1. Introduction
The sympathetic nervous system (SNS) is part of the autonomic nervous system and plays a central role in the body’s stress response. It regulates physiological processes by releasing catecholamines, such as adrenaline and noradrenaline, which modulate cardiovascular function, the metabolism, and the immune system [
1]. In recent years, it has become increasingly clear that the SNS plays a crucial role in modulating inflammatory processes. Under normal conditions, the SNS helps maintain homeostasis, but in cases of stress exposure, its prolonged activation can lead to immune dysregulation [
2].
Inflammatory processes in periodontal tissues are associated with the increased release of pro-inflammatory cytokines; simultaneously, sympathetic activation stimulates the release of hormones that should limit this inflammation. However, in patients with chronic stress or high SNS activity, this regulation becomes disrupted, resulting in increased inflammation and periodontal tissue destruction [
3].
In patients with periodontitis, increased sympathetic activity enhances the inflammatory response to periodontal pathogens, demonstrated by elevated levels of pro-inflammatory cytokines and neutrophil activation and further accelerating periodontal tissue damage [
4]. Persistent SNS activation might also suppress anti-inflammatory mechanisms, hindering tissue repair and sustaining chronic inflammation.
Stress conditions lead to prolonged SNS activation, which is measurable through various biomarkers in saliva. Chromogranin A (CgA) and alpha-amylase (sAA) are among the most commonly utilized markers for SNS activity under stress conditions, reflecting the body’s physiological response [
5,
6,
7,
8].
Chromogranin A is a protein released from sympathetic nerve endings upon SNS activation. Studies indicate significantly elevated salivary CgA levels in patients with periodontitis, particularly those experiencing stress [
9,
10,
11].
Alpha-amylase, another marker of sympathetic activity, is an enzyme produced by the salivary glands. Elevated salivary alpha-amylase levels correlate with heightened SNS activity and stress [
7,
8]. Recent studies have confirmed higher sAA levels in patients with periodontitis compared with healthy individuals, indicating its potential as a biomarker for periodontitis severity [
12,
13].
Studies suggest significantly higher salivary levels of CgA and sAA in periodontitis patients than in healthy controls, supporting a connection between inflammatory-destructive periodontal changes and SNS activity [
9,
10,
11,
12,
13]. However, the literature remains limited regarding SNS activity marker levels across varying degrees of periodontitis severity and their relationship with host inflammatory response.
We hypothesize that patients with more severe periodontitis would exhibit elevated levels of salivary stress-related (CgA, sAA) and inflammatory (IL-1β, IL-6) biomarkers, reflecting increased sympathetic activity and immune dysregulation.
The aim of our study is to biochemically characterize SNS activity through salivary biomarkers (CgA and sAA) and objectively assess inflammation by measuring salivary concentrations of pro-inflammatory cytokines interleukin-1β (IL-1β) and interleukin-6 (IL-6) in patients with different stages of periodontitis.
2. Materials and Methods
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and approved by the Research Ethics Committee of Medical University—Sofia (KENIMUS), under protocol No. 06/01.06.2023. All participants were thoroughly informed about the study objectives and procedures and provided written informed consent prior to their inclusion.
This study included 67 adult patients diagnosed with periodontitis, who sought consultation and treatment at the Department of Periodontology, Faculty of Dental Medicine, Medical University—Sofia, between 2022 and 2023. The study population consisted of 35 females and 32 males, with a mean age of 51.4 years (range: 26–72 years).
This study enrolled patients who met the following inclusion criteria:
Interdental CAL is detectable at ≥2 non-adjacent teeth, or
Buccal/oral CAL ≥ 3 mm with probing pocket depth ≥3 mm is detectable at ≥2 teeth, but the observed CAL cannot be attributed to non-periodontitis-related causes (e.g., gingival recession, dental caries, endodontic lesion, etc.),
Absence of systemic diseases or medications known to influence periodontal status, inflammatory response, or evaluated salivary biomarkers.
The participants were classified into two groups based on disease severity, following the criteria of the 2017 Classification of Periodontal and Peri-Implant Diseases and Conditions [
14]:
- ➢
Group 1 (Stage I/II Periodontitis):
Interdental clinical attachment loss (CAL) of 1–4 mm;
Radiographic bone loss limited to the coronal third (<15% for Stage I; 15–33% for Stage II);
Maximum probing depth ≤5 mm;
Mostly horizontal bone loss;
No tooth loss due to periodontitis.
- ➢
Group 2 (Stage III/IV Periodontitis):
Interdental CAL ≥ 5 mm;
Radiographic bone loss extending to the middle or apical third of the root;
Probing depth ≥ 6 mm;
Vertical bone loss ≥ 3 mm;
Furcation involvement Class II or III;
Tooth mobility Grade II or higher;
At least one tooth lost due to periodontitis (Stage III) or masticatory dysfunction, bite collapse, or less than 20 remaining teeth (Stage IV).
Exclusion criteria:
Pregnancy or breastfeeding;
Diagnosed diabetes or autoimmune diseases influencing periodontal pathogenesis;
Use of hormonal contraceptives or medications affecting the menstrual cycle or menopause;
Recent (within 6 months) use of antimicrobial, anti-inflammatory or immunomodulatory medications;
Corticosteroid therapy in the past 6 months;
Periodontal treatment within the past year.
All clinical periodontal examinations were performed by two trained periodontists. Before the study commenced, examiners underwent a calibration process to ensure consistent and reliable clinical measurements. This was conducted on a separate group of 10 patients not involved in this study. Each examiner measured the probing pocket depth (PPD) and clinical attachment level (CAL) twice, and the intra- and inter-examiner agreements were assessed by determining the percentage of repeated measurements falling within ±1 mm. A reproducibility rate of ≥90% for both PPD and CAL was considered acceptable and was achieved by both examiners. Additionally, inter-examiner reliability was quantified using the intraclass correlation coefficient (ICC), which yielded a value of 0.87, indicating high agreement. Periodontal and oral hygiene assessments were carried out using a UNC-15 periodontal probe (Hu-Friedy®, Chicago, IL, USA).
Full Mouth Plaque Score (FMPS)/Full Mouth Bleeding Score (FMBS): Presence or absence of plaque and bleeding was recorded at four sites per tooth.
Probing Pocket Depth (PPD): Measured as the distance from the gingival margin to the base of the pocket in millimeters.
Clinical Attachment Level (CAL): Measured from the cementoenamel junction to the base of the pocket.
Bleeding on Probing (BoP): Bleeding during probing was recorded.
Furcation Involvement (F): Evaluated using a Nabers probe and classified according to Hamp’s classification (F1, F2, F3).
Tooth Mobility (M): Assessed using the Miller Index.
Periapical radiographs were used to assess the severity and morphology of alveolar bone loss for the staging and grading of periodontitis. The bone loss/age (BL/Age) ratio was calculated using the site with the most severe bone loss.
To minimize diurnal variation—a particularly relevant matter for salivary alpha-amylase, which is known to increase significantly in the late afternoon—unstimulated whole saliva samples were collected between 9:00 and 11:00 a.m. under resting conditions. Participants were instructed to abstain from eating, drinking, or performing oral hygiene procedures for at least one hour prior to collection. After rinsing with water and resting for 10 min, the saliva was gathered over a 5-min period using the passive drool method, yielding approximately 2 mL per participant. The collected samples were immediately frozen and stored at −80 °C until further analysis.
Before analysis, saliva samples were thawed and centrifuged at 3000 rpm for 15 min to remove cellular debris. The supernatant was used for analysis. Depending on the biomarker, the samples were diluted with an assay buffer as recommended by the kit.
The salivary concentrations of alpha-amylase, chromogranin A, interleukin-1β, and interleukin-6 were quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kits, following the protocols provided by the manufacturers. Alpha-amylase was measured using the kinetic enzyme assay kit (Salimetrics®, State College, PA, USA; Cat. No. 1-1902). CgA was assessed by ELISA (Yanaihara Institute Inc., Shizuoka, Japan; Cat. No. YK070). IL-1β and IL-6 were quantified using sandwich ELISA kits (BioVendor®, Brno, Czech Republic; Cat. Nos. RD194559200R and RD194015200R, respectively).
All samples were analyzed in duplicate and the mean values were used for statistical analysis. Standard curves were generated using 4-parameter logistic regression. Results with intra-assay CVs >10% were excluded.
All statistical analyses were performed using SPSS v.21 (IBM, Armonk, NY, USA). Data distribution was assessed using the Shapiro–Wilk test for normality. Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for non-normally distributed variables. Categorical data were summarized as frequencies and percentages. Comparisons between the two groups (Stage I/II vs. Stage III/IV periodontitis) were made using the Mann–Whitney U test for non-normally distributed variables. The Chi-square test was used for categorical variables. The correlations between the salivary biomarker levels and clinical parameters were assessed using Spearman’s rank correlation coefficient (ρ). A p-value < 0.05 was considered statistically significant.
To evaluate the adequacy of the sample size, a post hoc power analysis was conducted using G*Power software (version 3.1.9.7) and confirmed in R (package version 4.4.2). The observed effect sizes and sample sizes were above 0.80 for the primary comparisons, indicating sufficient statistical sensitivity.
4. Discussion
The findings of our study support the initial hypothesis that patients with more severe forms of periodontitis exhibit heightened SNS activity and amplified inflammatory responses. Notably, salivary levels of chromogranin A and interleukin-1β were significantly elevated in Stage III/IV patients compared with those with milder diseases. These biomarkers represent two complementary pathways—neuroendocrine stress signaling and immune-mediated tissue breakdown—that may act synergistically to accelerate periodontal destruction. The increased CgA levels reflect sustained SNS activation, while elevated IL-1β reinforces the role of innate immunity in advanced periodontal pathology. Together, these results support the concept that neuroimmune interactions play a critical role in periodontitis progression.
One of the challenges in studying salivary concentrations of stress-related markers are the observed differences due to circadian rhythm. Chromogranin A has a valuable advantage, as its saliva concentrations remain stable throughout the day and are not influenced by the timing of sample collection [
15]. Our results are consistent with previous studies that demonstrated higher chromogranin concentrations in patients with periodontitis [
10,
11,
16,
17]. Develioglu et al., in their study of salivary chromogranin, did not find statistically significant differences between groups of patients with varying periodontitis severity [
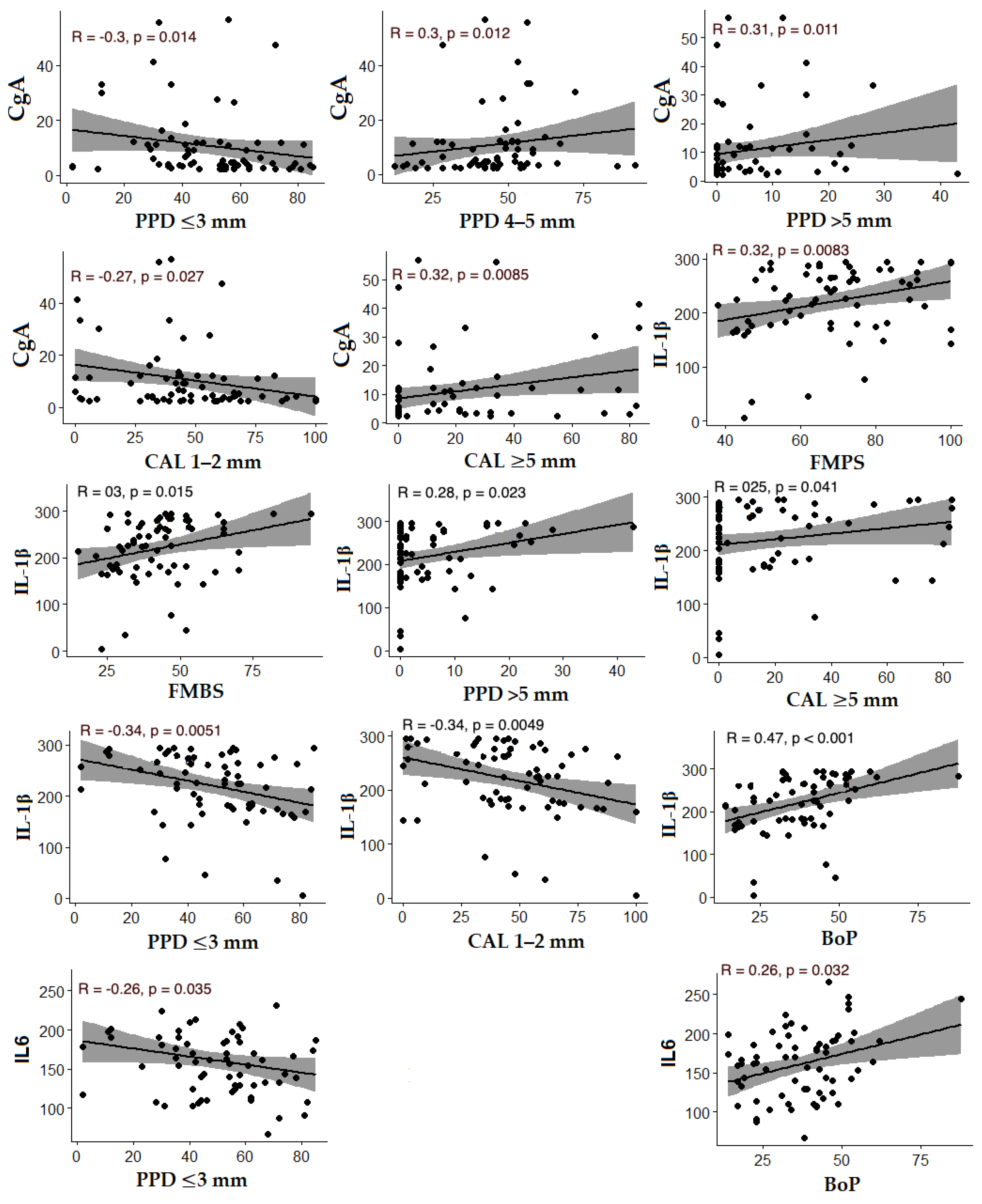
18]. The statistically significant positive correlations we found between chromogranin A levels and the percentage of sites with PPD > 5 mm and CAL ≥ 5 mm, along with negative correlations between chromogranin levels and PPD ≤ 3 mm and CAL = 1–2 mm, indicate increased concentrations of this neuroendocrine marker in patients with deeper pockets and more severe periodontal destruction.
Chromogranin A is a glycoprotein released by the adrenal medulla and sympathetic nerve endings during the activation of the sympathetic nervous system. Its peptide derivatives have demonstrated immunomodulatory properties [
19,
20]. The elevated levels of chromogranin A observed in patients with Stage III/IV periodontitis may be linked to neuroendocrine responses that stimulate immune system activity. Persistent psychological stress can lead to increased chromogranin A secretion, which may activate polymorphonuclear cells, promote cellular degranulation, and enhance the production of pro-inflammatory cytokines such as interleukin-1β and interleukin-6. This activation of the sympathetic nervous system under stress conditions contributes to neuro-immune interactions that drive cytokine release and shift the Th1/Th2 balance toward Th2 dominance—an immune profile associated with more severe periodontal tissue destruction [
21]. Additionally, local chromogranin A release from polymorphonuclear cells and Merkel cells may contribute to increases in clinical attachment loss (CAL) and probing pocket depth (PPD) [
17].
It should be noted that the link between stress and periodontitis is likely bidirectional. While elevated CgA levels may reflect stress-related sympathetic activation, persistent periodontal inflammation could itself lead to neuroendocrine response. The relationship between elevated chromogranin A levels and periodontal inflammation is further supported by findings from Lihala et al., who reported decreased levels of this neuroendocrine marker following non-surgical periodontal therapy [
17]. Further longitudinal studies are needed to clarify the directionality of this association.
In a study by Kogawa et al., significantly higher levels of salivary chromogranin A were observed in patients with type 2 diabetes who had PPD and CAL values of 5–6 mm [
22]. The authors suggested that this elevation was due to chromogranin A being produced by endocrine cells in the striated ducts of the salivary glands [
23]. Their findings point to a link between elevated chromogranin A levels and increased periodontal severity in diabetic patients, which is potentially driven by neuroendocrine changes from SNS activation under stress and immune-inflammatory processes that may also impact glucose metabolism [
22].
According to Breivik et al., the immune response depends on the degree of reaction of the stress system, which determines whether periodontal tissues remain healthy or develop periodontal disease [
24]. The balance between Th1/Th2 and Th17/Treg cells, which dictates the outcome of an immune reaction, is controlled by cytokines produced by innate immune cells [
25]. This balance can be disrupted by external environmental factors, including emotional stress [
24].
Cytokines are proteins that play a key role in the communication between the nervous, immune, and endocrine systems. Interleukin-1β and interleukin-6 are among the most studied cytokines in patients with periodontal diseases in relation to chronic stress [
26]. Barrett et al. found that high levels of stress lead to the increased production of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and TNF-α, by myeloid cells [
27].
Alpha-amylase and chromogranin A, which we studied, are indicative of SNS activity, which actively interacts with the immune system. However, they cannot be directly considered markers of periodontal inflammation, which is why we used pro-inflammatory cytokines IL-1β and IL-6 to biochemically characterize the inflammatory response in periodontitis, in addition to clinical periodontal parameters.
IL-1β is a powerful inflammatory mediator with a broad spectrum of activity. It has an immunomodulatory role, stimulating T-cell differentiation and B-cell proliferation [
28]. There are contradictory findings in the literature regarding the influence of stress on IL-1β concentrations [
29]. Some studies have found increased levels of IL-1β in relation to stress [
30,
31], while others have not found significant differences [
32,
33].
In our study, we investigated IL-1β levels in saliva among patients with varying severity of periodontitis and found significantly higher levels in the Stage III/IV group compared with the Stage I/II group (
p = 0.03). This finding underscores the pivotal role of IL-1β in the inflammatory processes that drive periodontal destruction. Our results are consistent with previous studies that reported elevated IL-1β concentrations in periodontitis patients compared with healthy controls, as well as in those with more severe forms of the disease [
34,
35,
36,
37]. In relation to periodontitis grade, we observed higher IL-1β levels in patients with Grade C compared with Grade B, though the difference was not statistically significant (
p = 0.06). This suggests that IL-1β levels in saliva are more strongly associated with the severity of the disease rather than the risk of progression, which contrasts with the findings of Reddahi et al., who identified significantly higher cytokine levels in Grade C patients [
35]. Further supporting the link between increased periodontitis severity and IL-1β levels, we found positive correlations between IL-1β and clinical periodontal parameters indicative of more advanced destruction, such as PPD > 5 mm, CAL ≥ 5 mm, BoP, and BL/Age. These findings highlight the relevance of IL-1β as a potential marker of periodontal disease severity in our study population.
The role of IL-6 in the pathogenesis of periodontal diseases is well established, with its involvement in osteoclast differentiation and the stimulation of bone resorption [
38]. Numerous studies have reported significantly higher IL-6 concentrations in patients with periodontitis compared with healthy controls [
35,
37,
39]. In our study, however, we did not observe statistically significant differences in IL-6 levels between patients with Stage III/IV and Stage I/II periodontitis (
p = 0.24). Similarly, while patients with Grade C periodontitis exhibited higher IL-6 levels compared with those with Grade B, the difference was not statistically significant (
p = 0.25), which aligns with findings from other studies [
35,
37]. These results suggest that while IL-6 may play a crucial role in periodontal inflammation, its levels in our study were not significantly associated with disease severity or progression, highlighting the complexity of its involvement in periodontal pathogenesis.
In terms of alpha-amylase levels, our study did not reveal statistically significant differences between patients with Stage III/IV periodontitis and those with Stage I/II. These findings are consistent with Develioglu et al., who also reported no significant differences among patients with varying disease severity [
18]. Similarly, Parlak et al. observed slightly elevated alpha-amylase levels in individuals with periodontitis and gingivitis compared with healthy controls, though without statistical significance [
40]. While these findings are consistent with existing research, our study offers additional insight by evaluating alpha-amylase levels in a mixed group of smokers and non-smokers, with an average enzyme level of 111.33 UI/mL—lower than the significantly elevated levels reported in smokers with periodontitis in a previous study (177.96 ± 14.5 vs. 94.04 ± 19.6 UI/mL) [
41]. This difference may be influenced by our standardized morning saliva collection protocol, as alpha-amylase activity is known to rise in the late afternoon [
15]. Furthermore, our findings align with studies showing a significant reduction in alpha-amylase levels following periodontal treatment, such as scaling and root planing [
42].
Our study has several strengths. First, it employed standardized clinical protocols carried out by calibrated periodontists with high intra- and inter-examiner agreement. Second, periodontitis was diagnosed and classified according to the 2017 World Workshop criteria [
14], using clearly defined, internationally accepted diagnostic thresholds, which enhances the reproducibility and clinical relevance of the findings. Third, the study uniquely combined neuroendocrine (CgA, sAA) and inflammatory (IL-1β, IL-6) salivary biomarkers to explore the biological interplay between stress and periodontal inflammation. However, certain limitations must be acknowledged. The cross-sectional design limits causal inference. The absence of a healthy control group restricts the ability to compare findings across the full health–disease spectrum. Additionally, the single-center setting and modest sample size may limit generalizability. Future longitudinal and multi-center studies are warranted to validate these findings and explore causal pathways.