Micro- and Nanoplastics and the Immune System: Mechanistic Insights and Future Directions
Abstract
1. Introduction
2. Characteristics and Immunological Relevance of MNPs
2.1. Definition of Micro- and Nanoplastics
2.2. Routes and Mechanisms of MNP Entry into the Human Body
2.2.1. Ingestion
2.2.2. Inhalation
2.2.3. Dermal Contact
2.3. Biodistribution and Immune System Impacts
2.4. MNP Surface Modification and Immune Reactivity
2.5. Indirect Effects of MNPs on the Immune System
2.5.1. Pathogen Carriage and Increased Susceptibility
2.5.2. Gut Microbiota Disruption and Cytotoxicity
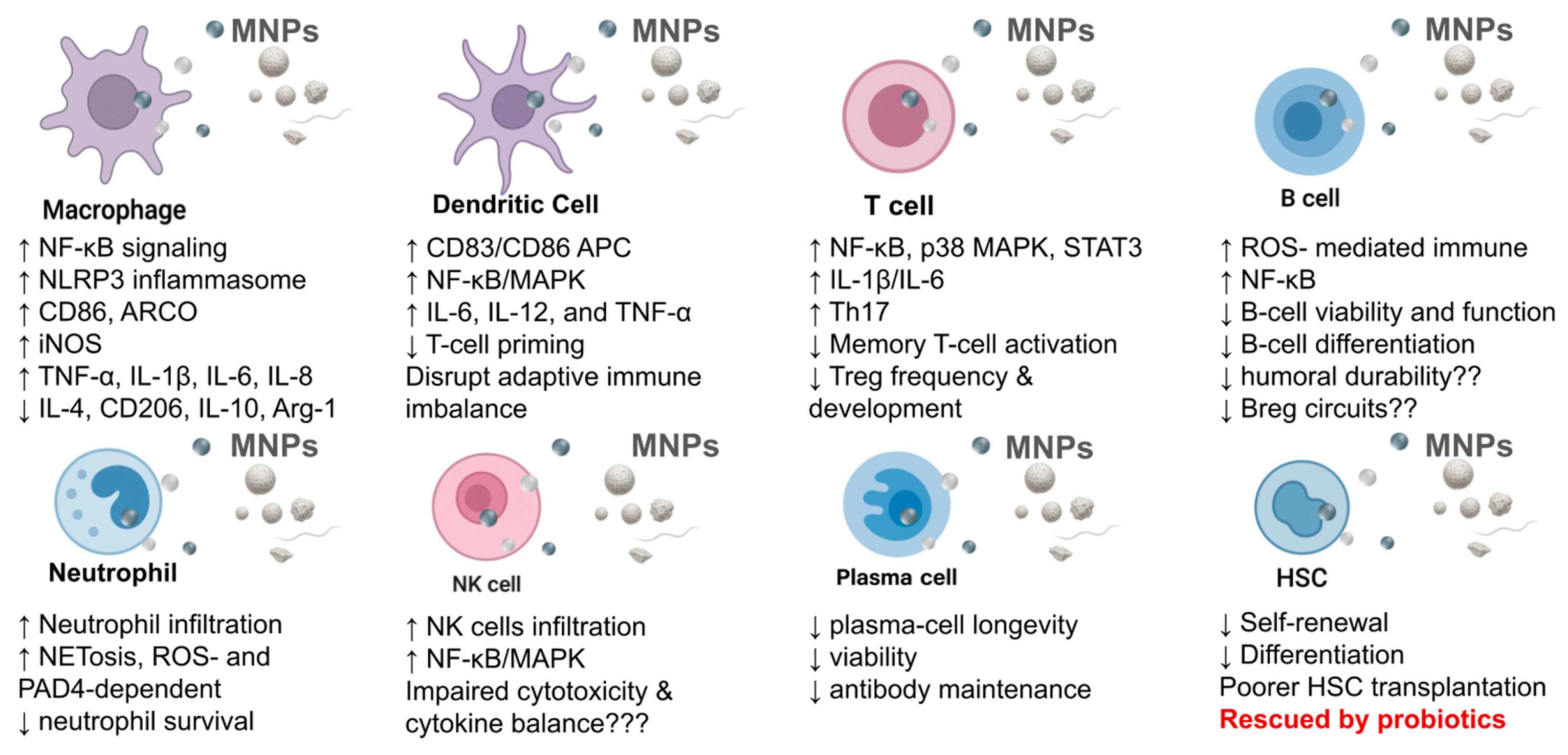
3. Direct Effects of MNPs on the Immune System
3.1. Effects on Innate Immune Cells
3.1.1. Effects of MNPs on Macrophages
Effects on Macrophage Phagocytosis
Effects on Macrophage Polarization
Effects on Macrophage Cytokine Secretion
3.1.2. Effects on the Dendritic Cells (DCs)
Antigen Presentation
Maturation
3.1.3. Effects on Neutrophils
3.1.4. Effects on Natural Killer (NK) Cells
3.2. Effects on Adaptive Immune Cells
3.2.1. Disruption of T Cell Compartments
Regulatory T (Treg) Cells
T Helper (Th) Cells
Natural Killer T (NKT) Cells
Memory T Cells
3.2.2. Effects on B Cells
Memory B Cells (Emerging View)
Regulatory B Cells (Bregs)
Long-Lived Plasma Cells
3.3. Effects of MNP Types on Immune Cells
3.4. Disruption of Hematopoietic Stem Cell (HSC) Function
4. Molecular Mechanisms and Signaling Pathways
4.1. Oxidative Stress and Mitochondrial Dysfunction
4.1.1. ROS Overproduction and Disruption of Antioxidant Defenses
4.1.2. Loss of Mitochondrial Membrane Potential (Δψm)
4.2. Inflammatory Signaling Pathways
4.2.1. NF-κB Pathway
4.2.2. Mitogen-Activated Protein Kinase (MAPK) Pathways
4.2.3. NLRP3 Inflammasome
4.3. Apoptosis and Cell Death Programs
4.3.1. Intrinsic and Extrinsic Apoptosis
4.3.2. Autophagy Dysregulation
4.3.3. Pyroptosis
4.3.4. Convergence of Programmed Cell-Death Pathways
5. Immunomodulatory and Autoimmune Implications
5.1. MNPs and Chronic Low-Grade Inflammation
5.2. Disruption of Immune Tolerance via Barrier Dysfunction and Innate Activation
5.3. Immunomodulatory and Autoimmune Consequences of MNP Exposure
5.4. Potential Links to Specific Autoimmune Diseases
5.4.1. Systemic Lupus Erythematosus (SLE)
5.4.2. Inflammatory Bowel Disease (IBD)
5.4.3. Rheumatoid Arthritis (RA) and TLR/NF-κB Signaling
5.5. MNP Exposure Routes and Specific Diseases
6. Current Knowledge Gaps and Research Challenges
6.1. Lack of Human Cohort and Longitudinal Studies
6.2. Poor Standardization of MNPs Parameters
6.2.1. Experimental Inconsistencies
6.2.2. Variable Particle Sizes and Dosing Protocols
6.2.3. Sampling and Quality Control
6.3. Interactions Between MNPs, Co-Pollutants, and the Gut Microbiota
6.3.1. Co-Exposures with Pollutants
6.3.2. Gut Microbiota Disruption
6.3.3. Ecosystem Perspective
6.4. Challenges in Tracing MNPs In Vivo
6.4.1. Lack of Reliable Tracking Methods
6.4.2. Limitations in Imaging and Quantification
6.4.3. Lack of Standardization in Particle Characterization
6.5. Discrepancies Between In Vitro Data and In Vivo Results
7. Future Perspectives and Research Directions
7.1. Advancing Human Epidemiological Evidence
7.2. Improving Environmental and Experimental Realism
7.3. Innovations in Detection, Imaging, and Tracing
7.4. Mechanistic Insights into Host–Microbe–Pollutant Interactions
7.5. Clinical and Immunological Endpoints
7.6. Policy, Mitigation, and Intervention Strategies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile butadiene styrene |
| APC | Antigen-presenting cell |
| BPA | Bisphenol A |
| Bregs | Regulatory B cells |
| DAMP | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| ER | Endoplasmic reticulum |
| FTIR | Fourier transform infrared spectroscopy |
| GC-MS | Gas chromatography–mass spectrometry |
| GSDMD | Gasdermin D |
| GSH | Glutathione |
| HSC | Hematopoietic stem cell |
| IBD | Inflammatory bowel disease |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MARCO | Macrophage receptor with collagenous structure |
| METs | Macrophage extracellular traps |
| MNPs | Micro- and nanoplastics |
| MPs | Microplastics |
| MRI | Magnetic resonance imaging |
| NETs | Neutrophil extracellular traps |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer |
| NKT | Natural killer T |
| NLRP3 | Nod-like receptor protein 3 |
| NPs | Nanoplastics |
| PET | Positron emission tomography |
| PMMA | polymethyl methacrylate |
| PPR | Pattern recognition receptor |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| RA | Rheumatoid arthritis |
| ROS | Reactive oxygen species |
| SLE | Systemic lupus erythematosus |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| Th | Helper T cells |
| TLR | Toll-like receptor |
| Treg | Regulatory T cells |
| UPR | Unfolded protein response |
| UV | Ultraviolet |
References
- Mohajan, H.K. Plastic Pollution: A Potential Threat on Health and Environment. Stud. Soc. Sci. Humanit. 2025, 4, 25–30. [Google Scholar] [CrossRef]
- Plastic Pollution. Available online: https://iucn.org/resources/issues-brief/plastic-pollution (accessed on 26 August 2025).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Abimbola, A.N.; Adejumobi, V.O.; Aribisala, O.C.; Oyeniyi, E.O. Influence of Plastic Waste Management on the Environment: A Review. Environ. Technol. Sci. J. 2023, 14, 56–64. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the Seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.-H. Adsorption of Environmental Contaminants on Micro- and Nano-Scale Plastic Polymers and the Influence of Weathering Processes on Their Adsorptive Attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research—What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Tuan Anuar, S.; Yusof, K.M.K.K.; Lai, L.A.; Brentnall, T. Detection of Microplastics in Human Tissues and Organs: A Scoping Review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Chakraborty, S.; Banerjee, M.; Jayaraman, G.; Rajeswari, V.D. Evaluation of the Health Impacts and Deregulation of Signaling Pathways in Humans Induced by Microplastics. Chemosphere 2024, 369, 143881. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of Microplastics on Immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef]
- Zeng, W.; He, S.; Zhao, Y.; Jiang, M.; Wang, W.; Yang, L.; Du, W.; Zhuang, W. Microplastics Exposure Aggravates Synovitis and Pyroptosis in SLE by Activating NF-κB and NRF2/KEAP1 Signaling. Toxics 2024, 12, 840. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Mcguinness, K. Nanoplastics. Futurist 1995, 29, 50–53. [Google Scholar]
- Yu, Q.; Chuang, C.-Y.A.; Jiang, Y.; Zhong, H.; Cundy, A.; Kwong, R.W.M.; Min, C.; Zhu, X.; Ji, R. Exploring Environmental Nanoplastics Research: Networks and Evolutionary Trends. Rev. Environ. Contam. Toxicol. 2023, 261, 12. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Nawab, A.; Ahmad, M.; Khan, M.T.; Nafees, M.; Khan, I.; Ihsanullah, I. Human Exposure to Microplastics: A Review on Exposure Routes and Public Health Impacts. J. Hazard. Mater. Adv. 2024, 16, 100487. [Google Scholar] [CrossRef]
- Jahedi, F.; Jaafarzadeh Haghighi Fard, N. Micro- and Nanoplastic Toxicity in Humans: Exposure Pathways, Cellular Effects, and Mitigation Strategies. Toxicol. Rep. 2025, 14, 102043. [Google Scholar] [CrossRef]
- Niccolai, E.; Colzi, I.; Amedei, A. Adverse Effects of Micro- and Nanoplastics on Humans and the Environment. Int. J. Mol. Sci. 2023, 24, 15822. [Google Scholar] [CrossRef]
- Sajedi, S.; An, C.; Chen, Z. Unveiling the Hidden Chronic Health Risks of Nano- and Microplastics in Single-Use Plastic Water Bottles: A Review. J. Hazard. Mater. 2025, 495, 138948. [Google Scholar] [CrossRef]
- Covello, C.; Di Vincenzo, F.; Cammarota, G.; Pizzoferrato, M. Micro(Nano)Plastics and Their Potential Impact on Human Gut Health: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 2658–2677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ran, L.; He, Y.; Huang, Y. Mechanisms of Microplastics on Gastrointestinal Injury and Liver Metabolism Disorder (Review). Mol. Med. Rep. 2025, 31, 98. [Google Scholar] [CrossRef]
- Vagner, M.; Boudry, G.; Courcot, L.; Vincent, D.; Dehaut, A.; Duflos, G.; Huvet, A.; Tallec, K.; Zambonino-Infante, J.-L. Experimental Evidence That Polystyrene Nanoplastics Cross the Intestinal Barrier of European Seabass. Environ. Int. 2022, 166, 107340. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Microplastics and Human Health: Integrating Pharmacokinetics. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1489–1511. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, X.-Y.; Chen, B.-J.; Yang, Y.-P.; Li, H.; Wang, F. Impact of Microplastics on the Human Digestive System: From Basic to Clinical. World J. Gastroenterol. 2025, 31, 100470. [Google Scholar] [CrossRef]
- Yakovenko, N.; Pérez-Serrano, L.; Segur, T.; Hagelskjaer, O.; Margenat, H.; Roux, G.L.; Sonke, J.E. Human Exposure to PM10 Microplastics in Indoor Air. PLoS ONE 2025, 20, e0328011. [Google Scholar] [CrossRef]
- Ningrum, P.T.; Keman, S.; Sulistyorini, L.; Sudiana, I.K.; Hidayat, A.; Negoro, A.H.S.; Junaidi, H.; Kustin, K. A Systematic Review of the Effects of Airborne Microplastic Contamination on Human Lungs. Afr. J. Reprod. Health 2024, 28, 430–448. [Google Scholar] [CrossRef]
- Gou, Z.; Wu, H.; Li, S.; Liu, Z.; Zhang, Y. Airborne Micro- and Nanoplastics: Emerging Causes of Respiratory Diseases. Part. Fibre Toxicol. 2024, 21, 50. [Google Scholar] [CrossRef]
- Mousseau, F.; Berret, J.-F. The Role of Surface Charge in the Interaction of Nanoparticles with Model Pulmonary Surfactants. Soft Matter 2018, 14, 5764–5774. [Google Scholar] [CrossRef]
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.-F. Pulmonary Surfactant Inhibition of Nanoparticle Uptake by Alveolar Epithelial Cells. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, J.D.; French, B.W.; Shrestha, U.; Adya, Z.K.; Wooten, R.M.; Fribley, A.M.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium. Antioxidants 2025, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Pat, Y.; Yazici, D.; D’Avino, P.; Li, M.; Ardicli, S.; Ardicli, O.; Mitamura, Y.; Akdis, M.; Dhir, R.; Nadeau, K.; et al. Recent Advances in the Epithelial Barrier Theory. Int. Immunol. 2024, 36, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Pileta-Labañino, M.; Crespo-Barrera, P.; Navarro-Frómeta, A. Human Skin and Micro- and Nanoplastics: A Mini-Review. MOJ Ecol. Environ. Sci. 2024, 9, 122–125. [Google Scholar] [CrossRef]
- Aristizabal, M.; Jiménez-Orrego, K.V.; Caicedo-León, M.D.; Páez-Cárdenas, L.S.; Castellanos-García, I.; Villalba-Moreno, D.L.; Ramírez-Zuluaga, L.V.; Hsu, J.T.S.; Jaller, J.; Gold, M. Microplastics in Dermatology: Potential Effects on Skin Homeostasis. J. Cosmet. Dermatol. 2024, 23, 766–772. [Google Scholar] [CrossRef]
- Hoang, H.G.; Nguyen, N.S.H.; Zhang, T.; Tran, H.-T.; Mukherjee, S.; Naidu, R. A Review of Microplastic Pollution and Human Health Risk Assessment: Current Knowledge and Future Outlook. Front. Environ. Sci. 2025, 13, 1606332. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Penetration of Microplastics and Nanoparticles Through Skin: Effects of Size, Shape, and Surface Chemistry. J. Xenobiotics 2025, 15, 6. [Google Scholar] [CrossRef]
- Martin, L.; Simpson, K.; Brzezinski, M.; Watt, J.; Xu, W. Cellular Response of Keratinocytes to the Entry and Accumulation of Nanoplastic Particles. Part. Fibre Toxicol. 2024, 21, 22. [Google Scholar] [CrossRef]
- Thapliyal, C.; Negi, S.; Nagarkoti, S.; Daverey, A. Mechanistic Insight into Potential Toxic Effects of Microplastics and Nanoplastics on Human Health. Discov. Appl. Sci. 2025, 7, 645. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; Boughanem, H.; Dávalos, A. Untoward Effects of Micro- and Nanoplastics: An Expert Review of Their Biological Impact and Epigenetic Effects. Adv. Nutr. 2022, 13, 1310–1323. [Google Scholar] [CrossRef]
- Jayavel, S.; Govindaraju, B.; Michael, J.R.; Viswanathan, B. Impacts of Micro and Nanoplastics on Human Health. Bull. Natl. Res. Cent. 2024, 48, 110. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Luo, W.-H. Cellular Biodistribution of Polymeric Nanoparticles in the Immune System. J. Control. Release 2016, 227, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wang, X.; Li, D.; Wang, H.; Xu, H.; Liu, Y.; Kang, R.; Chen, Q.; Zheng, L.; et al. Discovery and Analysis of Microplastics in Human Bone Marrow. J. Hazard. Mater. 2024, 477, 135266. [Google Scholar] [CrossRef]
- Deng, X.; Gui, Y.; Zhao, L. The Micro(Nano)Plastics Perspective: Exploring Cancer Development and Therapy. Mol. Cancer 2025, 24, 30. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, W.H.; Choi, K.J.; Koh, B.; Kim, K.Y. A Comparative Systematic Analysis of The Influence of Microplastics on Colon Cells, Mouse and Colon Organoids. Tissue Eng. Regen. Med. 2022, 20, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Li, J.; Yin, K.; Hou, L.; Zhang, Y.; Lu, H.; Ma, C.; Xing, M. Polystyrene Microplastics Mediate Inflammatory Responses in the Chicken Thymus by Nrf2/NF-κB Pathway and Trigger Autophagy and Apoptosis. Environ. Toxicol. Pharmacol. 2023, 100, 104136. [Google Scholar] [CrossRef]
- Han, S.; Bang, J.; Choi, D.; Hwang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. Surface Pattern Analysis of Microplastics and Their Impact on Human-Derived Cells. ACS Appl. Polym. Mater. 2020, 2, 4541–4550. [Google Scholar] [CrossRef]
- Visalli, G.; Laganà, A.; Facciolà, A.; Iaconis, A.; Curcio, J.; Pollino, S.; Celesti, C.; Scalese, S.; Libertino, S.; Iannazzo, D.; et al. Enhancement of Biological Effects of Oxidised Nano- and Microplastics in Human Professional Phagocytes. Environ. Toxicol. Pharmacol. 2023, 99, 104086. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zhang, Z.; Halimu, G.; Li, Y.; Li, Y.; Gu, W.; Zhang, B.; Wang, X. In Vitro Study on the Toxicity of Nanoplastics with Different Charges to Murine Splenic Lymphocytes. J. Hazard. Mater. 2022, 424, 127508. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xuan, Y.; Shen, H.; Tang, Y.; Zhang, T.; Xu, J. Surface Functionalization-Dependent Inflammatory Potential of Polystyrene Nanoplastics through the Activation of MAPK/ NF-κB Signaling Pathways in Macrophage Raw 264.7. Ecotoxicol. Environ. Saf. 2023, 251, 114520. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Boraschi, D. Association between Microorganisms and Microplastics: How Does It Change the Host–Pathogen Interaction and Subsequent Immune Response? Int. J. Mol. Sci. 2023, 24, 4065. [Google Scholar] [CrossRef]
- Stunnenberg, M.; de Roda Husman, A.M. It’s a Matter of Microbes: A Perspective on the Microbiological Aspects of Micro- and Nanoplastics in Human Health. Front. Nanotechnol. 2024, 6, 1368437. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Z.; Su, Z.; Wang, J. Immunotoxicity of Microplastics: Carrying Pathogens and Destroying the Immune System. TrAC Trends Anal. Chem. 2024, 177, 117817. [Google Scholar] [CrossRef]
- Gross, N.; Muhvich, J.; Ching, C.; Gomez, B.; Horvath, E.; Nahum, Y.; Zaman, M.H. Effects of Microplastic Concentration, Composition, and Size on Escherichia coli Biofilm-Associated Antimicrobial Resistance. Appl. Environ. Microbiol. 2025, 91, e02282-24. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Banerjee, G.; De, J.; Dsouza, N.; Sur, S.; Scott, J.W.; Banerjee, P. Nanoplastics-Mediated Physiologic and Genomic Responses in Pathogenic Escherichia coli O157:H7. J. Nanobiotechnol. 2025, 23, 304. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Ngiam, L.; Guo, J. Microplastics as Potential Carriers of Viruses Could Prolong Virus Survival and Infectivity. Water Res. 2022, 225, 119115. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, K.; Wang, Y.; Hou, X.; He, Y.; Wang, Z.; Zhang, J.; Chen, X.; Liu, X. Microplastics and Viruses in the Aquatic Environment: A Mini Review. Front. Microbiol. 2024, 15, 1433724. [Google Scholar] [CrossRef]
- Cai, B.; De Jesus Andino, F.; McGrath, J.L.; Romanick, S.S.; Robert, J. Ingestion of Polyethylene Terephthalate Microplastic Water Contaminants by Xenopus Laevis Tadpoles Negatively Affects Their Resistance to Ranavirus Infection and Antiviral Immunity. Environ. Pollut. 2024, 356, 124340. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Zhou, Q.; Peijnenburg, W.J.G.M.; Luo, Y. Uptake of Microplastics and Their Effects on Plants. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 279–298. ISBN 978-3-030-56271-7. [Google Scholar]
- Mattioda, V.; Benedetti, V.; Tessarolo, C.; Oberto, F.; Favole, A.; Gallo, M.; Martelli, W.; Crescio, M.I.; Berio, E.; Masoero, L.; et al. Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines. Biomolecules 2023, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pei, W.; Li, J.; Feng, Y.; Gao, X.; Jiang, P.; Wu, Q.; Li, L. Microplastics Induced Apoptosis in Macrophages by Promoting ROS Generation and Altering Metabolic Profiles. Ecotoxicol. Environ. Saf. 2024, 271, 115970. [Google Scholar] [CrossRef]
- Alijagic, A.; Hedbrant, A.; Persson, A.; Larsson, M.; Engwall, M.; Särndahl, E. NLRP3 Inflammasome as a Sensor of Micro- and Nanoplastics Immunotoxicity. Front. Immunol. 2023, 14, 1178434. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, Y.; Shi, P.; Ni, Y.; Zeng, H.; Zhang, Z.; Zhao, C.; Sun, W.; Yi, Q. Mitigating Microplastic-Induced Organ Damage: Mechanistic Insights from the Microplastic-Macrophage Axes. Redox Biol. 2025, 84, 103688. [Google Scholar] [CrossRef]
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Hou, L.; Guo, T.; Zhao, H.; Xing, M. Polystyrene Microplastics Promote Liver Inflammation by Inducing the Formation of Macrophages Extracellular Traps. J. Hazard. Mater. 2023, 452, 131236. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Wu, Y.; Zhang, L.; Zhang, B.; Zhou, S.; Zhang, P.; Xu, T.; Wu, M.; Lv, S. Polystyrene Nanoplastics of Different Particle Sizes Regulate the Polarization of Pro-Inflammatory Macrophages. Sci. Rep. 2024, 14, 16329. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Casati, L.; Sauro, G.; Taurino, G.; Griffini, E.; Milani, C.; Ventura, M.; Bussolati, O.; Chiu, M. Biological Effects of Micro-/Nano-Plastics in Macrophages. Nanomaterials 2025, 15, 394. [Google Scholar] [CrossRef]
- Collin-Faure, V.; Vitipon, M.; Torres, A.; Tanyeres, O.; Dalzon, B.; Rabilloud, T. The Internal Dose Makes the Poison: Higher Internalization of Polystyrene Particles Induce Increased Perturbation of Macrophages. Front. Immunol. 2023, 14, 1092743. [Google Scholar] [CrossRef]
- van den Berg, A.E.T.; Adriaans, K.J.; Parker, L.A.; Höppener, E.M.; Dusza, H.M.; Legler, J.; Pieters, R.H.H. Top-down Generated Micro- and Nanoplastics Reduce Macrophage Viability without Eliciting a pro-Inflammatory Response. Microplastics Nanoplastics 2025, 5, 32. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Luo, G.; Yuan, Y.; Hu, D.; Xiao, F. The Crosstalk between M1 Macrophage Polarization and Energy Metabolism Disorder Contributes to Polystyrene Nanoplastics-Triggered Testicular Inflammation. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 180, 114002. [Google Scholar] [CrossRef]
- Skaba, D.; Fiegler-Rudol, J.; Dembicka-Mączka, D.; Wiench, R. Nanoplastics and Immune Disruption: A Systematic Review of Exposure Routes, Mechanisms, and Health Implications. Int. J. Mol. Sci. 2025, 26, 5228. [Google Scholar] [CrossRef]
- van den Berg, A.E.T.; Plantinga, M.; Vethaak, D.; Adriaans, K.J.; Bol-Schoenmakers, M.; Legler, J.; Smit, J.J.; Pieters, R.H.H. Environmentally Weathered Polystyrene Particles Induce Phenotypical and Functional Maturation of Human Monocyte-Derived Dendritic Cells. J. Immunotoxicol. 2022, 19, 125–133. [Google Scholar] [CrossRef]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics Affect the Inflammatory Cytokine Release by Primary Human Monocytes and Dendritic Cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef]
- Lopez, G.L.; Lamarre, A. The Impact of Micro- and Nanoplastics on Immune System Development and Functions: Current Knowledge and Future Directions. Reprod. Toxicol. 2025, 135, 108951. [Google Scholar] [CrossRef]
- Park, K.-M.; Kim, B.; Woo, W.; Kim, L.K.; Hyun, Y.-M. Polystyrene Microplastics Induce Activation and Cell Death of Neutrophils through Strong Adherence and Engulfment. J. Hazard. Mater. 2024, 480, 136100. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, L.; Song, E.; Song, Y. Polystyrene Nanoplastics Induce Neutrophil Extracellular Traps in Mice Neutrophils. Chem. Res. Toxicol. 2022, 35, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Xu, T.; He, Y.; Li, Z.; Tang, X.; Fan, X.; Li, S. Polystyrene Nanoparticle Exposure Supports ROS-NLRP3 Axis-Dependent DNA-NET to Promote Liver Inflammation. J. Hazard. Mater. 2022, 439, 129502. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xiao, Y.; Zhang, X.; Xu, Y.; Zhu, K.; Zhang, K.; Li, X.; Zhou, H.; Chen, G.; Guo, X. Dietary Exposure to Polystyrene Microplastics Exacerbates Liver Damage in Fulminant Hepatic Failure via ROS Production and Neutrophil Extracellular Trap Formation. Sci. Total Environ. 2024, 907, 167403. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Hasegawa, Y.; Okamura, T.; Ono, Y.; Ichikawa, T.; Nakanishi, N.; Tsuchimura, Y.; Morioka, T.; Tanaka, S.; Takano, H.; et al. Negative Impact of Oral Exposure to Polystyrene Microplastics on Glucose Tolerance and Intestinal Environment in Mice Is Independent of Particle Size. Environ. Sci. Eur. 2025, 37, 125. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Serna, J.B. de la The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Woo, J.-H.; Seo, H.J.; Lee, J.-Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene Nanoplastic Exposure Leads to Lung Inflammation through P38-Mediated NF-κB Pathway Due to Mitochondrial Damage. Part. Fibre Toxicol. 2023, 20, 2. [Google Scholar] [CrossRef]
- Trushina, E.N.; Riger, N.A.; Mustafina, O.K.; Timonin, A.N. Effect of microand nanoplastics as food contaminants on the immune system. Vopr. Pitan. 2023, 92, 6–15. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Bai, L.; Cui, J. Microplastics: An Often-Overlooked Issue in the Transition from Chronic Inflammation to Cancer. J. Transl. Med. 2024, 22, 959. [Google Scholar] [CrossRef]
- Shang, Q.; Wu, H.; Wang, K.; Zhang, M.; Dou, Y.; Jiang, X.; Zhao, Y.; Zhao, H.; Chen, Z.-J.; Wang, J.; et al. Exposure to Polystyrene Microplastics during Lactational Period Alters Immune Status in Both Male Mice and Their Offspring. Sci. Total Environ. 2024, 951, 175371. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.-B.; Yoo, J.Y.; Min, H. The Emerging Threat of Micro-and Nanoplastics on the Maturation and Activity of Immune Cells. Biomol. Ther. 2025, 33, 95–105. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.-X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Zwollo, P.; Quddos, F.; Bagdassarian, C.; Seeley, M.E.; Hale, R.C.; Abderhalden, L. Polystyrene Microplastics Reduce Abundance of Developing B Cells in Rainbow Trout (Oncorhynchus Mykiss) Primary Cultures. Fish Shellfish Immunol. 2021, 114, 102–111. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef] [PubMed]
- Slamanig, S.A.; Nolte, M.A. The Bone Marrow as Sanctuary for Plasma Cells and Memory T-Cells: Implications for Adaptive Immunity and Vaccinology. Cells 2021, 10, 1508. [Google Scholar] [CrossRef]
- Koo, J.; Jeong, B.; Yeob Baek, J.; Sik Lee, W.; Gong, J.; Park, S.; Hong, J.; Sim, Y.; Soo Kim, D.; Ryong Kim, S.; et al. Type-Dependent Effects of Nanoplastics on Microglial Activation and CXCR2-Mediated Chemotactic Migration. Nanoscale 2025, 17, 17274–17284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ye, Y.; Han, Y.; Wang, Q.; Lu, H.; Li, J.; Qian, W.; Zeng, X.; Zhang, Z.; Zhao, Y.; et al. Microplastics Dampen the Self-Renewal of Hematopoietic Stem Cells by Disrupting the Gut Microbiota-Hypoxanthine-Wnt Axis. Cell Discov. 2024, 10, 35. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, L.; Han, L.; Wang, J.; Zhang, W.; Liu, Z.; Gao, A. Polystyrene Micro-/Nanoplastics Induced Hematopoietic Damages via the Crosstalk of Gut Microbiota, Metabolites, and Cytokines. Environ. Int. 2022, 161, 107131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, J.; Han, L.; Liu, Z.; Wang, J.; Zhang, W.; Gao, A. Melatonin and Probiotics Ameliorate Nanoplastics-Induced Hematopoietic Injury by Modulating the Gut Microbiota-Metabolism. Nano Res. 2023, 16, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897. [Google Scholar] [CrossRef]
- Yöntem, F.D.; Ahbab, M.A. Mitochondria as a Target of Micro- and Nanoplastic Toxicity. Camb. Prism. Plast. 2024, 2, e6. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, R.; Guo, S.; Li, S.; Huang, Z.; Wang, Y.; Yu, C.; Hou, Z.; Zhang, Y.; Zhang, Y.; et al. Large-Sized Polystyrene Microplastics Induce Oxidative Stress in AML12 Cells. Sci. Rep. 2025, 15, 26616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Zhang, J.; Feng, G.; Miao, S.; Lu, R.; Tian, X.; Ye, Y. Antioxidant Intervention Against Microplastic Hazards. Antioxidants 2025, 14, 797. [Google Scholar] [CrossRef]
- Pei, J.; Chen, S.; Li, L.; Wang, K.; Pang, A.; Niu, M.; Peng, X.; Li, N.; Wu, H.; Nie, P. Impact of Polystyrene Nanoplastics on Apoptosis and Inflammation in Zebrafish Larvae: Insights from Reactive Oxygen Species Perspective. Sci. Total Environ. 2024, 948, 174737. [Google Scholar] [CrossRef]
- Sivakumar, R.; Senghor Kadalangudi Aravaanan, A.; Vellore Mohanakrishnan, V.; Kumar, J. From Environment to Endothelium: The Role of Microplastics in Vascular Aging. Microplastics 2025, 4, 52. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene Nanoplastic Exposure Induces Excessive Mitophagy by Activating AMPK/ULK1 Pathway in Differentiated SH-SY5Y Cells and Dopaminergic Neurons in Vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, X.; Wang, X.; Qi, Y.; Hu, S.; Liu, R. Polystyrene Nanoplastics Elicit Multiple Responses in Immune Cells of the Eisenia Fetida (Savigny, 1826). Toxics 2025, 13, 18. [Google Scholar] [CrossRef]
- Krause, S.; Ouellet, V.; Allen, D.; Allen, S.; Moss, K.; Nel, H.A.; Manaseki-Holland, S.; Lynch, I. The Potential of Micro- and Nanoplastics to Exacerbate the Health Impacts and Global Burden of Non-Communicable Diseases. Cell Rep. Med. 2024, 5, 101581. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Liu, S.; Wang, Z.; Li, F.; Bu, Q.; An, X. Polystyrene Microplastics Induce Potential Toxicity through the Gut-Mammary Axis. npj Sci. Food 2025, 9, 139. [Google Scholar] [CrossRef]
- Lu, H.; Guo, T.; Zhang, Y.; Liu, D.; Hou, L.; Ma, C.; Xing, M. Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome Activation as a Novel Mechanism of Polystyrene Microplastics (PS-MPs)-Induced Pulmonary Inflammation in Chickens. J. Zhejiang Univ. Sci. B 2024, 25, 233–243. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Waag, F.; Rahimi, K.; Ramachandran, H.; Bessel, T.; Barcikowski, S.; Herrmann, A.; Rossi, A.; Schins, R.P.F. Assessing the NLRP3 Inflammasome Activating Potential of a Large Panel of Micro- and Nanoplastics in THP-1 Cells. Biomolecules 2022, 12, 1095. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Wang, F.; Geng, Y.; Xu, T.; Zhu, M.; Lv, H.; Xu, S.; Guo, M. Polyethylene Microplastics Trigger Cell Apoptosis and Inflammation via Inducing Oxidative Stress and Activation of the NLRP3 Inflammasome in Carp Gills. Fish Shellfish Immunol. 2023, 132, 108470. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, X.; Chen, W.; Wang, M.; Zhao, J.; Xu, Z.; Wang, R.; Mi, C.; Zheng, Z.; Zhang, H. Exposure to High Dose of Polystyrene Nanoplastics Causes Trophoblast Cell Apoptosis and Induces Miscarriage. Part. Fibre Toxicol. 2024, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, D.Y.; Jeong, T.S.; Park, Y.S. Micro- and Nano-Plastic-Induced Adverse Health Effects on Lungs and Kidneys Linked to Oxidative Stress and Inflammation. Life 2025, 15, 392. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Fu, R.; Zhang, P.; Chen, H.; Cao, H.; Jiang, Z.; Hong, Y.; Li, Y.; He, C.; et al. Molecular Mechanism Differences between Nanoplastics and Microplastics in Colon Toxicity: Nanoplastics Induce Ferroptosis-Mediated Immunogenic Cell Death, While Microplastics Cause Cell Metabolic Reprogramming. J. Nanobiotechnol. 2025, 23, 505. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, M.; Xiong, F.; Xu, K.; Huang, J.; Liu, J.; Wang, D.; Pu, Y. Polystyrene Micro- and Nanoplastics Induce Gastric Toxicity through ROS Mediated Oxidative Stress and P62/Keap1/Nrf2 Pathway. Sci. Total Environ. 2024, 912, 169228. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Xiong, Y. Chronic Polystyrene Microplastic Exposure Reduces Testosterone Levels in Mice through Mitochondrial Oxidative Stress and BAX/BCL2-Mediated Apoptosis. Toxics 2024, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Luzio, A.; Félix, L.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Microplastics and Copper Induce Apoptosis, Alter Neurocircuits, and Cause Behavioral Changes in Zebrafish (Danio Rerio) Brain. Ecotoxicol. Environ. Saf. 2022, 242, 113926. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Shi, X.; Wang, Y.; Xu, S. Polystyrene Microplastics with Different Sizes Induce the Apoptosis and Necroptosis in Liver through the PTEN/PI3K/AKT/Autophagy Axis. Sci. Total Environ. 2023, 899, 165461. [Google Scholar] [CrossRef] [PubMed]
- Annangi, B.; Villacorta, A.; López-Mesas, M.; Fuentes-Cebrian, V.; Marcos, R.; Hernández, A. Hazard Assessment of Polystyrene Nanoplastics in Primary Human Nasal Epithelial Cells, Focusing on the Autophagic Effects. Biomolecules 2023, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, J.; Sun, J.; Zhu, Y.; Li, X.; Liu, X.; Zhang, X.; Liu, L.; Li, L.; Yang, J.; et al. Dust Fall Microplastics from a Megacity of China Inhibit Autophagy via the PI3K/Akt/mTOR Pathway. Environ. Health 2025, 3, 469–481. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alqahtani, S.; Saquib, Q.; Mohiddin, F. Toxicological Impact of Microplastics and Nanoplastics on Humans: Understanding the Mechanistic Aspect of the Interaction. Front. Toxicol. 2023, 5, 1193386. [Google Scholar] [CrossRef]
- Florance, I.; Cordani, M.; Pashootan, P.; Moosavi, M.A.; Zarrabi, A.; Chandrasekaran, N. The Impact of Nanomaterials on Autophagy across Health and Disease Conditions. Cell. Mol. Life Sci. 2024, 81, 184. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Chen, T.; Yu, F.; Lin, Q.; Zhao, H.; Jin, L.; Peng, R. Research Progress on Micro(Nano)Plastic-Induced Programmed Cell Death Associated with Disease Risks. Toxics 2024, 12, 493. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, Y.; Zhang, P.; Zhu, B.; Feng, J.; Deng, T.; Fu, Z.; Liu, C.; Chen, C.; Zhang, Y. Polystyrene Nanoplastics Trigger Pyroptosis in Dopaminergic Neurons through TSC2/TFEB-Mediated Disruption of Autophagosome-Lysosome Fusion in Parkinson’s Disease. J. Transl. Med. 2025, 23, 631. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Vanetti, C.; Broggiato, M.; Pezzana, S.; Clerici, M.; Fenizia, C. Effects of Microplastics on the Immune System: How Much Should We Worry? Immunol. Lett. 2025, 272, 106976. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Tang, Z. Microplastics Aggravates Rheumatoid Arthritis by Affecting the Proliferation/Migration/Inflammation of Fibroblast-like Synovial Cells by Regulating Mitochondrial Homeostasis. Int. Immunopharmacol. 2023, 120, 110268. [Google Scholar] [CrossRef]
- Zeyneloglu, C.; Babayev, H.; Ogulur, I.; Ardicli, S.; Pat, Y.; Yazici, D.; Zhao, B.; Chang, L.; Liu, X.; D’Avino, P.; et al. The Epithelial Barrier Theory Proposes a Comprehensive Explanation for the Origins of Allergic and Other Chronic Noncommunicable Diseases. FEBS Lett. 2025. [Google Scholar] [CrossRef]
- Rawle, D.J.; Dumenil, T.; Tang, B.; Bishop, C.R.; Yan, K.; Le, T.T.; Suhrbier, A. Microplastic Consumption Induces Inflammatory Signatures in the Colon and Prolongs a Viral Arthritis. Sci. Total Environ. 2022, 809, 152212. [Google Scholar] [CrossRef]
- Chen, H.; Wan, L.; Qiu, Y.; Qiu, F.; Wen, C.; Mao, Y.; He, Z. Microplastics Exposure Induced and Exacerbated the Development of Systemic Lupus Erythematosus in Mice. Sci. Total Environ. 2024, 909, 168586. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, N.; Dzhalilova, D.; Tsvetkov, I.; Makarova, O. Influence of Microplastics on Morphological Manifestations of Experimental Acute Colitis. Toxics 2023, 11, 730. [Google Scholar] [CrossRef]
- Chen, Y.; Williams, A.M.; Gordon, E.B.; Rudolph, S.E.; Longo, B.N.; Li, G.; Kaplan, D.L. Biological Effects of Polystyrene Micro- and Nano-Plastics on Human Intestinal Organoid-Derived Epithelial Tissue Models without and with M Cells. Nanomedicine Nanotechnol. Biol. Med. 2023, 50, 102680. [Google Scholar] [CrossRef]
- Kumar, N.; Pachar, A.K.; Singh, J.K.; Mandal, M.; Lamba, M.; Yadav, S.; Sharda, N.; Acharya, A. Investigating the Hypothesis Role of the Hidden Poison Microplastics in Lymphoma Development. Discov. Environ. 2025, 3, 73. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Xu, S.; Cui, J.; Li, K.; Shiwen, X.; Guo, M.-Y. Polystyrene Microplastics Induce Myocardial Inflammation and Cell Death via the TLR4/NF-κB Pathway in Carp. Fish Shellfish Immunol. 2023, 135, 108690. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Visalli, G.; Facciolà, A.; Saija, C.; Bertuccio, M.P.; Baluce, B.; Celesti, C.; Iannazzo, D.; Di Pietro, A. Sterile Inflammation Induced by Respirable Micro and Nano Polystyrene Particles in the Pathogenesis of Pulmonary Diseases. Toxicol. Res. 2024, 13, tfae138. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene Microplastics Exacerbate Experimental Colitis in Mice Tightly Associated with the Occurrence of Hepatic Inflammation. Sci. Total Environ. 2022, 844, 156884. [Google Scholar] [CrossRef] [PubMed]
- Andonotopo, W.; Bachnas, M.A.; Dewantiningrum, J.; Pramono, M.B.A.; Sanjaya, I.N.H.; Stanojevic, M.; Kurjak, A. Microplastics in the Perinatal Period: Emerging Evidence on Maternal Exposure, Placental Transfer, and Fetal Health Outcomes. Sarvodaya Int. J. Med. 2025, 1, 82. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The Potential Impact of Nano- and Microplastics on Human Health: Understanding Human Health Risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef]
- Seewoo, B.J.; Goodes, L.M.; Mofflin, L.; Mulders, Y.R.; Wong, E.V.; Toshniwal, P.; Brunner, M.; Alex, J.; Johnston, B.; Elagali, A.; et al. The Plastic Health Map: A Systematic Evidence Map of Human Health Studies on Plastic-Associated Chemicals. Environ. Int. 2023, 181, 108225. [Google Scholar] [CrossRef]
- Valamontes, A. Impact of Microplastics on Global Public Health: A Systematic Review and Meta-Analysis. 2025. Available online: https://www.preprints.org/manuscript/202502.1675 (accessed on 26 October 2025).
- Weis, J.S.; Palmquist, K.H. Reality Check: Experimental Studies on Microplastics Lack Realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choi, K.-H. The Current Status of Studies of Human Exposure Assessment of Microplastics and Their Health Effects: A Rapid Systematic Review. Environ. Anal. Health Toxicol. 2021, 36, e2021004. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Microplastics Research. Available online: https://www.epa.gov/water-research/microplastics-research (accessed on 14 September 2025).
- Prata, J.C.; Padrão, J.; Khan, M.T.; Walker, T.R. Do’s and Don’ts of Microplastic Research: A Comprehensive Guide. Water Emerg. Contam. Nanoplastics 2024, 3, 8. [Google Scholar] [CrossRef]
- Boettcher, H.; Kukulka, T.; Cohen, J.H. Methods for Controlled Preparation and Dosing of Microplastic Fragments in Bioassays. Sci. Rep. 2023, 13, 5195. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Hsueh, Y.; Zhang, C.; Yu, J.; Zhu, J.; Niu, J.; Yin, N.; Zhang, J.; Cui, X.; et al. Interactions between Environmental Pollutants and Gut Microbiota: A Review Connecting the Conventional Heavy Metals and the Emerging Microplastics. Environ. Res. 2025, 269, 120928. [Google Scholar] [CrossRef]
- Demarquoy, J. Microplastics and Microbiota: Unraveling the Hidden Environmental Challenge. World J. Gastroenterol. 2024, 30, 2191–2194. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET Microplastics Affect Human Gut Microbiota Communities during Simulated Gastrointestinal Digestion, First Evidence of Plausible Polymer Biodegradation during Human Digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.C.; Soggiu, A.; Urbani, A.; Roncada, P. Interactions between Microplastics and Microbiota in a One Health Perspective. One Health 2025, 20, 101002. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in Wastewater: State of the Knowledge on Sources, Fate and Solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 201–227. ISBN 978-3-319-16510-3. [Google Scholar]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental Occurrences, Fate, and Impacts of Microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Bucci, K.; Tulio, M.; Rochman, C.M. What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-Analysis and Systematic Review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Merly, L.; Smith, S.L. Murine RAW 264.7 Cell Line as an Immune Target: Are We Missing Something? Immunopharmacol. Immunotoxicol. 2017, 39, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Du, Y.; Yang, B.; Tian, W.; Li, J.; Xie, J. Comprehensive Review of Macrophage Models: Primary Cells and Immortalized Lines across Species. Front. Immunol. 2025, 16, 1640935. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Ha, Y. Micro- and Nanoplastics and the Immune System: Mechanistic Insights and Future Directions. Immuno 2025, 5, 52. https://doi.org/10.3390/immuno5040052
Fan J, Ha Y. Micro- and Nanoplastics and the Immune System: Mechanistic Insights and Future Directions. Immuno. 2025; 5(4):52. https://doi.org/10.3390/immuno5040052
Chicago/Turabian StyleFan, Jeffrey, and Yang Ha. 2025. "Micro- and Nanoplastics and the Immune System: Mechanistic Insights and Future Directions" Immuno 5, no. 4: 52. https://doi.org/10.3390/immuno5040052
APA StyleFan, J., & Ha, Y. (2025). Micro- and Nanoplastics and the Immune System: Mechanistic Insights and Future Directions. Immuno, 5(4), 52. https://doi.org/10.3390/immuno5040052




