Analysis of Acute Leukemia-Associated Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment of HLH
2.3. Assessment of HLH Treatment
2.4. Definitions
2.4.1. Evaluation Criteria for Treatment Response in Acute Leukemia
2.4.2. Diagnostic Criteria for HLH
2.4.3. Criteria for Infection
2.5. Statistical Analysis
3. Results
3.1. Study Population Patients
3.2. Analysis of Clinical Manifestations and Laboratory Findings
3.3. Treatment Outcomes of HLH
3.4. Treatment of AL
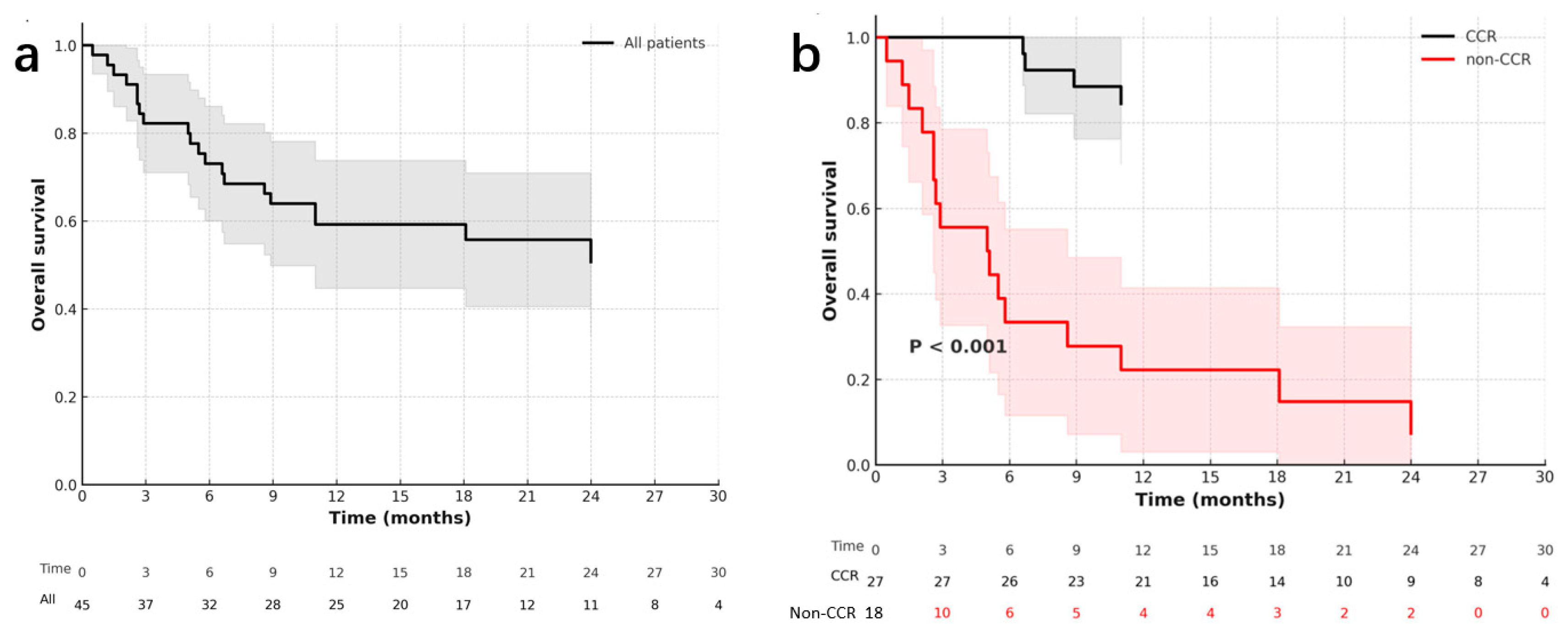
3.5. Survival and Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Aclarubicin + Etoposide |
| AEG | Aclarubicin, Etoposide and G-CSF |
| AL | Acute Leukemia |
| ALL | Acute Lymphoblastic Leukemia |
| ALT | Alanine Aminotransferase |
| AML | Acute Myeloid Leukemia |
| ANC | Absolute Neutrophil Count |
| APL | Acute Promyelocytic Leukemia |
| ATRA | All-trans Retinoic Acid |
| AZA | Azacitidine |
| BAL | Bronchoalveolar Lavage |
| CCR | Composite Complete Remission |
| CI | Confidence Interval |
| CMV | Cytomegalovirus |
| CR | Complete Response |
| CRi | Complete Remission with Incomplete Hematologic Recovery |
| CRAL | Complete Remission after Leukemia treatment |
| CT | Computed Tomography |
| DAH | Diffuse Alveolar Hemorrhage |
| DEP | Doxorubicin, Etoposide, and Methylprednisolone |
| EBV | Epstein–Barr Virus |
| ELN | European LeukemiaNet |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| HLH | Hemophagocytic Lymphohistiocytosis |
| HR | Hazard Ratio |
| HSCT | Hematopoietic Stem Cell Transplantation |
| IA | Idarubicin + Cytarabine |
| IDH | Isocitrate Dehydrogenase |
| IL | Interleukin |
| MLFS | Morphologic Leukemia-Free State |
| MOF | Multiple Organ Failure |
| MPAL | Mixed-Phenotype Acute Leukemia |
| NGS | Next-Generation Sequencing |
| NK | Natural Killer |
| NR | No Response |
| OR | Odds Ratio |
| ORR | Overall Remission Rate |
| OS | Overall Survival |
| PCR | Polymerase Chain Reaction |
| PR | Partial Response |
| sCD25 | Soluble Interleukin-2 Receptor α chain |
| sHLH | Secondary Hemophagocytic Lymphohistiocytosis |
| VP-16 | Etoposide |
| VDP | Vincristine, Daunorubicin, Prednisone |
| VDLP | Vincristine, Daunorubicin, L-asparaginase, Prednisone |
References
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Berliner, N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 2015, 125, 2908–2914. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zeron, P.; Lopez-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, M.; Wu, W.C.; Zhou, H.J.; Zou, L.Q. Lymphoma-associated hemophagocytic syndrome: A retrospective study from a single center. Hematology 2022, 27, 909–916. [Google Scholar] [CrossRef]
- Takahashi, N.; Miura, I.; Chubachi, A.; Miura, A.B.; Nakamura, S. A clinicopathological study of 20 patients with T/natural killer (NK)-cell lymphoma-associated hemophagocytic syndrome with special reference to nasal and nasal-type NK/T-cell lymphoma. Int. J. Hematol. 2001, 74, 303–308. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Kostov, B.; Moral-Moral, P.; Martinez-Zapico, A.; Diaz-Pedroche, C.; Fraile, G.; Pérez-Guerrero, P.; Fonseca, E.; Robles, A.; Vaquero-Herrero, M.P.; et al. Prognostic factors of death in 151 adults with hemophagocytic syndrome: Etiopathogenically driven analysis. Mayo. Clin. Proc. Innov. Qual. Outcomes 2018, 2, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kobayashi, R.; Tanaka, J.; Hashino, S.; Ota, S.; Torimoto, Y.; Kakinoki, Y.; Yamamoto, S.; Kurosawa, M.; Hatakeyama, N.; et al. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: A multicentre study. Br. J. Haematol. 2014, 165, 786–792. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Jin, F.; Dehoedt, C.; Rao, J.; Zhou, Y.; Li, P.; Yang, G.; Wang, M.; Zhang, R.; et al. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: A retrospective study of increasing awareness of a disease from a single-center in China. Orphanet. J. Rare. Dis. 2015, 10, 20. [Google Scholar] [CrossRef]
- Hannah, A.Y.; Jenny, O.N.; Andrew, J.W.; Satyen, G.; Jessica, J.M.; Elspeth, M.P. Safety and efficacy of anakinra in hemophagocytic lymphohistiocytosis associated with acute leukemia. Haematologica 2024, 109, 1947–1950. [Google Scholar] [CrossRef]
- Roe, C.; Bennett, J.; Zhang, L.; Chavez, J.; Shah, B.; Sokol, L.; Komrokji, R. Hemophagocytic lymphohistiocytosis in malignant hematology: Uncommon but should not be forgotten? Clin. Lymphoma Myeloma Leuk. 2015, 15, S147–S150. [Google Scholar] [CrossRef]
- Jamy, O.; Nunnery, S.; Giri, S.; Wiedower, E.; Johnson, B.; Yaghmour, G.; Martin, M.G. Under-recognition of hemophagocytic syndrome in United States’ rural, non-teaching hospitals. Leuk. Lymphoma 2016, 57, 2911–2913. [Google Scholar] [CrossRef]
- Delavigne, K.; Berard, E.; Bertoli, S.; Corre, J.; Duchayne, E.; Demur, C.; Mas, V.M.-D.; Borel, C.; Picard, M.; Alvarez, M.; et al. Hemophagocytic syndrome in patients with acute myeloid leukemia undergoing intensive chemotherapy. Haematologica 2014, 99, 474–480. [Google Scholar] [CrossRef]
- Bertozzi, A.I.; Suc, A.; Rubie, H.; Duchayne, E.; Demur, C.; Robert, A. Hemophagocytic syndrome associated with neutropenia after chemotherapy. Arch. Pediatr. 2002, 9, 125–129. [Google Scholar] [CrossRef]
- Henter, J.I.; Elinder, G.; Soder, O.; Ost, A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistio-cytosis. Acta. Paediatr. Scand. 1991, 80, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Samuelsson-Horne, A.; Arico, M.; Egeler, R.M.; Elinder, G.; Filipovich, A.H.; Gadner, H.; Imashuku, S.; Komp, D.; Ladisch, S.; et al. Treatment of hemophagocytic lym-phohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002, 100, 2367–2373. [Google Scholar] [CrossRef]
- Chinese Society of Hematology, Chinese Society of Pediatrics, Chinese Expert Alliance on Hemophagocytic Syndrome. Guidelines for the diagnosis and treatment of hemophagocytic syndrome in China (2022 edition). Chin. Med. J. 2022, 102, 1492–1499. [Google Scholar]
- Lai, W.; Wang, Y.; Wang, J.; Wu, L.; Jin, Z.; Wang, Z. Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults and adolescents: A life-threatening disease: Analysis of 133 cases from a single center. Hematology 2018, 23, 810–816. [Google Scholar] [CrossRef]
- Yu, W.J.; Kong, J.; Zheng, F.M.; Mo, X.D.; Zhang, X.H.; Xu, L.P.; Zhang, Y.-Y.; Sun, Y.-Q.; Jin, J.; Huang, X.-J.; et al. Treatment of minimal residual disease in myeloid malignancies after allo-HSCT with venetoclax-based regimens in patients ineligible for or failed in the immunotherapy. Hematology 2024, 29, 2418653. [Google Scholar] [CrossRef]
- Yu, W.J.; Mo, X.D.; Zhang, X.H.; Xu, L.P.; Wang, Y.; Yan, C.H.; Chen, H.; Chen, Y.-H.; Han, W.; Wang, F.-R.; et al. Occurrence and severity of donor lymphocyte infusion-associated chronic graft-versus-host disease influence the clinical outcomes in relapsed acute leukemia after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Melissa, R.G. Hemophagocytic lymphohistiocytosis: Review of etiologies and management. J. Blood Med. 2014, 5, 69–86. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the man-agement of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Machowicz, R.; Janka, G.; Wiktor-Jedrzejczak, W. Similar but not the same: Differential diagnosis of HLH and sepsis. Crit. Rev. Oncol. Hematol. 2017, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Galicier, L.; Coppo, P.; Marzac, C.; Aumont, C.; Lambotte, O.; Fardet, L. Reactive hemophagocytic syndrome in adults: A retrospective analysis of 162 patients. Am. J. Med. 2014, 127, 1118–1125. [Google Scholar] [CrossRef]
- Henter, A.; Park, S.; Gatt, D.; Yu, A.Y.L.; Chan, L.Y.C. Hemophagocytic syndromes (HPSs) including hemophagocytic lympho-histiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. 2016, 30, 411–420. [Google Scholar]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; DeFor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.; Nichols, K.E.; Albeituni, S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistio-cytosis. Front. Immunol. 2021, 12, 614704. [Google Scholar] [CrossRef]
- Wei, W.; Li, N.; Li, Z.; Yan, L. The notch pathway promotes NF-κB activation through Asb2 in T cell acute lymphoblastic leukemia cells. Cell Mol. Biol. Lett. 2018, 23, 37. [Google Scholar] [CrossRef]
- Sulis, M.L.; Aiello, A.I.; Qian, Y.; Hsieh, D.; Bhatia, M.; Garg, T.; Paietta, E.; Tallman, M.S.; Rowe, J.M.; De Keersmaecker, K.; et al. Synergistic antileukemic therapies in NOTCH1-induced T-ALL. Proc. Natl. Acad. Sci. USA 2017, 114, 2006–2011. [Google Scholar]
- Zhang, K.; Jordan, M.B.; Marsh, R.A.; Johnson, J.A.; Kissell, D.; Meller, J.; Villanueva, J.; Risma, K.A.; Wei, Q.; Klein, P.S.; et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011, 118, 5794–5798. [Google Scholar] [CrossRef] [PubMed]
- Borel, C.; Jeannin, J.P.; Gendron, M.; Benamara, M.; Legrand, Y.; Boucher, B.; Mahlaoui, N.; Garcelon, N.; Lambotte, O.; Launay, D.; et al. Severe adult hemophagocytic lymphohistiocytosis correlates with HLH-related gene variants. J. Allergy Clin. Immunol. 2023, 153, 256–264. [Google Scholar]
| Patient Characteristics | N (%) |
|---|---|
| Patient age, year, median (range) | 49(18–75) |
| Patient sex | |
| Male | 28(62%) |
| Female | 17 (38%) |
| Hematological malignancies | |
| AML | 40 (89%) |
| ALL | 4 (9%) |
| Other | 1 (2%) |
| ELN Risk category of AL | |
| Favorable | 16 (35%) |
| Intermediate | 3 (7%) |
| Poor | 26 (58%) |
| AL treatment status | |
| Patients with de novo AL before treatment | 25 (55.5%) |
| Patients with de novo AL after consolidation chemotherapy | 7 (15.6%) |
| Patients with refractory or recurrent acute leukemia | 13 (28.9%) |
| AL status at HLH onset | |
| CCR | 7 (15.6%) |
| Non-CCR | 38 (84.4%) |
| AL status after treatment | |
| CCR | 27 (60%) |
| Non-CCR | 18 (40%) |
| Sites of infection | |
| Lung | 30 (66.7%) |
| Digestive tract | 6 (13.3) |
| Soft tissue | 12 (33.3%) |
| Thyroid | 1 (2%) |
| Bacteremia | 12 (33.3%) |
| More than 2 sites | 9 (20%) |
| Etiologic features | |
| Bacteria | 11 (24.4%) |
| Fungi | 3 (6.7%) |
| Bacteria and fungi | 1 (2%) |
| EBV | 1 (2%) |
| Mycoplasma | 2(4%) |
| Cytogenetic and molecular biologic abnormalities * | |
| RUNX1::RUNX1T1 fusion, C-kit negative | 4 |
| RUNX1::RUNX1T1 fusion, C-kit D816 mutation | 2 |
| ASXL1 mutation | 7 |
| WT1 mutation | 3 |
| NPM1 mutation | 8 |
| IDH1/IDH2 mutation | 4 |
| FLT3-ITDhigh | 2 |
| bZIP-CEBPA mutation | 1 |
| TP53 mutation | 5 |
| MLL AF9 fusion | 1 |
| EVI1 mutation | 2 |
| Complex karyotype | 7 |
| Characteristics | N = 45 |
|---|---|
| Fever | 95.6% (43/45) |
| Hepatomegaly and splenomegaly | 36.4% (12/33) |
| Ferritin (ng/mL), median (range) | 2631 (1122–31,129) |
| Plasma fibrinogen concentration (g/L) | 3.13 (0.85–6.72) |
| NK cell activity (%) | 12.39 (3.77–19.28) |
| sCD25 level (pg/mL) | 10,249 (1982–70,375) |
| Alanine aminotransferase (U/L) | 53.5 (8.0–1899.0) |
| Aspartate aminotransferase (U/L) | 34.0 (14.0–725.0) |
| Cause of Death | Case IDs | Number of Patients |
|---|---|---|
| Leukemia relapse | 7, 8, 11, 17, 28, 37, 44 | 7 |
| No response to treatment (NR) | 9, 22, 23, 24, 35, 38, 40, 41, 42 | 9 |
| Diffuse alveolar hemorrhage (DAH) | 4, 21, 34, 36 | 4 |
| Total | 20 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| AL treatment status (new vs. refractory/relapsed) | 2.528 (1.039–6.153) | 0.041 | ||
| Risk category (favorable vs. intermediate and poor) | 7.006 (1.619–30.319) | 0.009 | 3.948 (0.884–17.642) | 0.072 |
| AL status after treatment (CCR vs. non-CCR) | 12.216 (4.031–37.017) | <0.001 | 9.268 (2.999–28.641) | <0.001 |
| AL status at HLH onset (CCR vs. non-CCR) | 5.215 (0.692–39.200) | 0.109 | ||
| HLH remission (CR vs. NR/PR) | 8.054 (2.312–28.055) | 0.001 | ||
| HSCT (no vs. yes) | 3.275 (1.929–5.185) | 0.999 | ||
| Age (>50 vs. ≤50, years old) | 3.525 (1.346–9.228) | 0.010 | ||
| Organ dysfunction (no vs. yes) | 1.787 (0.712–4.481) | 0.216 | ||
| Serum ferritin level (lower than median vs. higher than median) | 0.520 (0.207–1.305) | 0.164 | ||
| sCD25 level (<10,000 vs. ≥10,000 pg/mL) | 1.389 (0.406–4.750) | 0.600 | ||
| Infection status-microbiologically proven (no vs. yes) | 1.100 (0.449–2.695) | 0.834 | ||
| Infection status-clinical proven (no vs. yes) | 1.435 (0.192–10.744) | 0.725 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.-J.; Wu, Y.; Duan, W.-B.; Chen, Q.; Pei, X.-Y.; Jia, J.-S.; Wang, J.; Zhu, X.-L.; Zhao, X.-S.; Huang, X.-J.; et al. Analysis of Acute Leukemia-Associated Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Experience. Immuno 2025, 5, 58. https://doi.org/10.3390/immuno5040058
Yu W-J, Wu Y, Duan W-B, Chen Q, Pei X-Y, Jia J-S, Wang J, Zhu X-L, Zhao X-S, Huang X-J, et al. Analysis of Acute Leukemia-Associated Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Experience. Immuno. 2025; 5(4):58. https://doi.org/10.3390/immuno5040058
Chicago/Turabian StyleYu, Wen-Jing, Ying Wu, Wen-Bing Duan, Qi Chen, Xu-Ying Pei, Jin-Song Jia, Jing Wang, Xiao-Lu Zhu, Xiao-Su Zhao, Xiao-Jun Huang, and et al. 2025. "Analysis of Acute Leukemia-Associated Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Experience" Immuno 5, no. 4: 58. https://doi.org/10.3390/immuno5040058
APA StyleYu, W.-J., Wu, Y., Duan, W.-B., Chen, Q., Pei, X.-Y., Jia, J.-S., Wang, J., Zhu, X.-L., Zhao, X.-S., Huang, X.-J., & Jiang, H. (2025). Analysis of Acute Leukemia-Associated Hemophagocytic Lymphohistiocytosis in Adults: A Single-Center Experience. Immuno, 5(4), 58. https://doi.org/10.3390/immuno5040058







