Prototyping and Evaluation of 1D Cylindrical and MEMS-Based Helmholtz Acoustic Resonators for Ultra-Sensitive CO2 Gas Sensing
Abstract
1. Introduction
- High Accuracy and Long-Term Stability: [7] Frequency-based outputs are less affected by drift and electronic noise compared with analog voltage or current signals, resulting in more reliable performance.
- Exceptional Sensitivity: [8] Resonant sensors can detect very small variations in mass or force, allowing the precise detection of trace gases.
- Lower System Complexity and Cost: [9] We can often process frequency outputs without complex analog-to-digital converters.
- Robust Thermal Stability: [10] Compared with piezoresistive sensors, resonant sensors exhibit reduced susceptibility to temperature fluctuations, ensuring more consistent readings under varying environmental conditions.
2. Theory
2.1. Acoustic Resonance
2.2. Acoustic Resonance in a 1-D Cylindrical Resonator
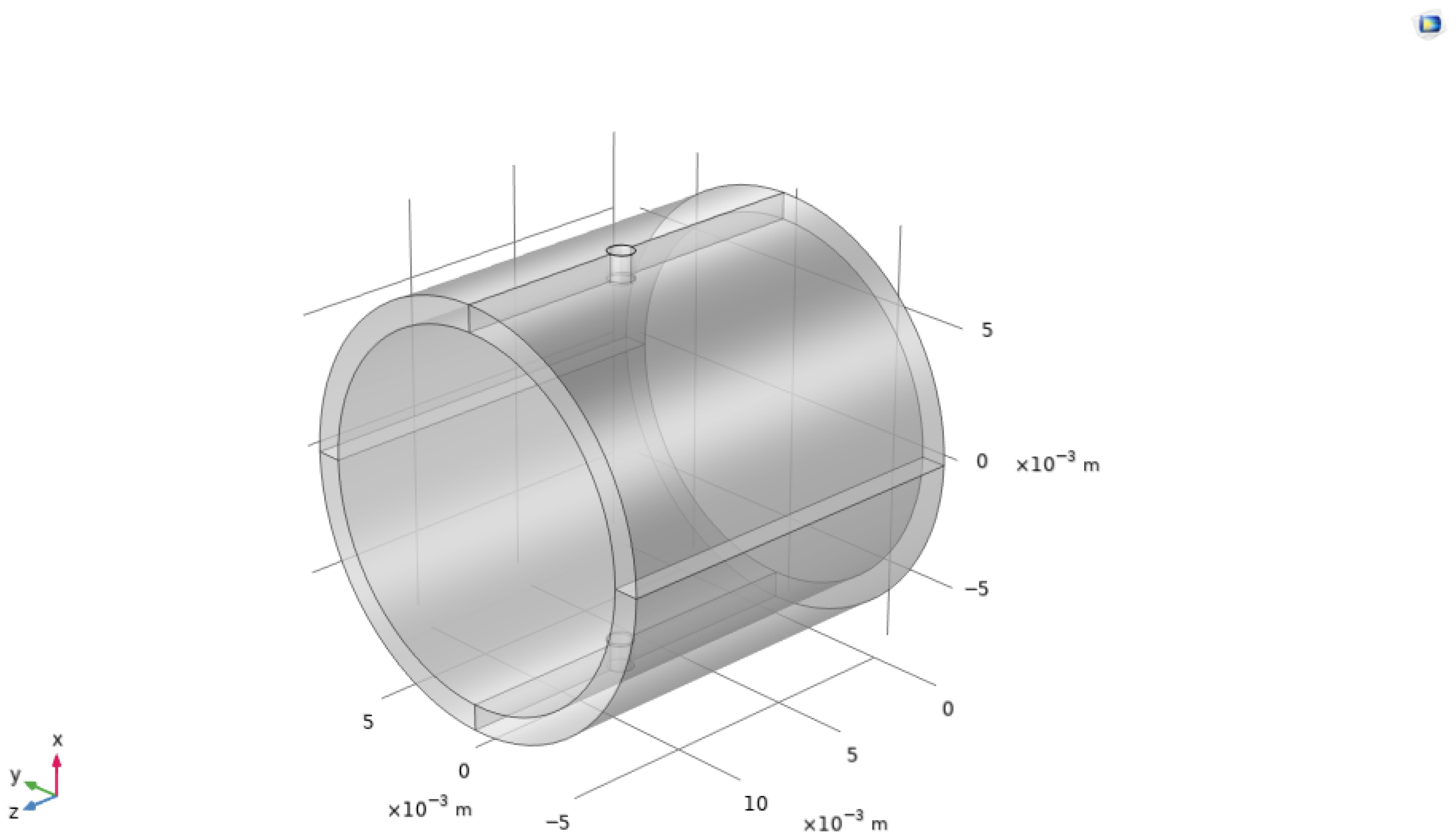
- The resonance frequency for a 1-dimensional acoustic resonator for an open–open/closed–closed system and open–closed system [15] is as follows:where and denote the resonance frequencies, c represents the speed of sound within the medium, L is the total length of the resonator, and is the end correction factor, which is conventionally zero for closed ends [6]. This end-correction factor accounts for the effective acoustic length, which may extend marginally beyond the physical dimensions of the resonator, particularly in configurations where two cavities are present, and the radius of the secondary cavity substantially exceeds that of the primary cylinder. Resonance in open–open or closed–closed pipes occurs at frequencies that are integer multiples of half the wavelength Equation (5) (left), in contrast with open–closed systems, which resonate at odd integer multiples, as indicated in Equation (5) (right). The experimental setup can be characterized as a one-dimensional acoustic resonator, given that the acoustic wavelength, calculated as m, exceeds the resonator length of 0.015 m (see Figure 2), and is a closed–closed system with the cylinder completely sealed, thus containing the acoustic waves. Within a closed–closed cylindrical resonator, standing waves establish at distinct frequencies, with pressure antinodes forming at both sealed ends. The fundamental frequency is characterized by half a wavelength fitting within the tube, with higher harmonics manifesting at integer multiples of this base frequency. This configuration promotes the amplification of acoustic signals at these resonant modes, thereby enhancing both the sensitivity and the signal-to-noise ratio critical for acoustic gas-sensing applications.
2.3. Resonance Frequency Shift with Gas Concentration
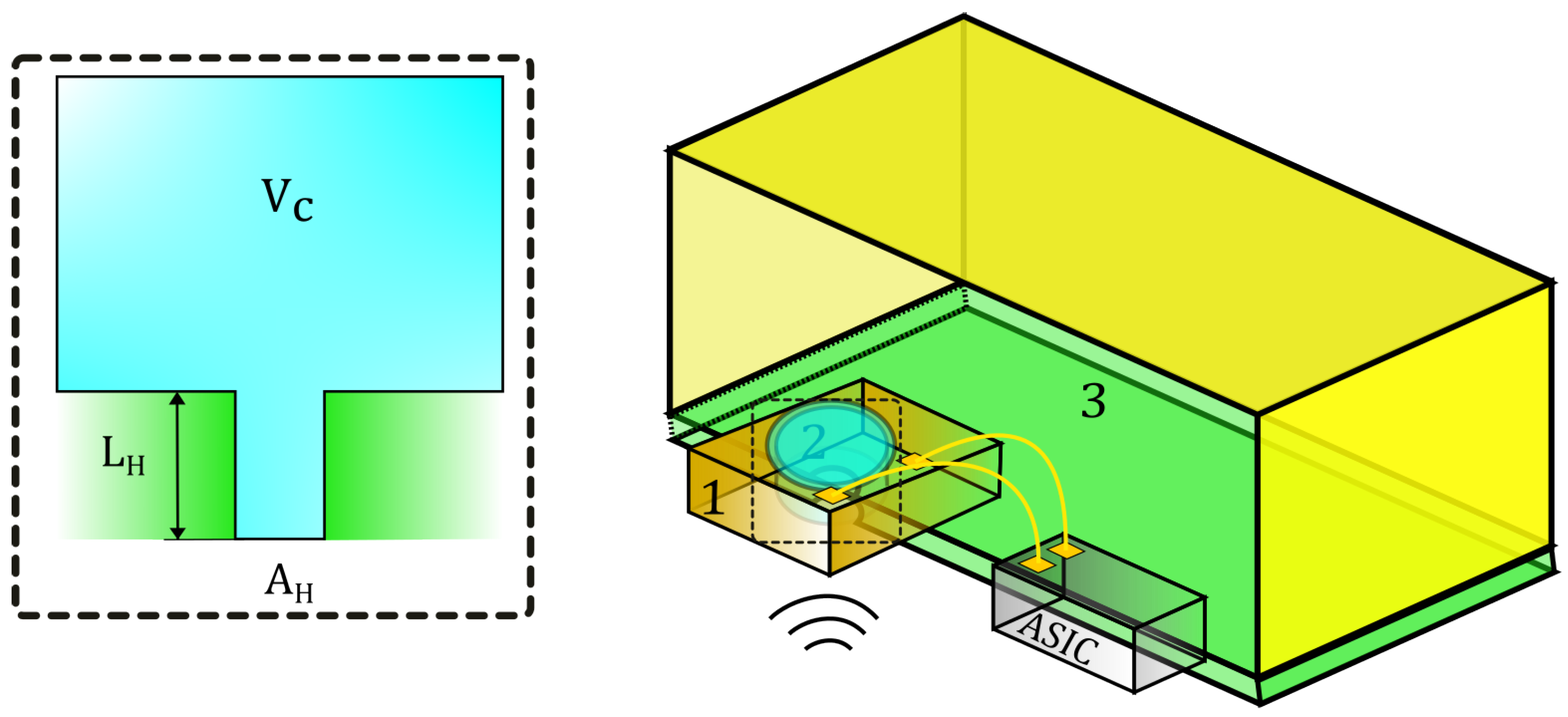
2.4. Microphone Helmholtz Resonance
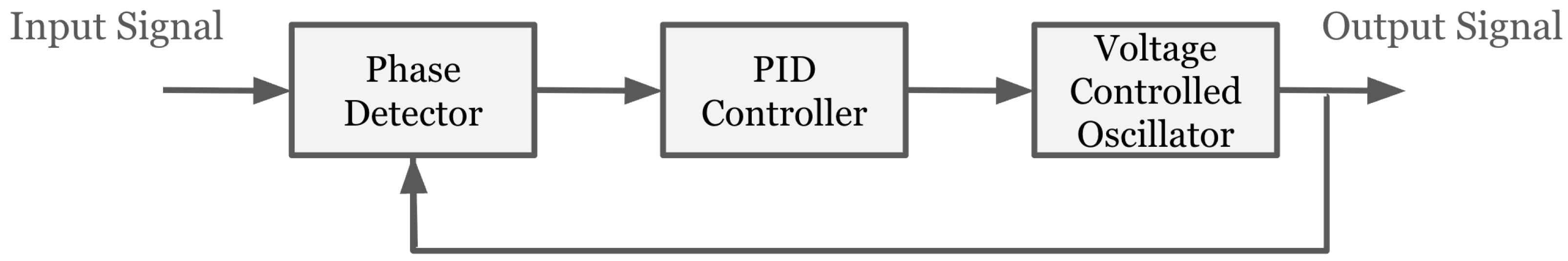
2.5. Frequency Tracking Using Lock-In and Phase-Locked Loop
3. Simulations
- Pressure Acoustics, Frequency Domain: The acoustic pressure in the chamber is generated using this module. We generate this pressure in COMSOL using the following Equations [20]:where are total pressure, background pressure field, and scattered field. Without background field, . and are complex valued quantities, and is the wave number in both the out-of-plane and azimuthal directions.
- Thermodynamics, Gas System: To simulate gas mixtures within the acoustic chamber, we use thermodynamic physics to manually define diluted gas compositions by specifying solvent and solute gases along with key mixture properties. This allows accurate replication of the behavior of the mass flow controller and enables the export of the mixture to the materials section for further simulation.
3.1. Boundary Conditions
3.1.1. Pressure Settings
- Left End of Cylinder: We apply a constant pressure of 1 Pa.
- Right End of Cylinder: Set to 0 Pa, simulating no pressure, but just a solid boundary.
3.1.2. Valve Configurations
3.1.3. Simulation Parameters
- We enable amplitude normalization to stabilize the solution.
- We disable port sweep to focus on stationary state analysis.
- The initial pressure settings across domains are set to 0 Pa.
- We select the external layer as an acoustic wall.
- We activate a domain probe for the inner cylinder.
3.2. Chemical and Thermodynamic Considerations
3.3. Selected Solvent and Bulk Species
- We select Nitrogen (N2) as the solvent, chosen for its mass and thermodynamic properties.
- Carbon dioxide (CO2) is designated as the bulk species, which primarily influences the system dynamics.
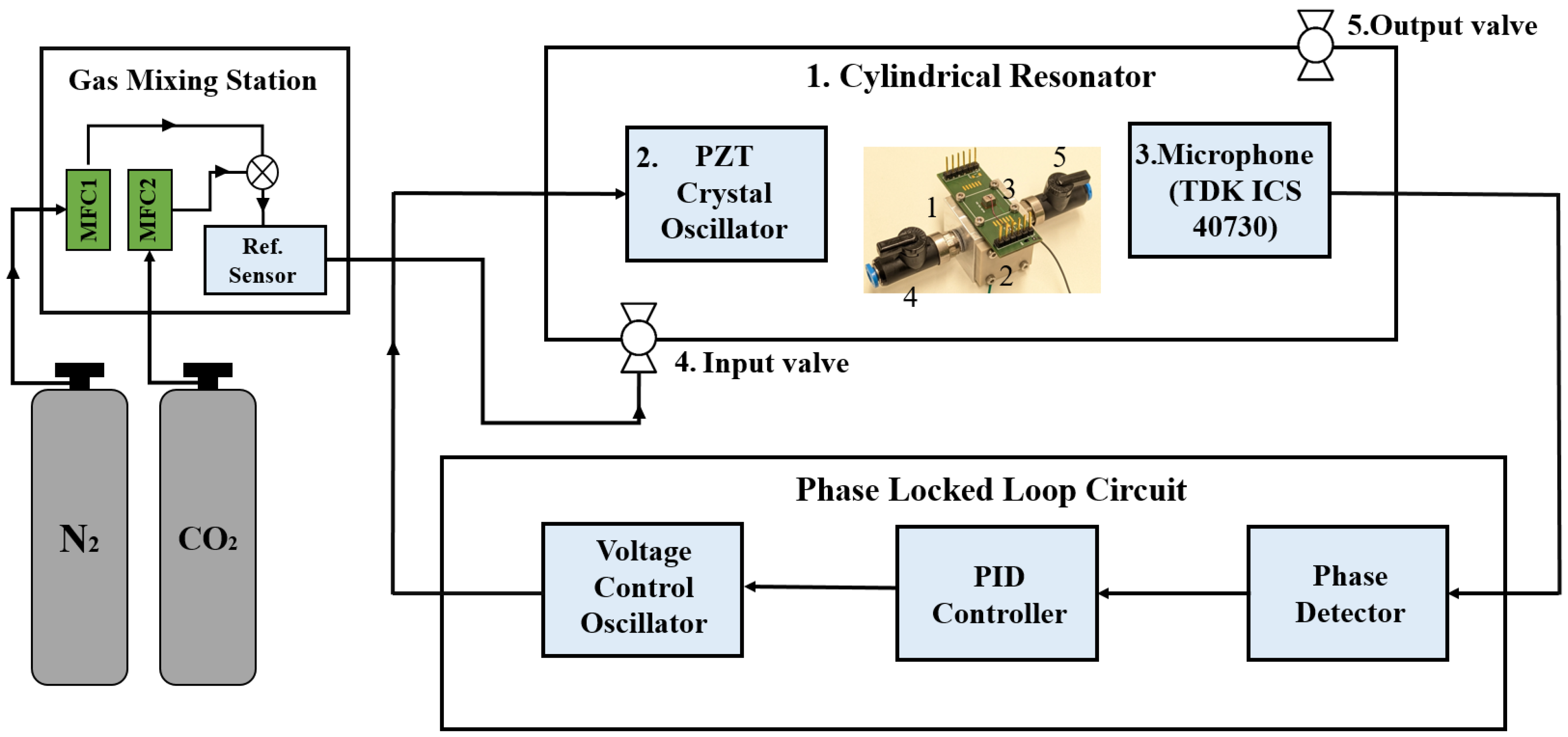
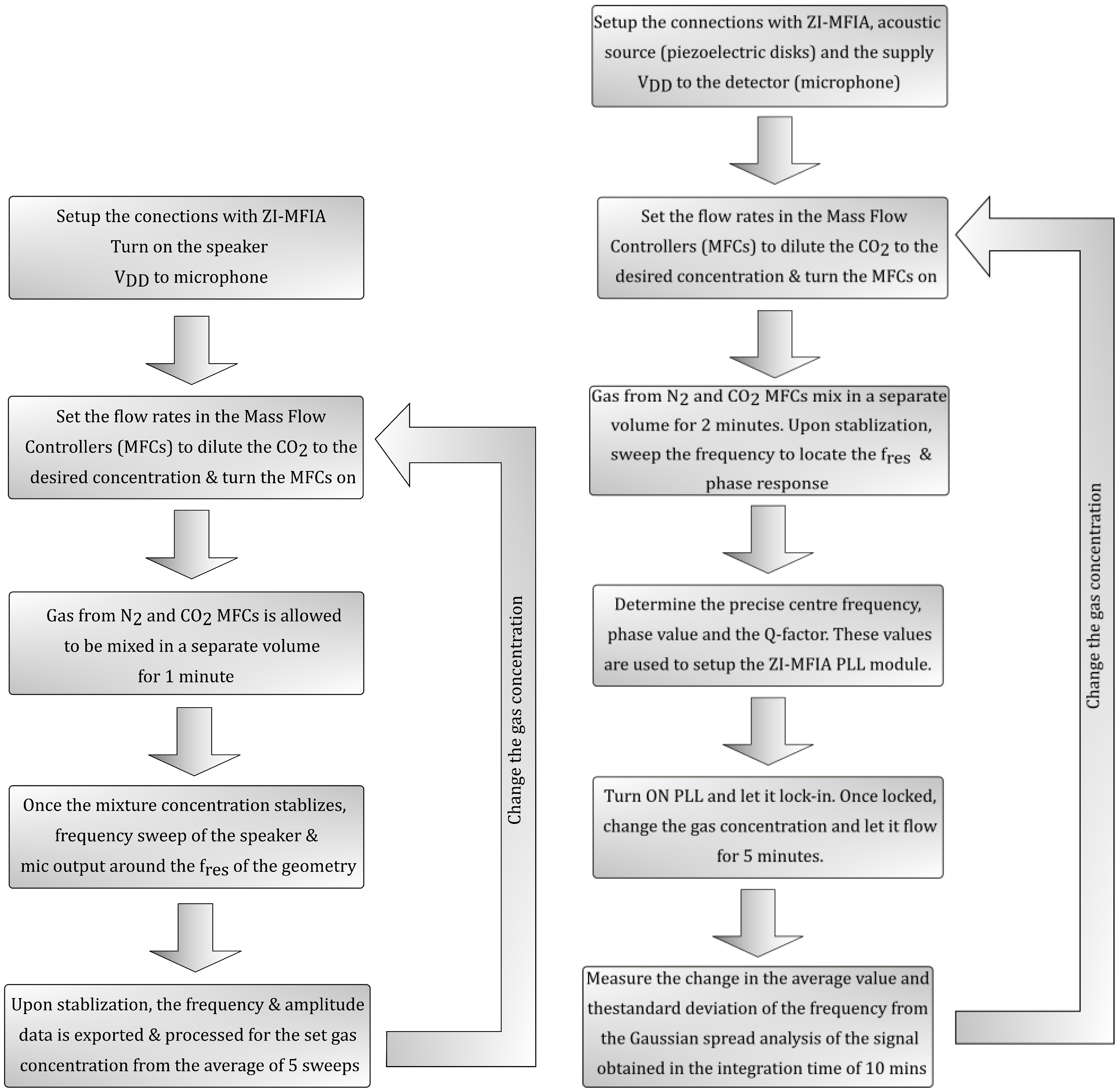
4. Experimental Setup and Methodology
4.1. Cylindrical Acoustic Resonator—Higher Concentrations
4.2. Cylindrical Acoustic Resonator—Trace Gas Concentrations
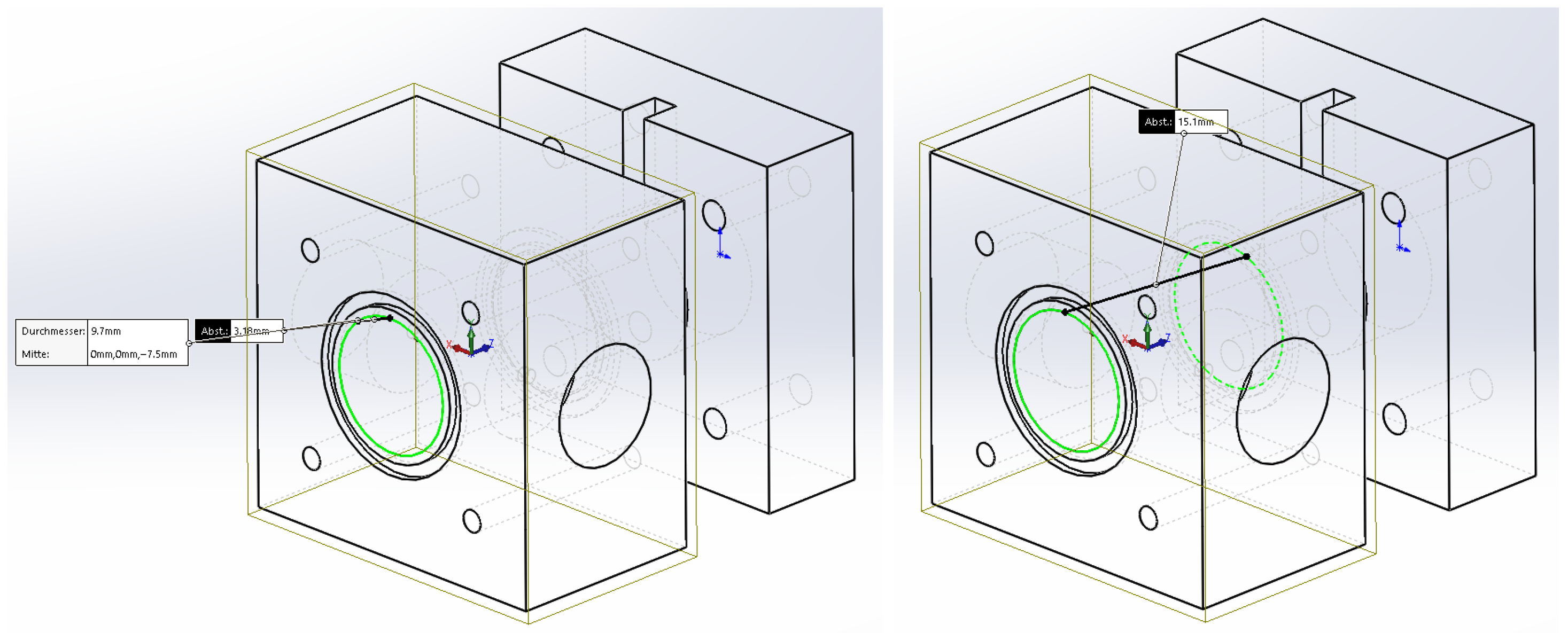
4.3. Helmholtz Resonator
5. Results and Discussion
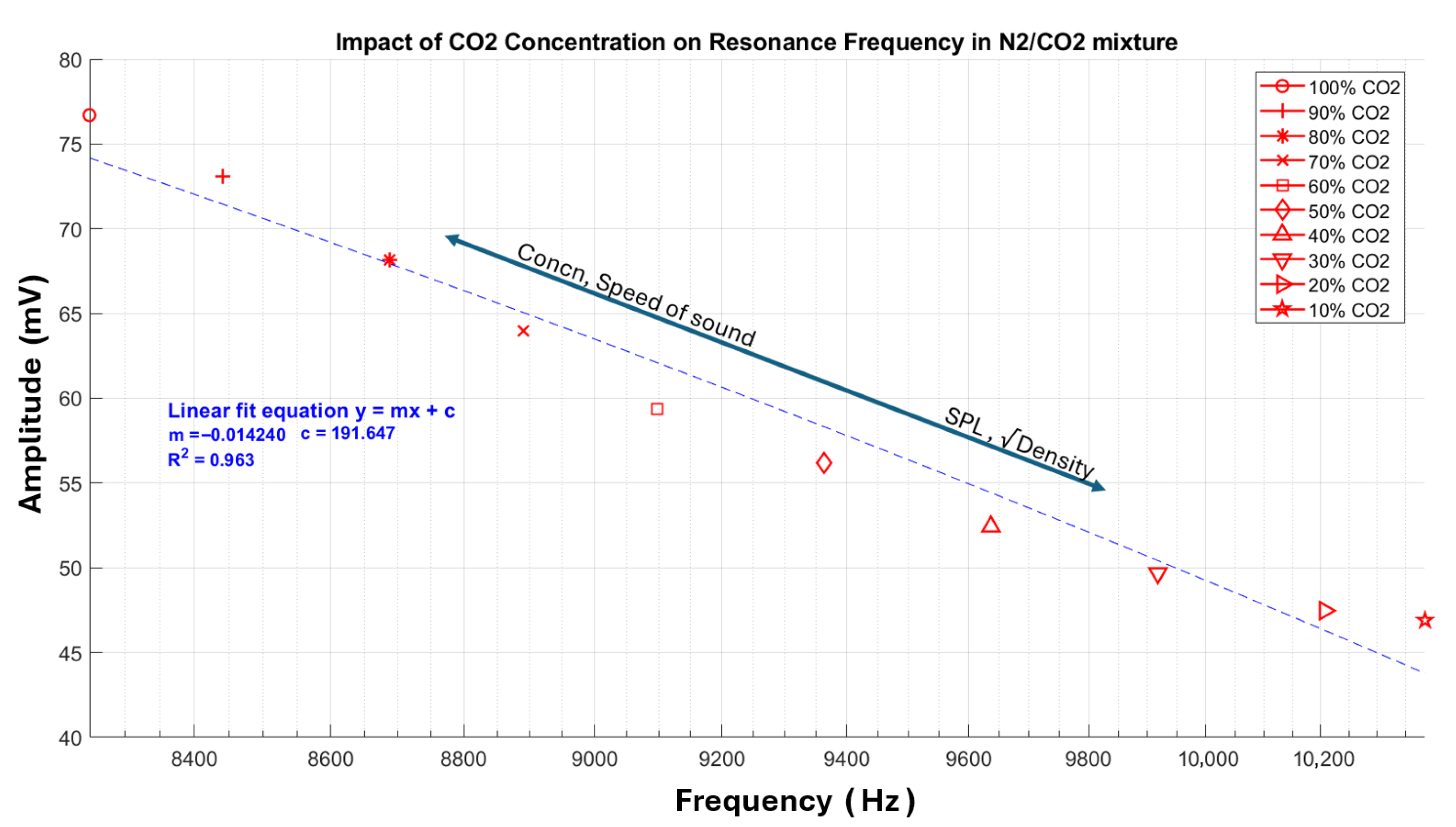
5.1. Geometric Acoustic Resonance—Higher Concentrations
- The linear relationship between resonance frequency and speed of sound is substantiated by applying a linear regression model, which produces a coefficient of determination (R2) of 0.96, which confirms direct proportionality.
- The small-magnitude negative slope of −11.8 mV/Hz indicates a gradual decrease in the resonance frequency with increasing CO2 concentration, although with a relatively small rate of change.
- Consequently, we observe an increase in amplitude, approximately 50%, from the lowest point to the highest point, which can be attributed to the relationship between SPL and density, expressed as SPL , where represents the gas density.
- The coefficient of determination (R2) of 0.96 obtained from the linear regression analysis suggests robust consistency and reliability in the measurements. This indicates that the technique has significant potential for gas detection applications, particularly for CO2 detection and quantification.
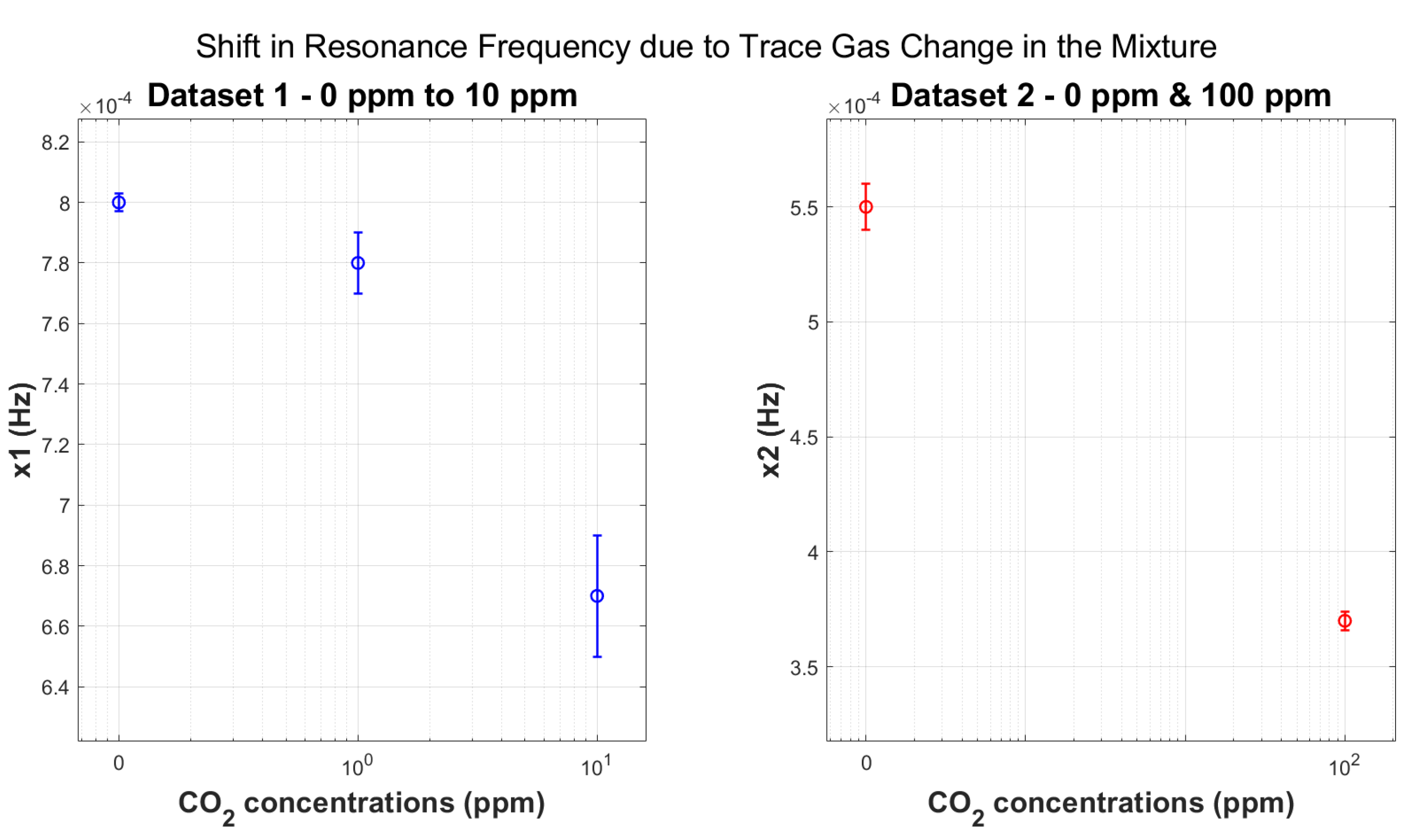
5.2. Geometric Acoustic Resonance—Trace Gas Concentrations
- Sensitivity: We observe significant frequency shifts in the transition from 100 ppm to 0 ppm CO2 concentrations (Table 3), as the change in the mole fraction becomes progressively smaller, resulting in increasingly smaller changes in CO2.
- Repeatability: The magnitude of the error bars is comparable to the frequency shifts observed, indicating high measurement repeatability and precision.These errors are derived from the standard deviations calculated from the respective group of multiple datasets. This underscores the reliability of the experimental setup and data acquisition process.
5.3. Geometric Acoustic Resonance—Simulation
- The observed trend of frequency, amplitude, and speed of sound remains consistent across both simulations and experiments.
- Variation in gas concentration resulted in larger frequency shifts in the simulations compared with the experimental results, since the simulations performed by us are simulations with no external influence of microphone sensitivity, pressure induced gas flow, open valves, etc. These ideal results serve as benchmarks for future experiments with better sensitivity and resolution.
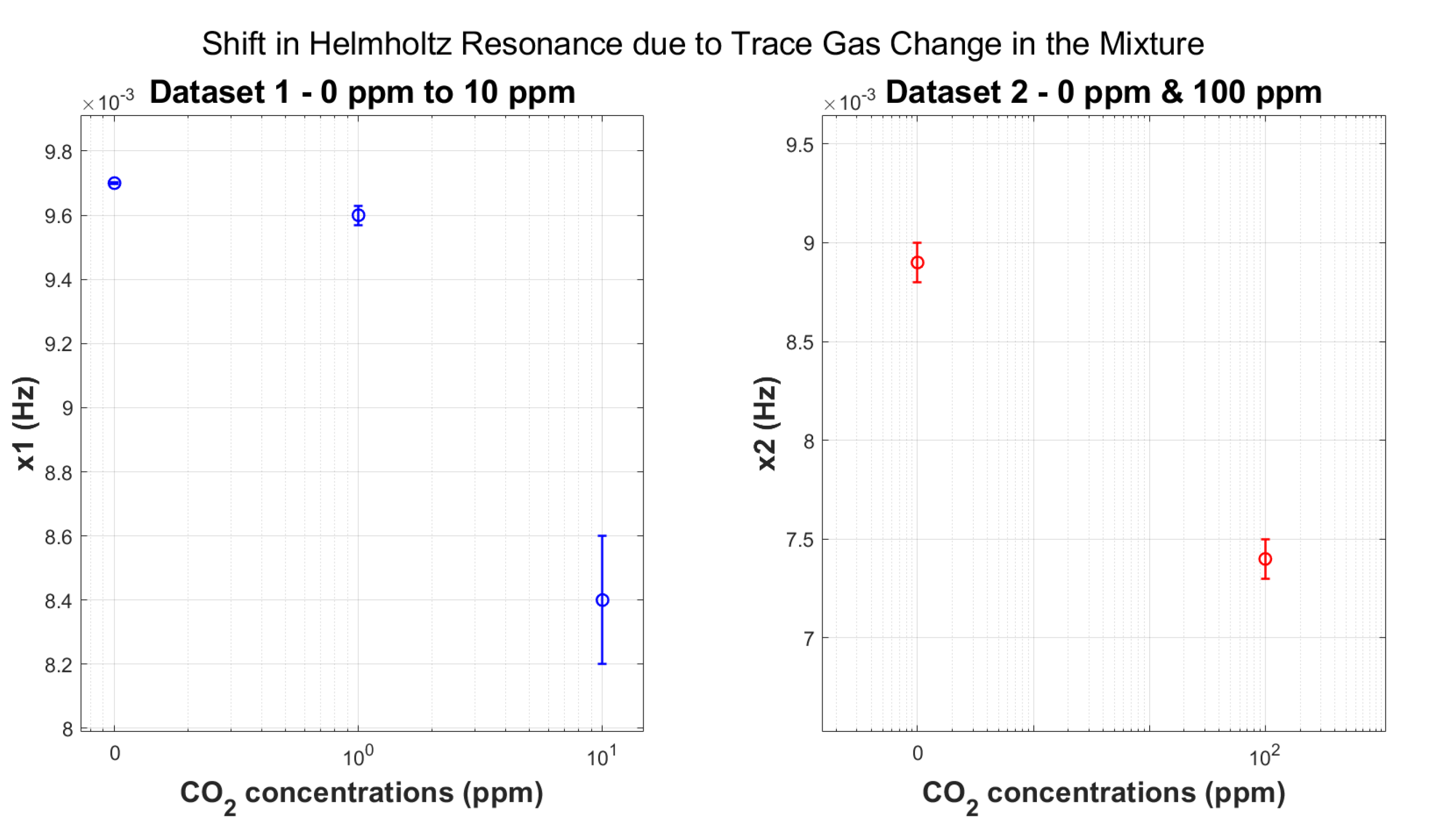
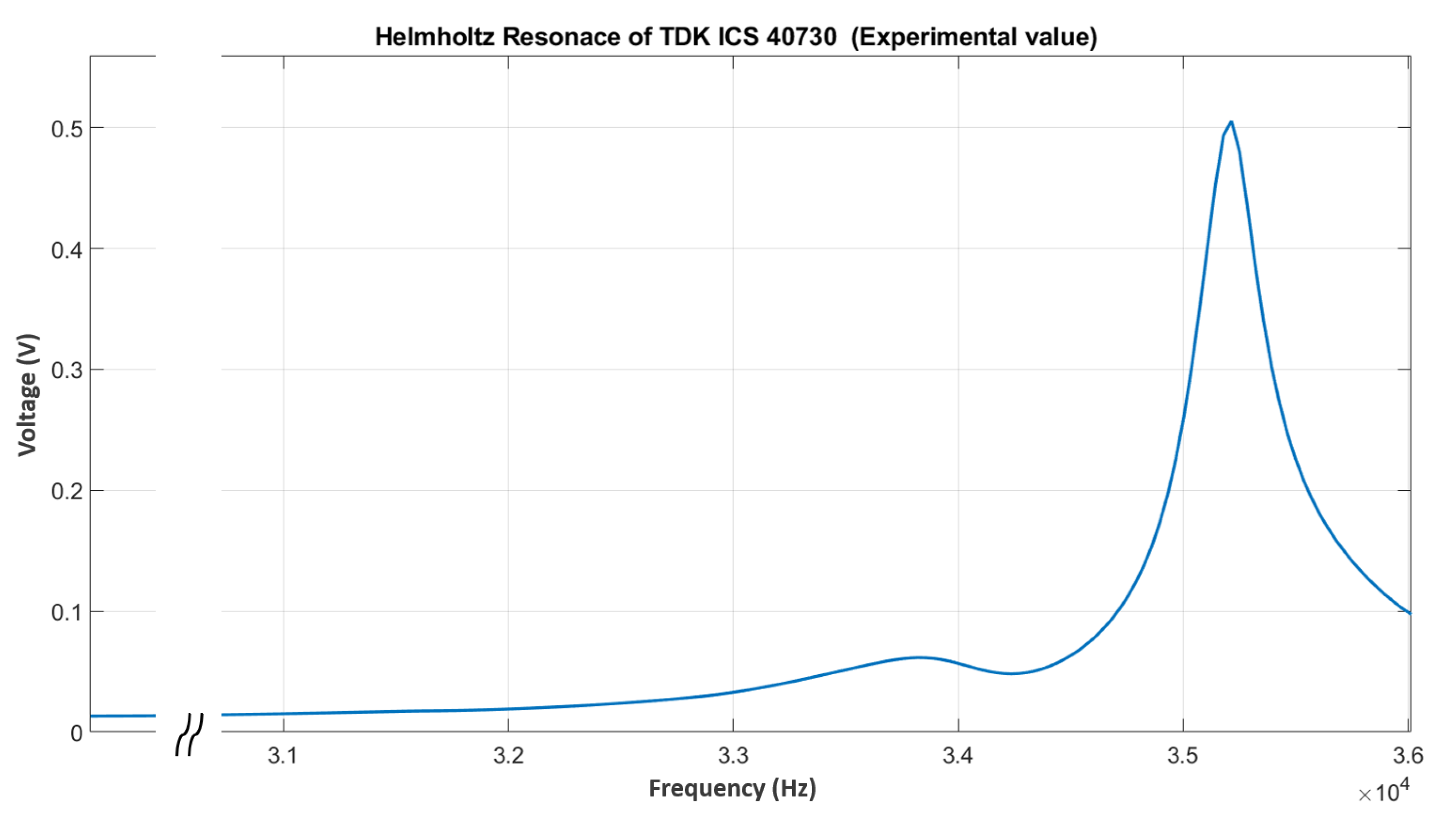
5.4. Helmholtz Resonance
- Repeatability: The raw frequency values seen in Figure 12 represent the mean of three consecutive measurement runs carried out for each gas concentration group. We computed the associated standard deviation and error values from this dataset, ensuring statistical reliability and measurement precision. This methodology improves the repeatability and precision of the reported results.
- Maximum Sensitivity: The Helmholtz resonance method exhibits the largest change in resonance frequency relative to pure N2 among all experiments carried out in this study so far (see Table 5). This indicates a high level of sensitivity to variations in gas composition. Equation (10) shows the direct proportionality between the speed of sound and the Helmholtz resonance.
- Geometry-Independent Tracking: These results demonstrate that the Helmholtz resonance, which is intrinsic to the microphone and independent of the geometry of the acoustic chamber, serves as an excellent parameter for tracking gas concentrations. This geometry independence offers potential advantages in sensor design and application versatility.
5.5. Comparison
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- What is a Gas Sensor. 2020. Available online: https://wiki.dfrobot.com/What_is_a_Gas_Sensor (accessed on 5 September 2024).
- Burge, S. 11 Most Common Flammable Gasses. ISFJ International Fire and Safety Journal, 1 March 2025. Available online: https://internationalfireandsafetyjournal.com/flammable-gasses/ (accessed on 11 April 2025).
- Sharma, A.; Eadi, S.B.; Noothalapati, H.; Otyepka, M.; Lee, H.D.; Jayaramulu, K. Porous materials as effective chemiresistive gas sensors. Chem. Soc. Rev. 2024, 53, 2530–2577. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Palzer, S. Photoacoustic-Based Gas Sensing: A Review. Sensors 2020, 20, 2745. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Feng, M.Q.; Ngo, T.A.; Shiba, K.; Minami, K.; Yoshikawa, G. Dual domain acoustic olfactory discriminator. Sens. Actuators A Phys. 2023, 350, 114102. [Google Scholar] [CrossRef]
- Hijmans, R.A.; Puers, R. Resonant sensors: A review. J. Micromech. Microeng. 2016, 26, 123001. [Google Scholar]
- Meijer, G. Smart Sensor Systems; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Eom, K.; Park, H.S.; Yoon, D.Y.; Kwon, T. Nanomechanical resonators and their applications in biological/chemical detection: Nanomechanics principles. Phys. Rep. 2011, 503, 115–163. [Google Scholar] [CrossRef]
- Nguyen, C.T.C. MEMS technology for timing and frequency control. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Frye, G.; Spates, J.; Butler, M. Gas sensing with acoustic devices. In Proceedings of the 1996 IEEE Ultrasonics Symposium, San Antonio, TX, USA, 3–6 November 1996; Volume 1, pp. 423–434. [Google Scholar] [CrossRef]
- Kuttruff, H. Introduction to Acoustics; Taylor and Francis: Abingdon, UK, 2007. [Google Scholar]
- Berg, R.E. Encyclopedia Britannica. Acoustics. 2025. Available online: https://www.britannica.com/science/acoustics (accessed on 8 March 2025).
- Yildirim, T.; Feng, M.Q.; Shiba, K.; Minami, K.; Yoshikawa, G. A portable audible-range acoustical approach for determining headspace vapour-phase properties. Sens. Actuators A Phys. 2023, 358, 114438. [Google Scholar] [CrossRef]
- Miklós, A.; Hess, P. Application of acoustic resonators in photoacoustic trace gas analysis and metrology. Rev. Sci. Instruments 2001, 72, 1937–1955. [Google Scholar] [CrossRef]
- EDN. Acoustic Design for MEMS Microphones; STMicroelectronics, Inc.: Geneva, Switzerland, 2014. [Google Scholar]
- STM. Ultrasound Behavior and Guidelines of Analog MEMS Microphone IMP23ABSU; Technical report; STMicroelectronics, Inc.: Geneva, Switzerland, 2020. [Google Scholar]
- Strahl, T.; Steinebrunner, J.; Weber, C.; Wöllenstein, J.; Schmitt, K. Photoacoustic methane detection inside a MEMS microphone. Photoacoustics 2023, 29, 100428. [Google Scholar] [CrossRef] [PubMed]
- Met One Instruments. User Manual Metone.com CAL-200 Dilution Calibrator Version 1.0; Met One Instruments: Pass, OR, USA, 2022. [Google Scholar]
- COMSOL Multiphysics. Pressure Acoustics—User’s Guide. Available online: https://doc.comsol.com/5.5/doc/com.comsol.help.aco/aco_ug_pressure.05.002.html (accessed on 27 July 2025).
- Senseair, A.B. Senseair tSENSE VAV Display. Available online: https://senseair.com/product/senseair-tsense-vav-display/ (accessed on 1 August 2025).
- Engineering Toolbox. Molecular Weight of Substances; Engineering Toolbox: Los Angeles, CA, USA, 2023. [Google Scholar]
- Duquesnoy, M. Tuning Forks in Photoacoustic Spectroscopy: Comparative Study and New Developments. Ph.D. Thesis, Université Paris-Saclay, Gif-sur-Yvette, France, 2021. [Google Scholar]

| No. | Sensor Type | Physical Change | Sensitivity (ppm) | Resolution (ppm) |
|---|---|---|---|---|
| 1 | Semiconductor | Electrical conductivity | 5–500 | 0.1–10 |
| 2 | Field effect | Electrical polarization | 0.1–100 | 0.01–1 |
| 3 | Piezoelectric | Mass | 0.1–100 | 0.01–1 |
| 4 | Optical | Optical parameters | 0.1–1000 | 0.01–10 |
| 5 | Catalytic | Heat/temperature | 100–10,000 | 10–100 |
| 6 | Electrochemical | EMF or current | 0.05–500 | 0.01–1 |
| Required CO2 in the Mixture (ppm) | Input CO2 (%) | CO2 Flow (slm) | N2 Flow (slm) |
|---|---|---|---|
| 100 | 0.5 | 0.001 | 0.049 |
| 10 | 0.02 | 0.001 | 0.019 |
| 1 | 0.01 | 0.001 | 0.099 |
| Reference | CO2 Concentration (ppm) | |
|---|---|---|
| N2—Group 1 | 1 | 25 |
| N2—Group 1 | 10 | 141 |
| N2—Group 2 | 100 | 200 |
| Change in CO2 Concentration (ppb) w.r.t N2 | Change in Frequency () (mHz) |
|---|---|
| 10 | 8 |
| 100 | 12 |
| 1000 | 28 |
| 10,000 | 76 |
| Reference | CO2 Concentration (ppm) | at Helmholtz (mHz) |
|---|---|---|
| N2—Group 1 | 1 | 0.085 |
| N2—Group 1 | 10 | 1.2 |
| N2—Group 2 | 100 | 1.4 |
| Type | Maximum Resolution (ppm) | (mHz) |
|---|---|---|
| Acoustic resonator (Simulated) | 0.001 | 8 |
| Acoustic resonator | 1 | 0.025 |
| Helmholtz resonator | 1 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, A.; Sonar, R.; Bittner, A.; Dehé, A. Prototyping and Evaluation of 1D Cylindrical and MEMS-Based Helmholtz Acoustic Resonators for Ultra-Sensitive CO2 Gas Sensing. Gases 2025, 5, 21. https://doi.org/10.3390/gases5030021
Srivastava A, Sonar R, Bittner A, Dehé A. Prototyping and Evaluation of 1D Cylindrical and MEMS-Based Helmholtz Acoustic Resonators for Ultra-Sensitive CO2 Gas Sensing. Gases. 2025; 5(3):21. https://doi.org/10.3390/gases5030021
Chicago/Turabian StyleSrivastava, Ananya, Rohan Sonar, Achim Bittner, and Alfons Dehé. 2025. "Prototyping and Evaluation of 1D Cylindrical and MEMS-Based Helmholtz Acoustic Resonators for Ultra-Sensitive CO2 Gas Sensing" Gases 5, no. 3: 21. https://doi.org/10.3390/gases5030021
APA StyleSrivastava, A., Sonar, R., Bittner, A., & Dehé, A. (2025). Prototyping and Evaluation of 1D Cylindrical and MEMS-Based Helmholtz Acoustic Resonators for Ultra-Sensitive CO2 Gas Sensing. Gases, 5(3), 21. https://doi.org/10.3390/gases5030021






