Complete Blood Count-Derived Biomarkers’ Association with Risk of PD-1 or PD-1/CTLA-4 Inhibitor-Induced Hypothyroidism in Patients with Solid Tumors
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Treatment and Data Collection
2.3. Study Assessments
2.4. Statistical Analysis
3. Results
- Multivariable Analysis
- A Power analysis
- Prognostic Evaluation of Hematological Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the definition of cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Shalit, A.; Sarantis, P.; Koustas, E.; Trifylli, E.M.; Matthaios, D.; Karamouzis, M.V. Predictive Biomarkers for Immune-Related Endocrinopathies following Immune Checkpoint Inhibitors Treatment. Cancers 2023, 15, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, L.; Xu, Y.; Wang, X.; Cheng, X.; Zhang, Y.; Wang, Y.; Fan, X.; Zhao, H.; Liu, H.; Chai, X.; et al. Thyroid dysfunction after immune checkpoint inhibitor treatment in a single-center Chinese cohort: A retrospective study. Endocrine 2023, 81, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, W.; Du, Y.; Hu, W.; Zhao, J. Biomarkers and risk factors for the early prediction of immune-related adverse events: A review. Hum. Vaccines Immunother. 2022, 18, 2018894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maekura, T.; Naito, M.; Tahara, M.; Ikegami, N.; Kimura, Y.; Sonobe, S.; Kobayashi, T.; Tsuji, T.; Minomo, S.; Tamiya, A.; et al. Predictive factors of nivolumab-induced hypothyroidism in patients with non-small cell lung cancer. Vivo 2017, 31, 1035–1039. [Google Scholar] [CrossRef]
- Goyal, I.; Pandey, M.R.; Sharma, R.; Chaudhuri, A.; Dandona, P. The side effects of immune checkpoint inhibitor therapy on the endocrine system. Indian J. Med. Res. 2021, 154, 559–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Liu, Y.; Ma, F.; Sun, B.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Peripheral Blood Markers Associated with Immune-Related Adverse Effects in Patients Who Had Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Manag. Res. 2021, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Seo, I.-H.; Lee, Y.-J. Usefulness of Complete Blood Count (CBC) to Assess Cardiovascular and Metabolic Diseases in Clinical Settings: A Comprehensive Literature Review. Biomedicines 2022, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, Z.; Fu, J.; Yi, X. Blood cell counts can predict adverse events of immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1117447. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, A.; Gundogan Bozdag, P. Evaluation of systemic inflammation markers in patients with Hashimoto’s thyroiditis. J. Int. Med. Res. 2024, 52, 9. [Google Scholar] [CrossRef]
- CLuongo, R.; Morra, C.; Gambale, T.; Porcelli, F.; Sessa, E.; Matano, V.; Damiano, M.; Klain, M.; Schlumberger, D. Salvatore Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J. Endocrinol. Investig. 2021, 44, 1927–1933. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I. Management of Immune-Related Adverse Events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2025; Available online: http://www.posit.co/ (accessed on 2 April 2025).
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cut-points obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, Z.; Fei, Q.; Hu, X.; Ouyang, Y.; Yi, X.; Xie, S.; Wang, L.; Huang, X.; He, Y.; et al. Treatment-related adverse events, immune-related adverse events and discontinuation in patients with solid tumors adding adjuvant immune checkpoint blockade: A meta-analysis of 38 randomized controlled trials. Int. J. Surg. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Klein-Brill, A.; Amar-Farkash, S.; Rosenberg-Katz, K.; Brenner, R.; Becker, J.C.; Aran, D. Comparative efficacy of combined CTLA-4 and PD-1 blockade vs. PD-1 monotherapy in metastatic melanoma: A real-world study. BJC Rep. 2024, 2, 14. [Google Scholar] [CrossRef]
- Zhan, L.; Feng, H.F.; Liu, H.Q.; Guo, L.T.; Chen, C.; Yao, X.L.; Sun, S.R. Immune Checkpoint Inhibitors-Related Thyroid Dysfunction: Epidemiology, Clinical Presentation, Possible Pathogenesis, and Management. Front. Endocrinol. 2021, 12, 649863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Ren, T.; Ji, C.; Zhao, L.; Wang, X. The baseline hemoglobin level is a positive biomarker for immunotherapy response and can improve the predictability of tumor mutation burden for immunotherapy response in cancer. Front. Pharmacol. 2024, 15, 1456833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adam Diehl, A.; Yarchoan, M.; Hopkins, A.; Jaffee, E.; Stuart, A.; Grossman, S.A. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017, 8, 114268–114280. [Google Scholar] [CrossRef]
| Cancer Type | Patient Number | Female/Male | ICI Therapy | Pretreatment | ||
|---|---|---|---|---|---|---|
| PD-1 | PD1/CTLA4 | |||||
| NSCLC | 27 (3) | 3/2 (0/3) | Pembrolizumab 24 (1) | Nivolumab 3 (2) | 0 | No |
| Ovarian Cancer | 7 (6) | 7/0 (6/0) | 0 | Nivolumab 1 (1) | Ipilimumab + Nivolumab 6 (5) | Paclitaxel/Carboplatine |
| Melanoma | 4 (3) | 4/0 (3/0) | Pembrolizumab 2 (1) | Nivolumab 2 (2) | 0 | No |
| Renal Cell Carcinoma | 2 (1) | 1/1 (1/0) | 0 | Nivolumab 2 (1) | 0 | No |
| Prostate Cancer | 2 (1) | 0/1 (0/1) | Pembrolizumab 2 (1) | 0 | 0 | Bicalutamide |
| Liver Cancer | 1 (1) | 0/1 (0/1) | Pembrolizumab 1 (1) | 0 | 0 | Sorafenib |
| Bladder Cancer | 1 (0) | 0/1 (0/0) | Pembrolizumab 1 (0) | 0 | 0 | No |
| Group 1 (n = 15) | Group 2 (n = 29) | Group 3 (n = 14) | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Age (years) | 63.6 ± 10.84 | 62.23 ± 6.96 | 57.5 ± 10.6 | NS | NS | 0.34 |
| F/M (%) | 66.67/33.33 | 20.69/79.31 | 80/20 | NS | NS | NS |
| TSH (N:0.4–4.0 µIU/mL) | 2.09 ± 1.19 | 1.68 ± 0.72 | 1.92 ± 0.72 | 0.860 | 0.668 | 0.303 |
| HGB (N:12/13–15/17 g/dl) | 11.88 ± 1.11 | 12.55 ± 1.98 | 13.85 ± 1.49 | 0.007 * | 0.054 | 0.428 |
| RBC (N:3.9/4.1–5.2/5.7 × 106 cells/µL) | 4.41 ± 0.76 | 4.49 ± 0.62 | 4.63 ± 0.55 | 0.620 | 0.773 | 0.919 |
| WBC (N:4–9 × 103 cells/µL) | 6.36 ± 2.07 | 11.07 ± 5.35 | 7.31 ± 1.26 | 0.801 | 0.015 | 0.001 * |
| Neutrophils (N: 2–7 × 103cells/µL) | 4.29 ± 1.55 | 7.87 ± 4.59 | 4.50 ± 1.05 | 0.985 | 0.010 | 0.005 * |
| Lymphocytes (N: 0.8–4 × 103cells/µL) | 1.23 ± 0.41 | 2.06 ± 1.06 | 2.10 ± 0.53 | 0.019 | 0.988 | 0.008 * |
| Platelets (N: 150–350 × 109 cells/L) | 269.60 ± 78.46 | 294.79 ± 102.49 | 264.57 ± 55.46 | 0.987 | 0.541 | 0.639 |
| WBC-Neutrophils (103 cells/µL) | 2.07 ± 0.66 | 3.20 ± 1.43 | 2.81 ± 0.64 | 0.191 | 0.529 | 0.007 * |
| dNLR | 2.13 ± 0.66 | 2.72 ± 1.79 | 1.69 ± 0.69 | 0.662 | 0.061 | 0.370 |
| SII | 1073.26 ± 667.46 | 1268.07 ± 1082.27 | 656.93 ± 462.33 | 0.410 | 0.088 | 0.763 |
| NLR | 3.80 ± 1.73 | 4.70 ± 4.04 | 2.33 ± 1.16 | 0.408 | 0.055 | 0.630 |
| PLR | 262.25 ± 162.95 | 172.00 ± 88.73 | 138.58 ± 67.51 | 0.009 * | 0.615 | 0.031 |
| WHR | 0.54 ± 0.19 | 0.88 ± 0.43 | 0.53 ± 0.11 | 0.998 | 0.004 * | 0.004 * |
| NRR | 0.96 ± 0.28 | 1.77 ± 1.09 | 0.97 ± 0.24 | 0.999 | 0.010 | 0.007 * |
| NHR | 0.36 ± 0.14 | 0.62 ± 0.37 | 0.32 ± 0.08 | 0.931 | 0.005 * | 0.012 |
| PHR | 22.92 ± 7.21 | 24.53 ± 10.83 | 19.21 ± 4.15 | 0.495 | 0.159 | 0.833 |
| FT4 (N: 12–22 pmol/L) | 16.16 ± 2.00 | 16.76 ± 2.70 | 16.04 ± 2.30 | 0.992 | 0.665 | 0.797 |
| Anti–TPO antibodies (N: <30 IU/mL) | 16.87 ± 7.88 | 19.31 ± 11.39 | 8.39 ± 3.86 | 0.070 | 0.003 * | 0.757 |
| Anti–Tg antibodies (N: <95IU/mL) | 34.69 ± 17.45 | 40.09 ± 21.60 | 18.06 ± 5.27 | 0.002 * | 0.064 | 0.694 |
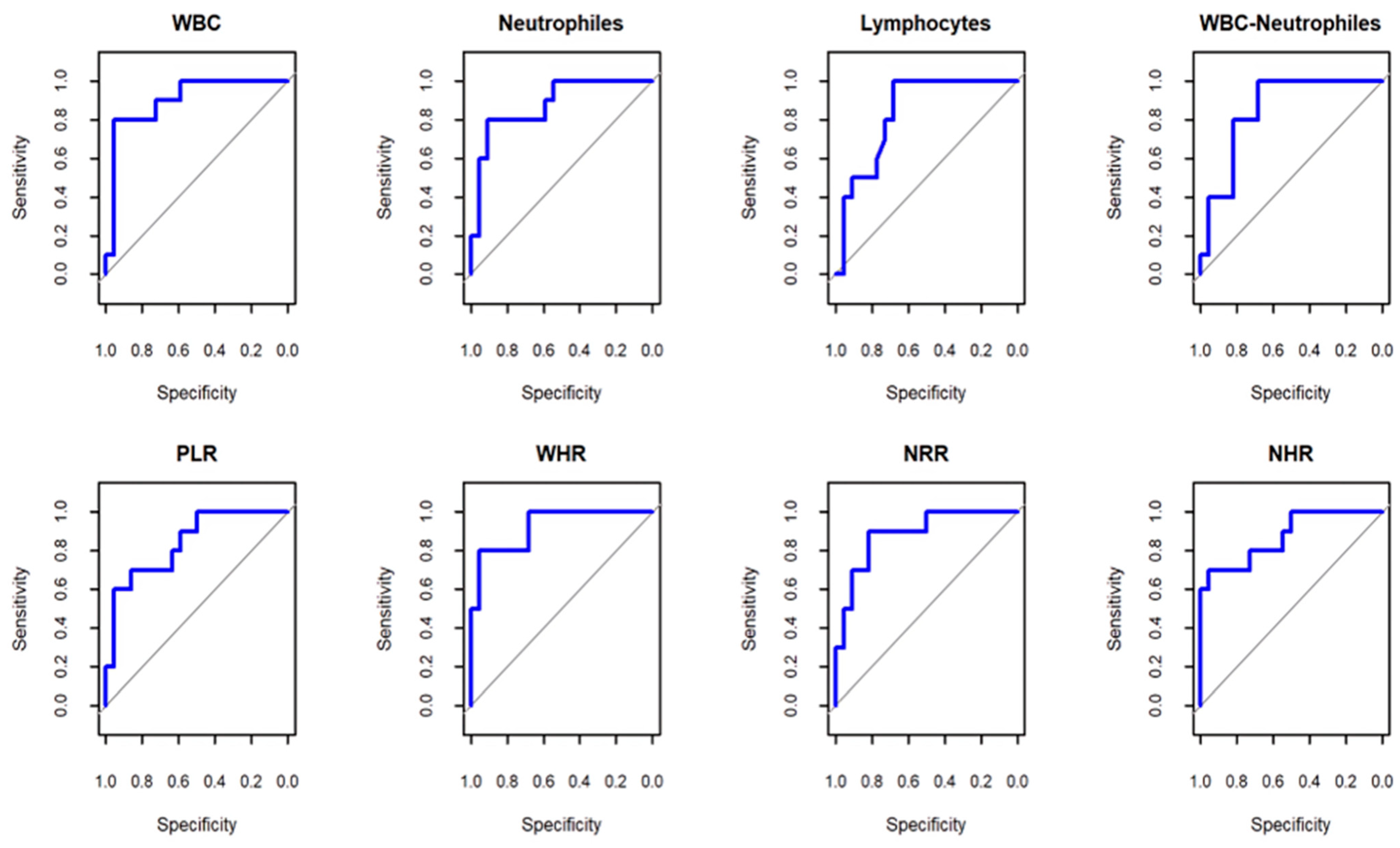
| Test Result Variables | AUC | Sensitivity with 95% CI | Specificity with 95% CI | Cut-Off Value |
|---|---|---|---|---|
| WBC | 0.9 | 0.8 (0.5–1) | 0.95 (0.86–1) | ≤6.6 |
| Neutrophils | 0.88 | 0.8 (0.5–1) | 0.91 (0.77–1) | ≤4.57 |
| Lymphocytes | 0.84 | 1 (1–1) | 0.68 (0.45–0.86) | ≤1.85 |
| WBCs-neutrophils | 0.85 | 1 (1–1) | 0.68 (0.45–0.86) | ≤2.59 |
| PLR | 0.84 | 0.7 (0.4–0.9) | 0.86 (0.73–1) | ≤222.97 |
| WHR | 0.92 | 0.8 (0.5–1) | 0.95 (0.86–1) | ≤0.52 |
| NRR | 0.89 | 0.9 (0.7–1) | 0.82 (0.64–0.95) | ≤1.06 |
| NHR | 0.87 | 0.7 (0.4–1) | 0.95 (0.86–1) | ≤0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomidze, K.; Kikodze, N.; Gordeladze, M.; Charkviani, N.; Chikovani, T. Complete Blood Count-Derived Biomarkers’ Association with Risk of PD-1 or PD-1/CTLA-4 Inhibitor-Induced Hypothyroidism in Patients with Solid Tumors. Immuno 2025, 5, 21. https://doi.org/10.3390/immuno5020021
Lomidze K, Kikodze N, Gordeladze M, Charkviani N, Chikovani T. Complete Blood Count-Derived Biomarkers’ Association with Risk of PD-1 or PD-1/CTLA-4 Inhibitor-Induced Hypothyroidism in Patients with Solid Tumors. Immuno. 2025; 5(2):21. https://doi.org/10.3390/immuno5020021
Chicago/Turabian StyleLomidze, Ketevan, Nino Kikodze, Marine Gordeladze, Nino Charkviani, and Tinatin Chikovani. 2025. "Complete Blood Count-Derived Biomarkers’ Association with Risk of PD-1 or PD-1/CTLA-4 Inhibitor-Induced Hypothyroidism in Patients with Solid Tumors" Immuno 5, no. 2: 21. https://doi.org/10.3390/immuno5020021
APA StyleLomidze, K., Kikodze, N., Gordeladze, M., Charkviani, N., & Chikovani, T. (2025). Complete Blood Count-Derived Biomarkers’ Association with Risk of PD-1 or PD-1/CTLA-4 Inhibitor-Induced Hypothyroidism in Patients with Solid Tumors. Immuno, 5(2), 21. https://doi.org/10.3390/immuno5020021







