolphins due to the limited tissue depth covering the transverse processes, and the proximity of some dart impacts (~1 cm). The post-impact skeletal damaged observed here is likely due to the difference in the way fixed structures with low surface areas, like ribs, absorb energy relative to soft tissue [37]. The carcass tissue appeared to dissipate some energy to the impact area before the dart rebounds (see the impact “crater” around the biopsy dart in Figure 3), yet this energy is likely not dissipated as well if the dart impacts a more rigid part of the body (e.g., over a rib). Several instances of significant over-penetration in all animals tested here resulted from impacts in grid partition A, B, and C, but the resulting damage in those grid partitions increased for the sub-adults (GA1794 and GA1817) and underweight animal (PA1031). Damage to ribs in the sub-adult dolphins was more severe, and the results from PA1031 suggest that an animal appearing underweight should be avoided for sampling, as even well-placed shots in grid partition S (the target area) over-penetrated.
Some improvements are recommended here for future studies, and some questions remain unresolved. Although it is logistically difficult, future studies should attempt to conduct sampling and examinations on fresh carcasses, as the integrity of some tissues may be compromised after death. Mckenna et al. [38] concluded the mechanical properties of blubber and muscle did not differ significantly between their fresh and thawed specimens, which were placed into the freezer 6–8 h post-mortem and stored for one year. Their results were consistent with the CT scan results of [39] who determined that the elastic quality of Ziphius cavirostris connective tissue is not affected by the freeze–thaw cycle. Although the freeze–thaw cycle may not bear on our results here, there is evidence that fresh post-mortem tissues lack typical ante-mortem resilience. Mckenna et al. [38] found small differences in blubber and muscle density between post-mortem tissues and live animals. Fitzgerald and Fitzgerald [40] determined that pilot whale blubber began to lose elasticity immediately after death. Whale muscle has been shown to reasonably approximate the post-mortem changes of other mammalian muscle [41]. If stiffness in the post-rigor muscle of common bottlenose dolphins is significantly changed compared to live tissues [42], then our study may be biased toward over-penetration compared to live animal biopsies.
Our laboratory shooting range could not duplicate all field environments. Among the several complicating factors that must be considered when assessing penetration depth of each impact are dart design and energy, dimensions of the biopsy punch, blubber thickness and body condition, as well as corporeal impact angle and location. The field angle of a dart trajectory may vary with the height of the shooting platform, skill of the sampler, distance to target, animal behavior, wind, and sea state. Since an impact perpendicular to a body will translate more energy than an impact at an angle [37] it may be possible that the over-penetrations of impacts to perpendicular areas of the carcass in the lab were intensified. Thus, future studies should consider an alternative to placing the carcass at table height. It is also possible that by placing the carcass on a solid surface (relative to a buoyant body in the water), that the carcass absorbed energy differently than a live animal in water. It is possible that the use of darts with variable amounts of foam backstop integrity resulted in more severe wounds in some cases, or that the rubber spacers used to standardize the length of exposed biopsy punch enabled a greater penetration depth (≤1 cm greater) if compressed upon impact; but if so, the results were consistently NSE for the target area except for the emaciated carcass (PA1031). The use of a dart with a metal backstop may be useful to minimize over-penetration as well as standardize the evaluation of dart impacts, but the corporeal location of dart impact, and dart energy, are also critical considerations. Remote biopsy projection systems using darts with less mass, and presumably less penetrating energy (e.g., “PAXARMS” [14]) may also be an alternative to consider for small cetaceans. Some researchers use biopsy punches with a length that corresponds to the seasonality and latitude of the study population (e.g., 17 mm [7]), and the blubber thickness measurements of the samples collected here seem to support that, although over-penetrations did not always occur when the blubber thickness was <25 mm. Visual examination of samples may not accurately indicate the penetration depth or severity of the wound, because soft tissue and bone fragments may not be retained in the sample. It is recommended that equipment be tested on carcasses of the same species, age-class, region, and season as the planned biopsy collection project due to possible differences in blubber thickness and body condition. Finally, these results likely will vary from the results obtained with other dart types and projection devices; we suggest that future research approaches similar assessments and tool development collaboratively to better understand the wider spectrum of remote biopsy device, dart, biopsy punch, and species combinations, with their respective variations in power, dimensions, and anatomy.
Author Contributions
Conceptualization: E.I.R.; Methodology, E.I.R., C.B.; Writing—Original Draft Preparation, E.I.R.; Writing—Review and Editing, E.I.R., C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable, all data used to support the results is available in the manuscript.
Acknowledgments
Stranded carcass recovery and logistics were generously provided by the staff and volunteers of the Texas Marine Mammal Stranding Network (TMMSN). The TMMSN operates under a Stranding Agreement with the National Marine Fisheries Service and was authorized to respond to marine mammal strandings on the Texas Gulf coast. No endorsement of any remote biopsy tools is implied by this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noren, D.P.; Mocklin, J.A. Review of cetacean biopsy techniques: Factors contributing to successful sample collection and physiological and behavioral impacts. Mar. Mammal Sci. 2012, 28, 154–199. [Google Scholar] [CrossRef]
- Hayes, S.A.; Josephson, E.; Maze-Foley, K.; Rosel, P.E. US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments—2018, NOAA Technical Memorandum NMFS-NE-258; US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Northeast Fisheries Science Center: Woods Hole, MA, USA, 2019; 306p.
- Kiszka, J.J.; Méndez-Fernandez, P.; Heithaus, M.R.; Ridoux, V. The foraging ecology of coastal bottlenose dolphins based on stable isotope mixing models and behavioural sampling. Mar. Biol. 2014, 161, 953–961. [Google Scholar] [CrossRef]
- Galligan, T.M.; Balmer, B.C.; Schwacke, L.; Bolton, J.L.; Quigley, B.M.; Rosel, P.; Ylitalo, G.M.; Boggs-Russell, A.S. Examining the Relationships Between Blubber Steroid Hormone and Persistent Organic Pollutant Measurements in Common Bottlenose Dolphins. Environ. Pollut. 2019, 249, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Righetti, B.P.H.; Mattos, J.J.; Siebert, M.N.; Daura-Jorge, F.G.; Bezamat, C.; Fruet, P.F.; Genoves, R.C.; Taniguchi, S.; da Silva, J.; Montone, R.C. Biochemical and molecular biomarkers in integument biopsies of free-ranging coastal bottlenose dolphins from southern Brazil. Chemosphere 2019, 225, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.; Barry, K.; Ronje, E.I.; Gorgone, A.; Martinez, A.; Speakman, T.; Mullin, K.D. Terrebonne Bay—Timbalier Bay, Lousiana Common Bottlenose Dolphin (Tursiops truncatus) Stock Photo-ID Capture-Recapture and Biopsy Field Summary; NOAA Technical Memorandum NMFS-SEFSC-717; NOAA: Washington, DC, USA, 2017; Volume 717, p. 21. [Google Scholar] [CrossRef]
- Parsons, K.; Durban, J.; Claridge, D. Comparing two alternative methods for sampling small cetaceans for molecular analysis. Mar. Mammal Sci. 2003, 19, 224–231. [Google Scholar] [CrossRef]
- Gorgone, A.M.; Haase, P.A.; Griffith, E.S.; Hohn, A.A. Modeling response of target and nontarget dolphins to biopsy darting. J. Wildl. Manag. 2008, 72, 926–932. [Google Scholar] [CrossRef]
- Wenzel, F.; Nicolas, J.; Larsen, F.; Pace, R.M., III. National Marine Fisheries Service, Northeast Fisheries Science Center Cetacean Biopsy Training Manual. (Northeast Fisheries Science Center Reference Document 10–11); National Oceanic and Atmospheric Administration: Woods Hole, MA, USA, 2010; 18p. Available online: https://repository.library.noaa.gov/view/noaa/3888 (accessed on 10 October 2021).
- Fruet, P.F.; Rosa, L.D.; Genoves, R.C.; Valiati, V.H.; de Freitas, T.R.; Möller, L.M. Biopsy darting of common bottlenose dolphins (Tursiops truncatus) in southern Brazil: Evaluating effectiveness, short-term responses and wound healing. Lat. Am. J. Aquat. Mamm. 2017, 11, 121–132. [Google Scholar] [CrossRef][Green Version]
- Pabst, D.A. Axial muscles and connective tissues of the bottlenose dolphin. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Patenaude, N.; White, B. Skin biopsy sampling of beluga whale carcasses: Assessment of biopsy darting factors for minimal wounding and effective sample retrieval. Mar. Mammal Sci. 1995, 11, 163–171. [Google Scholar] [CrossRef]
- Weller, D.W.; Cockcroft, V.G.; Würsig, B.; Lynn, S.K.; Fertl, D. Behavioral responses of bottlenose dolphins to remote biopsy sampling and observations of surgical biopsy wound healing. Aquat. Mamm. 1997, 23, 49–58. [Google Scholar]
- Krutzen, M.; Barre, L.M.; Moller, L.M.; Heithaus, M.R.; Simms, C.; Sherwin, W.B. A biopsy system for small cetaceans: Darting success and wound healing in Tursiops spp. Mar. Mammal Sci. 2002, 18, 863–878. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Hung, S.K. Effects of biopsy sampling on Indo-Pacific humpback dolphins (Sousa chinensis) in a polluted coastal environment. Aquat. Mamm. 2008, 34, 310. [Google Scholar] [CrossRef]
- Kiszka, J.; Simon-Bouhet, B.; Charlier, F.; Pusineri, C.; Ridoux, V. Individual and group behavioural reactions of small delphinids to remote biopsy sampling. Anim. Welf. 2010, 19, 411–417. [Google Scholar]
- Tezanos-Pinto, G.; Baker, C.S. Short-term reactions and long-term responses of bottlenose dolphins (Tursiops truncatus) to remote biopsy sampling. N. Z. J. Mar. Freshw. Res. 2012, 46, 13–29. [Google Scholar] [CrossRef]
- Kowarski, K.A.; Augusto, J.F.; Frasier, T.R.; Whitehead, H. Effects of Remote Biopsy Sampling on Long-Finned Pilot Whales (Globicephala melas) in Nova Scotia. Aquat. Mamm. 2014, 40, 117. [Google Scholar] [CrossRef]
- Reisinger, R.R.; Oosthuizen, W.C.; Peron, G.; Toussaint, D.C.; Andrews, R.D.; de Bruyn, P.J. Satellite tagging and biopsy sampling of killer whales at subantarctic Marion Island: Effectiveness, immediate reactions and long-term responses. PLoS ONE 2014, 9, e111835. [Google Scholar] [CrossRef]
- Gales, N.J.; Bowen, W.D.; Johnston, D.W.; Kovacs, K.M.; Littnan, C.L.; Perrin, W.F.; Reynolds, J.E.; Thompson, P.M. Guidelines for the treatment of marine mammals in field research. Mar. Mammal Sci. 2009, 25, 725–736. [Google Scholar] [CrossRef]
- Palsbøll, P.J.; Larsen, F.; Hansen, E.S. Sampling of skin biopsies from free-ranging large cetaceans in West Greenland: Development of new biopsy tips and bolt designs. Genetic ecology of whales and dolphins. Int. Whal. Comm. Spec. Issue 1991, 13, 71–79. [Google Scholar]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; John Schmitz & Sons Inc.: Sparks, MD, USA, 2005. [Google Scholar]
- Read, A.J.; Wells, R.S.; Hohn, A.A.; Scott, M.D. Patterns of Growth in Wild Bottle-Nosed Dolphins, Tursiops-truncatus. J. Zool. 1993, 231, 107–123. [Google Scholar] [CrossRef]
- Mazzoil, M.S.; McCulloch, S.D.; Youngbluth, M.J.; Kilpatrick, D.S.; Murdoch, E.M.; Mase-Guthrie, B.; Odell, D.K.; Bossart, G.D. Radio-Tracking and Survivorship of Two Rehabilitated Bottlenose Dolphins (Tursiops truncatus) in the Indian River Lagoon, Florida. Aquat. Mamm. 2008, 34, 54–64. [Google Scholar] [CrossRef]
- Sinclair, C.; Sinclair, J.; Zolman, E.; Martinez, A.; Balmer, B.; Barry, K. Remote Biopsy Sampling Field Procedures for Cetaceans Used during the Natural Resource Damage Assessment of the MSC252 Deepwater Horizon Oil Spill (NOAA Technical Memorandum NMFS-SEFSC-670); National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Fisheries Science Center: Pascagoula, MS, USA, 2015; 36p. [CrossRef]
- Kellar, N.M.; Trego, M.L.; Chivers, S.J.; Archer, F.I.; Minich, J.J.; Perryman, W.L. Are there biases in biopsy sampling? Potential drivers of sex ratio in projectile biopsy samples from two small delphinids. Mar. Mammal Sci. 2013, 29, E366–E389. [Google Scholar] [CrossRef]
- Ronje, E.I. Dart Speed and Energy for Potential Cetacean Remote Sampling Devices. Aquat. Mamm. 2020, 46, 454–460. [Google Scholar] [CrossRef]
- Bruce-Allen, L.J.; Geraci, J.R. Wound-healing in the bottlenose dolphin (Tursiops truncatus). Can. J. Fish. Aquat. Sci. 1985, 42, 216–228. [Google Scholar] [CrossRef]
- Corkeron, P.; Morris, R.; Bryden, M. A note on healing of large wounds in bottlenose dolphins, Tursiops truncatus. Aquat. Mamm. 1987, 13, 96–98. [Google Scholar]
- Bloom, P.; Jager, M. The injury and subsequent healing of a serious propeller strike to a wild bottlenose dolphin (Tursiops truncatus) resident in cold waters off the Northumberland coast of England. Aquat. Mamm. 1994, 20, 59–64. [Google Scholar]
- Orams, M.; Deakin, R. Report on the healing of a large wound in a bottlenose dolphin (Tursiops truncatus). In Marine Mammal Research in the Southern Hemisphere: Status, Ecology and Medicine; Hindell, M.A., Kempner, C.M., Eds.; Surrey Beatty & Sons: Chipping Norton, UK, 1997; Volume 1. [Google Scholar]
- Wells, R.S.; Allen, J.B.; Hofmann, S.; Bassos-Hull, K.; Fauquier, D.A.; Barros, N.B.; DeLynn, R.E.; Sutton, G.; Socha, V.; Scott, M.D. Consequences of injuries on survival and reproduction of common bottlenose dolphins (Tursiops truncatus) along the west coast of Florida. Mar. Mammal Sci. 2008, 24, 774–794. [Google Scholar] [CrossRef]
- Dwyer, S. Cookie cutter shark (Isistius sp.) bites on cetaceans, with particular reference to killer whales (orca) (Orcinus orca). Aquat. Mamm. 2011, 37, 111–138. [Google Scholar] [CrossRef]
- Best, P.B.; Photopoulou, T. Identifying the “demon whale-biter”: Patterns of scarring on large whales attributed to a cookie-cutter shark (Isistius sp.). PLoS ONE 2016, 11, e0152643. [Google Scholar] [CrossRef]
- Bearzi, G. First report of a common dolphin (Delphinus delphis) death following penetration of a biopsy dart. J. Cetacean Res. Manag. 2000, 2, 217–222. [Google Scholar]
- Barrett-Lennard, L.; Smith, T.G.; Ellis, G.M. A cetacean biopsy system using lightweight pneumatic darts, and its effect on the behavior of killer whales. Mar. Mammal Sci. 1996, 12, 14–27. [Google Scholar] [CrossRef]
- Fierro, M.F.; Ongley, J.P. Blunt force injuries. In Handbook of Forensic Pathology; Froede, R.C., Ed.; College of American Pathologists: Northfield, IL, USA, 1990. [Google Scholar]
- Mckenna, M.F.; Goldbogen, J.A.; Leger, J.S.; Hildebrand, J.A.; Cranford, T.W. Evaluation of postmortem changes in tissue structure in the bottlenose dolphin (Tursiops truncatus). Anat. Rec.-Adv. Integr. Anat. Evol. Biol. 2007, 290, 1023–1032. [Google Scholar] [CrossRef]
- Soldevilla, M.S.; McKenna, M.F.; Wiggins, S.M.; Shadwick, R.E.; Cranford, T.W.; Hildebrand, J.A. Cuvier’s beaked whale (Ziphius cavirostris) head tissues: Physical properties and CT imaging. J. Exp. Biol. 2005, 208, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.R.; Fitzgerald, J.W. Blubber and compliant coatings for drag reduction in water I. Viscoelastic properties of blubber and compliant coating materials. Mater. Sci. Eng. C 1995, 2, 209–214. [Google Scholar] [CrossRef]
- Marsh, B.B. Observations on rigor mortis in whale muscle. Biochim. Biophys. Acta 1952, 9, 127–132. [Google Scholar] [CrossRef]
- Van Ee, C.; Chasse, A.; Myers, B. Quantifying skeletal muscle properties in cadaveric test specimens: Effects of mechanical loading, postmortem time, and freezer storage. J. Biomech. Eng. 2000, 122, 9–14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).