Abstract
Honey and other bee products, including propolis, royal jelly, and bee pollen, are widely recognized for their medicinal properties. Among their numerous biological activities, their anti-inflammatory and immunomodulatory effects have gained significant attention in recent years. Immune and inflammatory disorders contribute significantly to the development of chronic conditions, including cancer and diabetes. Bee-derived products, along with their bioactive compounds such as polyphenols, have shown promising therapeutic effects in modulating inflammatory mediators. Studies indicate that these products help regulate tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), and interleukin-7 (IL-7) levels while reducing reactive oxygen species (ROS) production. Additionally, both in vitro and in vivo research, along with clinical studies, highlight their role in enhancing immune responses by activating B and T lymphocytes. This review explores the molecular mechanisms underlying these properties, emphasizing the role of bioactive compounds such as flavonoids, phenolic acids, and proteins in modulating immune responses and reducing inflammation. Evidence from in vitro, in vivo, and clinical studies suggests that honey and bee products influence cytokine production, regulate immune cell activity, and mitigate oxidative stress, making them potential therapeutic agents for inflammatory and immune-related disorders. To gather relevant information, databases such as Google Scholar, PubMed, and ScienceDirect were searched using various keyword combinations, including immunomodulatory, anti-inflammatory, bee products, honey, propolis, royal jelly, bee venom, and bee pollen. Given their anti-inflammatory, immune-protective, antioxidant, anti-apoptotic, and antimicrobial properties, bee products remain a subject of interest for further clinical evaluation.
1. Introduction
Inflammation is a fundamental biological response triggered by injury or infection, acting as a universal mechanism to address tissue disturbances and initiate repair [1,2]. This complex process involves distinct vascular changes that result in swelling and the subsequent recruitment of white blood cells to the affected area, forming an inflammatory site [3]. The primary objective of inflammation is to identify and eliminate harmful agents while promoting tissue healing and regeneration [4].
Cytokines, which are signaling proteins, play a pivotal role in orchestrating the inflammatory response. These molecules are classified into pro-inflammatory cytokines, which amplify inflammation, and anti-inflammatory cytokines, which help regulate and resolve it [5]. Persistent or chronic inflammation has been linked to a wide range of diseases, including atherosclerosis, myocardial infarction, asthma, diabetes, psoriasis, osteoporosis, hypertension associated with angiotensin II, tumor progression, and cardiovascular disease (CVD) [1,2,6,7]. Among these conditions, CVD remains one of the most significant global health concerns, contributing to high rates of morbidity and mortality. It encompasses disorders such as hypertension, coronary artery disease, peripheral vascular disease, heart failure, and various cardiomyopathies [6,7].
Natural anti-inflammatory agents, including dietary components, have gained attention for their potential in modulating inflammatory responses. Among these, honey has been widely recognized for its medicinal properties, exhibiting both anti-inflammatory and antioxidant activities (Figure 1). Its bioactive components, particularly phenolic compounds and flavonoids, contribute to the downregulation of pro-inflammatory cytokines while supporting tissue repair and immune regulation. It is important to note the distinction between immunomodulation and immunostimulation, as the terms are not interchangeable. Immunomodulation refers to the balanced regulation of immune function, including both upregulation and suppression of immune activity, to maintain homeostasis. Immunostimulation, in contrast, specifically involves enhancing or activating immune responses. This differentiation is clinically significant—while immunostimulation may be beneficial in combating infections or cancer, immunomodulation is particularly relevant for managing chronic inflammation, autoimmune diseases, and tissue repair, where excessive immune activation can be harmful. Studies suggest that honey’s ability to reduce oxidative stress and inflammation makes it a promising therapeutic option for managing chronic inflammatory conditions, including CVD (Figure 1 and Figure 2) [8].
Figure 1.
Properties of honey bee products.
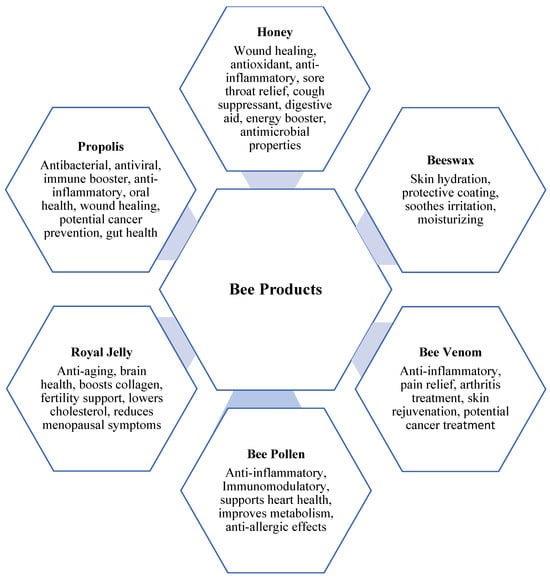
Figure 2.
Honey bee products and their medical uses.
The diverse medicinal benefits of honey can be attributed to its complex composition, which varies depending on its botanical source. Produced by bees from the nectar of various plant flowers, honey contains a rich array of nutritional and bioactive compounds that modern science continues to explore. While carbohydrates make up approximately 80–85% of its dry weight, honey also includes organic acids, proteins, amino acids, minerals, polyphenols, vitamins, and aromatic compounds. These components, influenced by floral origin, contribute to honey’s therapeutic effects, reinforcing its role as a natural remedy with both nutritional and medicinal value [9].
In the Arab-Islamic medical tradition, honey has long been valued for its healing properties, particularly in treating wounds. Rhazes (AD 864–932) recommended honey-based ointments for skin conditions and nerve injuries, as well as honey water for bladder wounds. His influential medical encyclopedia, Al Hawi, emphasized honey’s benefits for oral health, suggesting a honey–vinegar mixture for daily mouthwash to strengthen gums and whiten teeth. He also noted honey’s preservative qualities, even for cadavers. Similarly, Avicenna (AD 980–1037) praised honey for promoting longevity and vitality, recommending its consumption after age 45, especially with chestnut powder. He advocated using honey mixed with flour as a wound dressing and honey with rose petals for lung diseases and early stage tuberculosis. Additionally, Avicenna suggested honey as a natural remedy for insomnia. Both scholars contributed to honey’s reputation as a medicinal staple in Islamic and global medical traditions. Their writings influenced medical practices for centuries, shaping the understanding of honey’s therapeutic potential [10].
Scientific research over the past four decades has reinforced honey’s effectiveness in treating wounds, burns, and infections (Figure 1) [11]. This renewed interest in honey’s medicinal properties has been fueled by the rise in drug-resistant infections [12]. Studies indicate that honey possesses antimicrobial properties, and some clinical case reports show that applying honey to infected wounds aids in clearing infections and promoting tissue repair [13]. The antibacterial effects of honey are attributed to its physicochemical properties, including osmotic effects and pH [14,15]. Research also suggests that honey may have anti-inflammatory properties and enhance immune responses, which contribute to reducing infections and accelerating wound healing in burns, ulcers, and other skin injuries [13]. Manuka honey, derived from the Leptospermum tree species in New Zealand and Australia, has attracted attention due to its antibacterial activity, which is independent of hydrogen peroxide effects and osmolarity. Certain types of honey, including Medihoney and Active Manuka honey, have been approved for therapeutic use [16].
Unlike conventional antibiotics that target bacterial cell walls or metabolic pathways, honey has been shown to inhibit approximately 60 bacterial species, including both aerobic and anaerobic, Gram-positive and Gram-negative bacteria [17]. Several studies also report honey’s antifungal activity against yeasts, Aspergillus, Penicillium, and common dermatophytes [17]. Its hygroscopic nature enables honey to draw moisture from the environment, dehydrating bacterial cells. Additionally, its high sugar concentration inhibits microbial growth; yet, even when diluted, honey maintains significant antibacterial properties. While over 100 compounds have been proposed to contribute to these effects, including hydrogen peroxide, methylglyoxal, polyphenols, and defensin-1, identifying the precise active ingredients and their synergistic roles remains an area of ongoing investigation. Recent studies have provided more detailed insights into the complex composition and mechanisms underpinning honey’s antimicrobial properties, highlighting key bioactive compounds and their potential therapeutic applications [18].
The antibacterial potency of honey varies significantly based on its floral source and processing methods, sometimes by as much as 100-fold. Historical figures such as Aristotle, Dioscorides, and Arab-Islamic physicians recognized that honey from specific regions and seasons could be more effective in treating particular ailments. Modern scientific research has confirmed that honey exhibits selective antimicrobial activity, with some bacterial strains being more sensitive than others. For clinical applications, honey should be evaluated in laboratories to determine its antibacterial strength. Staphylococcus aureus is among the bacterial species most susceptible to honey’s antibacterial effects [14,15,18,19].
Oxidative stress results from an imbalance between free radical production and antioxidant defenses, contributing to chronic diseases [20]. Honey has been identified as a source of significant antioxidant activity, containing compounds such as glucose oxidase, catalase, ascorbic acid, flavonoids, phenolic acids, carotenoids, organic acids, and amino acids [8,9,19,20].
2. Composition and Bioactive Compounds of Honey and Bee Products
Honey and other bee products possess a complex and diverse chemical composition that contributes to their health beneficial and therapeutic properties. Their therapeutic potential is largely attributed to the presence of essential nutrients, bioactive compounds, and natural antioxidants, which vary depending on the botanical and geographical origins [8,9,21].
Honey is primarily composed of carbohydrates, making up approximately 75–80% of its total content. The dominant sugars include fructose (38%) and glucose (31%), which provide honey’s natural sweetness and energy value. Other minor sugars, such as maltose and sucrose, contribute to its physicochemical properties [9]. Apart from sugars, honey contains water (15–20%), proteins, amino acids, organic acids, vitamins, and minerals. Enzymes such as glucose oxidase and catalase contribute to honey’s antimicrobial properties by producing hydrogen peroxide and breaking down harmful oxidants. Organic acids, including gluconic, citric, and malic acids, not only provide honey’s characteristic acidity (pH 3.2–4.5) but also enhance its preservative and antibacterial effects. The vitamin content of honey includes vitamin C, B-complex vitamins (B1, B2, B6, B3, B5), and small amounts of folic acid. These vitamins contribute to honey’s antioxidant and immune-boosting properties. Essential minerals such as potassium, calcium, magnesium, iron, and zinc are also found in honey, further enhancing its nutritional and therapeutic profile [8,9,21].
The biological activity of honey and bee products is primarily attributed to their polyphenolic content. Polyphenols, including flavonoids and phenolic acids, play a crucial role in honey’s antioxidant, anti-inflammatory, and antimicrobial activities [22]. Flavonoids, such as quercetin, kaempferol, apigenin, and luteolin, are potent antioxidants that help neutralize free radicals, reducing oxidative stress and inflammation [22]. These compounds have also been shown to modulate immune responses by regulating cytokine production. Phenolic acids, including gallic acid, ferulic acid, and caffeic acid, further contribute to honey’s anti-inflammatory effects by inhibiting pro-inflammatory mediators [8,9,22].
The composition and bioactivity of honey vary significantly depending on the floral source from which it is derived. Different plant nectars contribute distinct polyphenol profiles, influencing honey’s therapeutic properties [21,22]. For example, manuka honey from Leptospermum species is known for its high levels of methylglyoxal (MGO), which exhibits strong antibacterial activity independent of hydrogen peroxide. Similarly, buckwheat honey is rich in phenolic acids, contributing to its superior antioxidant capacity [23]. Regional and environmental factors, such as soil composition, climate, and beekeeping practices, further impact honey’s nutrient content and bioactive profile. Raw, unprocessed honey tends to retain a higher concentration of bioactive compounds compared to commercially processed honey, which may undergo heat treatment and filtration that reduce its polyphenol content [21,22].
Overall, the diverse composition of honey and bee products underscores their potential as natural therapeutic agents. Their bioactive compounds contribute to a range of health benefits, including anti-inflammatory, antioxidant, and antimicrobial effects, making them valuable in both traditional and modern medicine.
3. Anti-Inflammatory Properties
Honey and other bee products, such as propolis and royal jelly, exhibit potent effects through multiple mechanisms. These products contain bioactive compounds, including flavonoids, phenolic acids, and enzymes, which modulate inflammatory pathways (Table 1) [24]. Honey suppresses the activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, leading to reduced inflammation [24]. Additionally, bee products enhance the production of anti-inflammatory mediators such as interleukin-10 (IL-10) while inhibiting pro-inflammatory responses (Figure 3) [25].
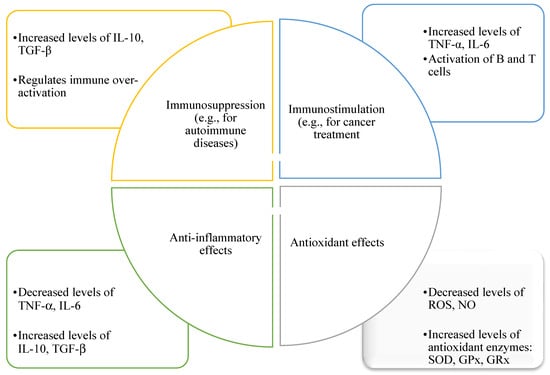
Figure 3.
Illustrates the shared anti-inflammatory, immunomodulatory (involving both immunostimulatory and immunosuppressive responses), and antioxidant properties of bee products, including honey, propolis, and royal jelly. These effects are mediated through components such as SOD (superoxide dismutase), GPx (glutathione peroxidase), and GRx (glutaredoxins), which act collectively against oxidative and inflammatory stressors like ROS (reactive oxygen species) and NO (nitric oxide).
A key aspect of honey’s anti-inflammatory action is its ability to regulate the expression of inflammatory cytokines. Studies indicate that honey and its components downregulate TNF-α, IL-1β, and IL-6, all of which play a crucial role in the inflammatory response [24,25]. Propolis, rich in caffeic acid phenethyl ester, has been shown to inhibit cytokine release from macrophages, thereby reducing systemic inflammation. Royal jelly also modulates immune cell activity, decreasing cytokine overproduction and promoting immune balance [24,25].
Studies have highlighted honey’s anti-inflammatory effects on immune cells. Research indicates that various commercial honeys can inhibit thrombin-induced oxidative respiratory bursts in human neutrophils and rodent peritoneal macrophages [26]. Similarly, another study demonstrated a significant, dose-dependent decrease in superoxide production by human neutrophils after exposure to three types of New Zealand honey—rewarewa, manuka, and kanuka [26]. Consistent with these findings, both pasture and manuka honeys exhibit strong antioxidant potential, with manuka honey demonstrating rapid free radical scavenging activity [27]. While methyl syringate, a compound particularly abundant in manuka honey, has been associated with this activity [28], it is important to note that honey’s antioxidant properties arise from a diverse array of bioactive compounds—including flavonoids, phenolic acids, enzymes, and vitamins—present in various types of honey. At a concentration of 400 µg/mL, manuka honey has also been shown to significantly inhibit TNF-α production by neutrophils [29]. However, encapsulation of manuka honey in alpha-cyclodextrin molecules reduces this bioactivity, suggesting that converting it into a cyclodextrin-based powder may diminish its anti-inflammatory effects [29].
The mechanisms underlying the anti-inflammatory effects of manuka honey have recently been explored. Research found that manuka honey treatment in LPS-induced RAW264.7 macrophages reduced cellular toxicity and enhanced cell viability. This improvement was linked to decreased ROS and nitrite accumulation, as well as the inhibition of cellular apoptosis [30]. Additionally, manuka honey was shown to suppress the TLR4/NF-κB signaling pathway, leading to lower secretion levels of key pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [30]. Comparable results were also observed in studies investigating Brazilian stingless bee honey [31].
Chronic inflammation is known to contribute to cancer development by disrupting normal tissue healing processes. This response is mediated by several pro-inflammatory cytokines and enzymes, among which cyclooxygenase-2 (COX-2) is particularly significant. COX-2 is involved in the conversion of arachidonic acid into prostaglandins—molecules implicated in both inflammation and tumor progression—and is frequently overexpressed in a variety of cancers [32,33,34]. The anti-inflammatory properties of honey are largely attributed to its rich content of phenolic compounds and flavonoids, which have been reported to inhibit the activity of COX-2 and inducible nitric oxide synthase (iNOS), thus contributing to an anti-inflammatory effect [33,35,36]. Studies on Malaysian honey have shown that its flavonoid and phenolic acid components possess both anti-inflammatory and cytoprotective capabilities [37]. For instance, in vitro experiments have demonstrated that honey extracts reduce nitric oxide (NO) production in activated RAW264.7 macrophages and protect these cells against cytotoxicity induced by tumor necrosis factor-alpha (TNF-α). This protective mechanism may be linked to the antioxidant effects of honey flavonoids [38], or to their ability to upregulate cytoprotective enzymes like heme oxygenase-1 (HO-1) [39,40]. Overall, the biological activity of honey-derived compounds appears to vary with concentration, cell type, and experimental conditions, resulting in either pro- or anti-inflammatory outcomes.
Mihajlovic et al. [41] demonstrated through both in vitro and in vivo experiments that active components found in honey and other bee products possess strong anti-inflammatory and immunomodulatory properties. One of these components, 10-hydroxydecenoic acid (10-HDA)—a medium-chain fatty acid (C8–C12) present in royal jelly—was shown to influence immune responses in a concentration-dependent fashion. Specifically, 10-HDA inhibited the maturation of dendritic cells derived from human monocytes that had been stimulated with lipopolysaccharides (LPS) and also reduced the production of inflammatory cytokines such as IL-8, IL-12, and TNF-α. Moreover, it downregulated both Th1 and Th2 immune pathways, leading to a decrease in helper T cells and monocyte-derived dendritic cells [41]. Bee venom and melittin have also been studied for their potential to suppress excessive immune activity and inflammation in experimental models; however, caution is warranted due to melittin’s cytotoxic nature [42]. The bacterium Porphyromonas gingivalis, along with its LPS (PgLPS), plays a role in periodontal disease, and bee venom has been observed to counteract the pro-inflammatory cytokines IFN-γ, IL-1β, IL-6, IL-8, and TNF-α by inhibiting the activation of NF-κB and AP-1 pathways in HaCaT keratinocyte cells under in vitro conditions [43]. Additionally, a polysaccharide derived from Chinese wolfberry bee pollen demonstrated immunomodulatory effects on RAW264.7 macrophages, as evidenced by reductions in IL-1, IL-6, TNF-α, and nitric oxide production [44]. The topical use of propolis was found to promote wound healing in a type I diabetes mouse model (induced by streptozotocin), likely due to lowered levels of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, as well as enhanced collagen synthesis mediated by TGF-β1 signaling through Smad2 and Smad3 activation [45]. Collectively, these findings suggest that bee-derived products exert anti-inflammatory and immunoregulatory effects by decreasing cytokine production (including IFN-γ, IL-1, IL-6, IL-8, and TNF-α), modulating signaling cascades, and influencing the function of B and T lymphocytes.
Propolis is a naturally found product obtained by bees from the diversity of plant resins, and it is one of the richest sources of polyphenols. For many years, propolis has gained substantial attention in providing a broad range of therapeutic, food, and cosmetic applications. The biological properties of propolis are associated with the bioactive chemical composition of propolis. Its chemical components and properties are linked directly with the species of bees, sources of plants, geographical areas, and environmental conditions. More than 300 components have been recognized in various varieties of propolis, including phenolic compounds, phenolic acids, flavonoids, mono- and sesquiterpenoids, diterpenoids, and triterpenoids. The bioactive components of propolis have been found to possess considerable antimicrobial, immunomodulatory, hepato-protective, antioxidant, antitumor, and antiparasitic effects. It is a widely applied natural product in pharmaceutic- and cosmetic-based products. Due to the presence of polyphenols in propolis and their antioxidant effect, it can be used topically, as it is a photoprotector that blocks solar radiation [46].
Research also supports honey’s efficacy in managing inflammatory gastrointestinal disorders. For instance, studies on ulcerative colitis have shown that natural honey significantly reduces serum levels of IL-1β and IL-6 while also lowering TNF-α, iNOS, and caspase-3 in colonic tissues. Experiments with natural Turkish honey demonstrated that a seven-day supplementation markedly alleviated macroscopic and microscopic ulcerative colitis symptoms in rats compared to synthetic drugs. Similarly, administering 2.5 g of manuka honey to rats with chronic gastric ulcers enhanced IL-10 levels while reducing TNF-α, IL-1β, and IL-6, promoting an anti-inflammatory response. Honey has been used in treating colitis and gastritis, with a seven-day intake showing reduced ulcerative lesions, lower microvascular permeability, and decreased gastric myeloperoxidase activity, thereby enhancing free radical scavenging efficiency [47].
Given its antioxidant and anti-inflammatory attributes, honey also exhibits cardioprotective effects. Consumption of honey has been associated with lower LDL cholesterol levels, likely due to its ability to neutralize free radicals. Its anti-inflammatory properties make honey beneficial in preventing metabolic and cardiovascular diseases, particularly when combined with other health-promoting foods [48]. Flavonoids, phenolic acids, and vitamin C found in honey have been shown to contribute positively to cardiovascular health by enhancing myocardial performance, helping to regulate blood pressure, and reducing the likelihood of arrhythmias. These bioactive compounds support coronary artery dilation and help lower the chances of blood clot formation. Evidence from both laboratory and animal studies indicates that honey can improve lipid metabolism, protect against oxidative stress, lower cardiac injury biomarkers such as CK-MB, AST, and ALT, and enhance the activity of antioxidant enzymes including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRx). Additionally, honey has been reported to increase the resistance of low-density lipoprotein (LDL) particles to oxidative modification [49,50].
Among various types of honey, buckwheat honey is considered particularly beneficial due to its high flavonoid content, specifically quercetin and rutin. These compounds enhance capillary strength and elasticity, reducing the risk of vascular damage and atherosclerotic plaque formation [23].
Touzani et al. [51] investigated in an in vitro study the therapeutic potential of propolis extract from northern Morocco (PNM), focusing on its chemical composition, physicochemical properties, and biological activities. They examined its immunomodulatory, antibacterial, antioxidant, and anticancer effects. High-performance liquid chromatography analysis revealed that pinocembrin, chrysin, and quercetin were the dominant phenolic compounds in PNM. The extract displayed significant antibacterial activity against both Gram-positive and Gram-negative bacteria and exhibited strong antioxidant potential by effectively neutralizing free radicals. Additionally, PNM demonstrated a dose-dependent inhibitory effect on MCF-7, HCT, and THP-1 cancer cell lines. In terms of its anti-inflammatory properties, it reduced TNF-α and IL-6 production in LPS-stimulated human peripheral blood mononuclear cells (PBMNCs) while increasing IL-10 production up to 15 times higher than in untreated PBMNCs. These findings suggest that the well-recognized multitarget therapeutic effects of PNM may be attributed, at least in part, to its immunomodulatory properties. Similar results were reported for other types of honey, for example, Castanopsis honey that showed evident antioxidant activities, and the anti-inflammatory activities of CHE were observed to inhibit the release of NO and reduce the content of TNF-α and improve the content of IL-10 by regulating the NF-κB pathway [52].
NO is a transient, bioactive free radical that serves as a crucial signaling molecule in various physiological and pathological processes. It plays a significant role in immune responses, vascular regulation, and neurotransmission [53]. In the context of inflammation, NO is primarily synthesized by iNOS in response to pro-inflammatory stimuli, including cytokines and bacterial endotoxins such as lipopolysaccharides (LPS) [53]. Excessive NO production is often associated with the severity and progression of both acute and chronic inflammatory conditions, as it contributes to oxidative stress and tissue damage [54].
In vitro studies have demonstrated that NO production is significantly elevated in immune cells activated by inflammatory stimuli. For instance, a recent study reported that when THP-1-derived macrophages were stimulated with LPS, NO production increased approximately five-fold compared to untreated control cells. However, treatment with non-toxic concentrations of Hyphaene thebaica honey led to a marked reduction in NO levels, restoring them to baseline levels observed in untreated cells. This suggests that Hyphaene thebaica honey may possess anti-inflammatory properties, potentially through its ability to modulate NO production [54]. While this study highlights a strong correlation between honey’s phenolic content and its NO-inhibitory activity, correlation does not imply causation. Additional investigations, including controlled mechanistic studies, are necessary to determine the precise roles of individual phenolic compounds and other bioactive constituents in NO regulation.
Previous research has also indicated that honey can suppress NO synthesis by downregulating iNOS expression. The inhibitory effect of honey on NO production has been attributed to its diverse phenolic composition. Different types of honey exhibit varying abilities to modulate nitrogen oxide levels, likely due to differences in their bioactive compound profiles. Studies have identified a strong negative correlation between the phenolic content of honey and its NO-scavenging activity, as observed in Moroccan Euphorbia honey [55,56]. A higher concentration of phenolic compounds in honey has been linked to a greater reduction in NO levels, suggesting that these compounds play a critical role in its anti-inflammatory properties [57,58].
Furthermore, honey’s ability to inhibit NO production aligns with its broader anti-inflammatory effects, which may be mediated by multiple bioactive constituents. These compounds may work through various mechanisms, such as scavenging reactive nitrogen species, modulating inflammatory signaling pathways, and reducing oxidative stress. However, the exact molecular mechanisms and the specific chemical constituents responsible for NO inhibition remain to be fully elucidated. Given the variability in honey composition based on botanical origin and geographical factors, further research is warranted to establish a clearer understanding of its role in NO modulation and inflammation control [57,58].
While these studies suggest a strong correlation between honey’s phenolic content and its NO-inhibitory activity, this relationship does not confirm direct causation. Additional investigations, including controlled mechanistic studies, are necessary to determine the precise role of individual phenolic compounds and other bioactive constituents in NO regulation. Understanding these mechanisms could provide valuable insights into the potential therapeutic applications of honey and natural plant-derived products in managing inflammatory conditions.
4. Effects on Oxidative Stress and Reactive Oxygen Species (ROS)
Inflammation and oxidative stress are closely linked, with ROS generated in mitochondria contributing to the production of pro-inflammatory cytokines and mediators. Conversely, cytokines such as TNF-α and IL-1β can promote ROS generation in mitochondria. This interplay between ROS and cytokines leads to metabolic and cellular alterations (Figure 3) [59].
Honey’s antioxidant properties extend beyond free radical scavenging, as polyphenols in honey play a significant role in preventing and managing various diseases [60]. Research indicates that phenolic compounds in honey enhance the body’s natural defense mechanisms against oxidative stress and chemical damage (Table 1) [60,61,62]. The mechanisms underlying this impact are diverse, as phenolic compounds contribute to gene regulation involved in oxidative stress reduction, amyloid fibril formation, and upregulation of the Nrf2 transcription factor, which triggers antioxidant gene expression [60,63]. Moreover, polyphenols stimulate the activity of antioxidant enzymes, including SOD, catalase (CAT), GPx, glutathione reductase (GR), and peroxiredoxins [60,63]. Studies have demonstrated that consuming honey rich in phenolic compounds boosts plasma antioxidant activity. Replacing conventional sugars and confectionery products with honey may enhance the body’s defense against free radicals [60]. Additionally, antioxidant compounds in honey protect pancreatic cells, which produce insulin and glucagon, from oxidative stress and its adverse effects [64,65].
A recent analysis of the antibacterial and antioxidant capabilities of four honey types collected from different regions of the West Bank, Palestine, found a positive link between their antioxidant effectiveness and polyphenol levels—an observation that supports earlier research findings [14,15,19]. The honey samples, derived from Medicago sativa, Centaurea dumulosa Boiss, Silybum, and Ziziphus spina-christi, reflect a range of botanical sources and environmental backgrounds, which may account for variations in their chemical makeup and biological effects. Antioxidant tests showed that each sample exhibited notable antioxidant activity [15].
Metabolic syndrome is a cluster of five risk factors, including central obesity, hyperglycemia, dyslipidemia, and hypertension, which increase the risk of cardiometabolic diseases [66]. Numerous studies have highlighted the beneficial effects of natural products and honey [67,68,69] in counteracting metabolic syndrome through its anti-obesity, hypoglycemic, hypolipidemic, and hypotensive properties. The health benefits of honey are largely linked to its rich polyphenol and flavonoid content, which contribute to its antioxidant and anti-inflammatory effects. Specific polyphenols such as caffeic acid, p-coumaric acid, and gallic acid have been associated with anti-obesity and lipid-lowering actions [67,68]. These compounds may downregulate the expression of sterol regulatory element-binding transcription factor 1 (SREBP-1) and its downstream enzyme, fatty acid synthase (FAS), which is involved in lipogenesis. Caffeic acid and quercetin, both present in honey, have been reported to help reduce body fat and overall body weight. In addition, honey contains fructooligosaccharides that influence lipid metabolism, partly by suppressing FAS activity. Its phenolic acids and natural sugars, particularly fructose, are also thought to support blood glucose regulation by acting on the PI3K/Akt insulin signaling pathway. Honey has been found to increase Akt expression while suppressing the activation of nuclear factor-kappa B (NF-κB). Moreover, quercetin—a prominent flavonoid in honey—has vasodilatory effects, enhancing nitric oxide release through endothelial nitric oxide synthase and activating calcium-sensitive potassium channels [69].
Since oxidative stress and inflammation are key contributors to metabolic syndrome, honey’s capacity to counteract these mechanisms highlights its potential role in prevention. An analysis of several Malaysian honey varieties reported a strong association between total phenolic content (TPC) and honey color intensity. Among the tested samples, Kelulut honey exhibited the highest TPC at 784.3 mg GAE/kg, followed by Tualang, Pineapple, and Borneo honeys with values of 589.2, 602.4, and 510.4 mg GAE/kg, respectively [70]. The polyphenols and flavonoids present in honey function as powerful antioxidants by donating hydrogen atoms and groups to neutralize reactive oxygen species, thereby reducing oxidative damage. For example, quercetin, caffeic acid (CA), and chlorogenic acid exhibit iron-chelating and iron-stabilizing properties, preventing free radical formation and enhancing antioxidant capacity [71,72,73]. Moreover, polyphenols such as apigenin, quercetin, and kaempferol may exert anti-inflammatory effects by modulating enzymes involved in pro-inflammatory processes, including nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), and nuclear factor erythroid 2-related factor 2 (Nrf2) [74,75,76,77]. The synergistic interaction of these bioactive compounds may contribute to honey’s antimetabolic properties.
5. Immunomodulatory Effects
Immunomodulatory agents, which can enhance or suppress immune responses, are of significant interest in treating autoimmune disorders, infections, and cancer. These agents influence immune mediators, leading to either pro-inflammatory or anti-inflammatory effects [78]. Immunostimulatory compounds often increase cytokines such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ), which promote immune activation. In contrast, immunosuppressive agents reduce pro-inflammatory mediators like TNF-α, IL-6, and IL-12—especially in conditions such as autoimmune diseases—while increasing anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta (TGF-β), thereby mitigating excessive immune responses [78]. Natural compounds, including polysaccharides, flavonoids, and alkaloids, have demonstrated potent immunomodulatory effects by modulating these cytokine profiles and influencing key signaling pathways like NF-κB and MAPK [79].
Bee venom has been found to suppress the activation of the pro-inflammatory NF-κB and AP-1 signaling pathways triggered by Porphyromonas gingivalis lipopolysaccharides (PgLPS) in human keratinocyte (HaCaT) cells [43]. In another study, a polysaccharide extracted from Chinese wolfberry bee pollen demonstrated immunoregulatory effects in RAW264.7 macrophages by lowering the secretion levels of inflammatory mediators such as IL-1, IL-6, TNF-α, and nitric oxide [44]. Additionally, when propolis was topically applied to wounds in a type I diabetes mouse model induced by streptozotocin (STZ), it significantly accelerated the healing process. This improvement was linked to a reduction in pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α, alongside enhanced collagen production mediated through the TGF-β1 signaling pathway involving Smad2 and Smad3 proteins [45].
As mentioned above, several scientific studies have highlighted the health benefits of manuka honey [80]. It has been found to exhibit immunomodulatory properties, as demonstrated by Masad et al. [81] using in vitro and in vivo approaches. Treatment of RAW 264.7 macrophages with 1% manuka honey significantly increased TNF-α gene expression (~26-fold) and secretion (~27-fold). The expression of other inflammatory cytokines (IL-1β, IL-6, iNOS) and chemokines (CXCL2, CCL2) also increased. In C57BL/6 mice, intraperitoneal manuka honey administration led to a significant rise in peritoneal exudate cells (PECs), mainly due to a 35-fold increase in neutrophil recruitment. This response was observed in TLR4-defective C3H/HeJ mice, indicating that it was independent of TLR4 and unlikely due to LPS contamination. Manuka honey altered macrophage phenotypic expression, shifting towards the CD11blo F4/80lo phenotype and increasing MHC class II expression. However, this peritoneal response was largely absent in MyD88-deficient mice, highlighting the role of TLR signaling.
In a recent study, Masad et al. [82] highlighted the immunostimulatory properties of MH and demonstrate its potential utilization in cancer prevention and treatment. They investigated the effects of orally administered manuka honey on tumor growth and immune responses. Manuka honey pretreatment enhanced anti-tumor immunity, leading to tumor suppression. Increased tumor immunogenicity was observed through upregulated MHC class-II on macrophages, MHC class-I on tumor cells, and greater T cell infiltration. Notably, manuka honey was also effective when administered therapeutically post-tumor implantation. Transcriptomic analysis revealed changes in chemokine and cytokine expression associated with these effects. The immunomodulatory impact of manuka honey was lost in IFNγ-deficient mice. Gut microbiota analysis showed manuka honey induced unique microbial changes, potentially driving the observed immune benefits. Manuka honey treatment appeared to enrich for a type I/II IFN signature, enhancing T cell activation and migration from the gut to peripheral tissues. These activated T cells exhibited superior effector function, contributing to tumor suppression. Further research is needed to validate the role of gut microbiota and explore manuka honey’s combination with chemotherapy or immunotherapy for enhanced anticancer efficacy.

Table 1.
Summary of bioactive compounds and their reported immunomodulatory and anti-inflammatory properties. The effects listed are primarily based on findings from in vitro and animal studies, as cited in the referenced literature. These studies investigated various mechanisms including cytokine modulation, inhibition of inflammatory mediators, and regulation of signaling pathways [41,81,82,83,84,85,86,87,88,89,90,91,92,93].
Table 1.
Summary of bioactive compounds and their reported immunomodulatory and anti-inflammatory properties. The effects listed are primarily based on findings from in vitro and animal studies, as cited in the referenced literature. These studies investigated various mechanisms including cytokine modulation, inhibition of inflammatory mediators, and regulation of signaling pathways [41,81,82,83,84,85,86,87,88,89,90,91,92,93].
| Principal Components | Honey | Propolis | Pollen | Royal Jelly | Medicinal Properties | |||
|---|---|---|---|---|---|---|---|---|
| Anti-Inflammatory | Immuno-Modulatory | Antioxidant | Wound Healing | |||||
| Acacetin | ✓ | ✓ | ✓ | |||||
| Apigenin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Caffeic acid | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Catechin | ✓ | ✓ | ✓ | |||||
| Chlorogenic acid | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Chrysin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Cinnamic | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Epicatechin | ✓ | ✓ | ||||||
| Ferulic | ✓ | ✓ | ✓ | |||||
| Fisetin | ✓ | ✓ | ✓ | |||||
| Galangin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Gallic acid | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hesperetin | ✓ | ✓ | ✓ | ✓ | ||||
| Isorhamnetin | ✓ | ✓ | ✓ | ✓ | ||||
| Kaempferol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Liquiritigenin | ✓ | ✓ | ✓ | ✓ | ||||
| Luteolin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Macarangin | ✓ | ✓ | ||||||
| Myricetin | ✓ | ✓ | ✓ | ✓ | ||||
| Naringenin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Neoflavonoid | ✓ | ✓ | ||||||
| Neovestitol | ✓ | ✓ | ||||||
| P-coumaric | ✓ | ✓ | ||||||
| P-hydroxybenzoic | ✓ | ✓ | ||||||
| Pinocembrin | ✓ | ✓ | ✓ | |||||
| Protocatechuic | ✓ | ✓ | ||||||
| Quercetin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Rosmarinic | ✓ | ✓ | ✓ | |||||
| Rutin | ✓ | ✓ | ✓ | ✓ | ||||
| Syringic | ✓ | ✓ | ||||||
6. Wound Healing
The wound-healing process is categorized into four sequential stages: hemostasis, inflammation, proliferation, and tissue remodeling [82]. Inflammation is a crucial biological response of living tissue to local injury, serving as a protective mechanism to prevent infections and facilitate tissue repair. While inflammation is essential for healing, an improper response can lead to excessive production of inflammatory mediators by immune cells, which fail to respond to the initial triggers, thereby hindering wound resolution [53]. The anti-inflammatory properties of honey contribute to wound healing through various mechanisms.
During the inflammatory phase, damaged tissues generate high levels of free radicals. The antioxidant compounds present in honey work in synergy to mitigate oxidative stress, thereby reducing tissue damage and preventing necrosis [14]. Additionally, both in vitro and in vivo studies suggest that honey inhibits the activity of cyclooxygenases 1 and 2 (COX1 and COX2), which are involved in prostaglandin synthesis [54,55]. Prostaglandins regulate the inflammatory response by promoting vasodilation, increasing vascular permeability, facilitating leukocyte migration, inhibiting platelet aggregation, and triggering pain receptors. A reduction in prostaglandin levels in plasma is associated with diminished inflammation, edema, and pain [56]. Furthermore, honey has been found to suppress the expression of TNF- α and reduce pro-inflammatory cytokine levels by modulating NF-κB [54]. NF-κB plays a role in activating inducible nitric oxide synthase (iNOS), an enzyme responsible for NO production. During inflammation, iNOS is stimulated by cytokines, TNF-α, interleukins, and bacterial endotoxins, leading to NO release. Although NO acts as a mediator in both acute and chronic inflammation, excessive or untimely production can be detrimental, contributing to inflammation-related disorders [56]. Another beneficial effect of honey’s anti-inflammatory activity is the reduction in edema, which alleviates pressure on the microvasculature of wound tissue, ensuring oxygen and nutrient availability necessary for tissue growth and repair [20]. This property also aids in controlling wound exudate and maintaining optimal moisture levels, a critical factor in effective wound healing [39].
The anti-inflammatory potential of honey is largely attributed to its phenolic compounds (Table 1) [53,57]. However, studies have not yet established a direct correlation between the anti-inflammatory activity of various honey samples and their phenolic content [58]. This discrepancy may result from the complex interactions between phenolic compounds and other bioactive constituents present in honey.
Honey has been shown to promote re-epithelialization by increasing TNF-α and IL-1β levels. Research indicates that a 1% concentration of honey stimulates monocytes to release TNF-α, IL-1, and IL-6, which enhance keratinocyte migration and proliferation—key cellular processes in wound healing. These cytokines also facilitate collagen synthesis by fibroblasts, further contributing to tissue regeneration [14,68,81,83].
A recent study [93] investigated the anti-inflammatory properties of seven honey varieties sourced from different regions of Romania using in vitro experimental models. The research focused on macrophage-like cells to assess the impact of honey on inflammatory cytokine production, a key factor in modulating inflammation. The findings confirmed that Romanian honey varieties exhibited anti-inflammatory effects by reducing TNF-α and IL-8 production by up to 12% in inflamed human macrophage-like cells. These anti-inflammatory properties were primarily influenced by the phenolic content of honey rather than its sugar composition. There was a strong positive correlation between phenolic compounds and biological activity, whereas glucose content showed a moderate negative correlation. This suggests that bioactive phenolic compounds, rather than sugars, play a crucial role in honey’s anti-inflammatory potential. Supporting evidence from previous studies indicates that phenolic-rich honey extracts from Malaysia inhibited nitric oxide production in LPS and interferon-γ-activated RAW 264.7 macrophages. Compounds such as ellagic acid, ferulic acid, myricetin, and hesperetin have been identified as contributors to honey’s anti-inflammatory activity [80,81,82,83,84,85,86,87,88,89,90,91,92]. In another study, McLoone et al. [94]. determined the ability of five honeys from Kazakhstan and manuka honey to stimulate TNF-α and TGF-β production by human keratinocytes. TNF-α and TGF-β levels increased over time in honey-treated and untreated keratinocytes, whereas cells treated with sugar solutions that matched those of the honeys had reduced levels of both cytokines. This suggests that the non-sugar phytochemical components of the honeys may have prevented this decrease. Analysis by LC-MS confirmed that the honeys contained a diverse range of phytochemicals. Some phytochemicals, e.g., pinobanksin and vanillin, were present at different levels across the honey types, whereas other components, e.g., dicarboxylic acids and their glycosides, were abundant in all the honeys. This study highlights the potential of honey’s non-sugar phytochemicals in modulating cytokine production and suggests that these components may contribute to the therapeutic properties of honey in skin-related inflammatory responses.
Similarly, recent research [95] has highlighted the significant role of propolis in wound healing due to its anti-inflammatory properties. However, its pharmacological efficacy varies depending on its geographic and botanical origins. A study analyzing three ethanolic extracts of Korean propolis assessed their chemical composition and biological activities, particularly their anti-inflammatory effects in wound repair. Flavonoids such as pinocembrin, chrysin, and apigenin were identified as major constituents. Among the extracts, Gongju Mountain propolis exhibited the highest anti-inflammatory activity, including the strongest inhibition of NO production. Gongju Mountain samples significantly suppressed inflammatory mediators such as iNOS, IL-1β, and IL-6. The high flavonoid content in Korean ethanolic propolis may contribute to its wound-healing, anti-inflammatory, and antioxidant effects. In vivo studies demonstrated that propolis accelerates wound healing by reducing inflammation, and acute dermal toxicity tests confirmed its safety for topical application. Tang et al. [96] recently conducted a systematic review and meta-analysis to assess the effectiveness and safety of honey-based dressings in the treatment of chronic wounds. The results indicated that honey dressings were more effective than conventional alternatives in promoting wound closure and speeding up the healing process. Despite these benefits, the analysis did not find significant differences in terms of overall healing rates, time required for bacterial clearance, or duration of hospitalization. While honey treatment may decrease the Visual Analog Scale pain score, it may also increase the incidence of painful discomfort during treatment.
Overall, these findings underscore the therapeutic potential of honey and propolis in wound healing. Their anti-inflammatory properties, largely driven by phenolic compounds and flavonoids, play a critical role in modulating inflammation and promoting tissue regeneration. Future research should aim to refine their applications, ensure product standardization, and explore their clinical efficacy through large-scale trials.
7. Safety Considerations of Honey and Bee Products
Although honey and other bee products are generally considered safe and beneficial, their therapeutic use requires awareness of potential adverse effects. Infant botulism remains a well-recognized risk associated with honey consumption in children under 12 months, due to the possible presence of Clostridium botulinum spores, and honey is therefore contraindicated in this age group [97]. Allergic reactions are another concern, particularly in individuals with sensitivities to pollen or bee-derived substances. Reactions may range from mild symptoms (e.g., skin irritation, gastrointestinal discomfort) to severe responses such as anaphylaxis [98]. Components like royal jelly and propolis have been linked to adverse effects including asthma exacerbation, contact dermatitis, and rare cases of nephrotoxicity or hepatotoxicity, especially with high doses or prolonged use [99,100]. Additionally, because of its high sugar content, honey may not be suitable for individuals with uncontrolled diabetes or those on strict glycemic diets [101]. Thus, while the therapeutic potential of bee products is significant, it is essential to consider these safety concerns and apply appropriate clinical judgment, especially in sensitive populations.
8. Therapeutic Ranges and Doses of Propolis and Bee Venom
Propolis and bee venom have long been studied for their therapeutic potential, particularly in the areas of wound healing, anti-inflammatory effects, and pain management. Below, we summarize the key findings from preclinical and clinical studies regarding the therapeutic doses and ranges for these substances.
Propolis is a resinous substance collected by honeybees from tree buds and other botanical sources. It contains a variety of bioactive compounds, including flavonoids, phenolic acids, and terpenoids, which contribute to its therapeutic properties. The therapeutic doses of propolis vary depending on the intended application and the form in which it is administered (e.g., oral, topical).
Oral Administration: Preclinical studies have shown that propolis is effective at doses ranging from 5 mg/kg to 50 mg/kg in animal models. Clinical trials, however, generally use lower doses in human patients, typically ranging from 200 mg to 1000 mg per day. These doses have been found to be well tolerated in humans, with promising results in the treatment of conditions like wound healing, infections, and inflammation [102,103].
Topical Application: Topically, propolis is often used in the form of creams or ointments. Clinical studies suggest that concentrations of 10–30% of propolis extract are effective for promoting wound healing and reducing inflammation [104]. In some cases, up to 500 mg of propolis in topical formulations has been applied daily for skin conditions like eczema or acne.
Therapeutic Applications: Propolis has been studied for its potential in treating a range of conditions, including oral ulcers, wound healing, and inflammatory diseases. Its use in oral form (e.g., 300–500 mg/day) has been shown to provide anti-inflammatory and antioxidant benefits, supporting its role in managing conditions like gingivitis and sore throat [105].
Bee venom is a complex mixture of enzymes, peptides, and biogenic amines, with melittin being the principal active component responsible for its anti-inflammatory and analgesic effects. Bee venom has been explored for therapeutic purposes in pain management, particularly in conditions like arthritis and chronic musculoskeletal pain.
Preclinical Studies: In animal models, bee venom is typically administered at doses ranging from 0.1 mg/kg to 1 mg/kg, depending on the specific condition being studied [106]. These doses have demonstrated significant anti-inflammatory and analgesic effects in conditions such as rheumatoid arthritis and osteoarthritis.
Clinical Administration: In clinical practice, bee venom is most commonly administered through localized injections as part of apitherapy. Doses of 0.1 mg to 2 mg per session have been reported in studies on chronic pain management [107]. Typically, patients receive 1–5 injections per week, with some protocols recommending treatment over several months depending on the severity of the condition.
Safety and Efficacy: Bee venom therapy has shown efficacy in reducing pain and inflammation in clinical settings, particularly for musculoskeletal disorders like rheumatoid arthritis. Most clinical trials report positive outcomes with doses ranging from 0.1 mg to 1 mg per injection, with minimal side effects when administered properly [108]. However, allergic reactions remain a concern, particularly in individuals with a history of insect stings.
Both propolis and bee venom have demonstrated therapeutic potential, with established dosing ranges based on preclinical and clinical studies. Propolis doses of 200 mg to 1000 mg per day are typically well tolerated in humans, while bee venom is most commonly administered in doses of 0.1 mg to 2 mg per session for pain and inflammation management. However, more extensive clinical trials are needed to refine the optimal therapeutic ranges and to explore their full potential in different medical conditions.
9. Concluding Remarks
Inflammation is a crucial defense mechanism, but its dysregulation can lead to inflammatory diseases. Bee products have been used as a wound dressing for thousands of years, but only in more recent times has a scientific explanation become available for its effectiveness. Bee products, rich in bioactive compounds, have shown significant potential in modulating immune responses, reducing inflammation, and supporting treatments for autoimmune conditions, diabetes, cancer, wound healing, and periodontal diseases. Their immunomodulatory, antioxidant, anti-inflammatory, neuroprotective, and antimicrobial properties further underscore their medicinal value. Despite these benefits, the clinical use of bee products remains limited due to a lack of extensive human trials and concerns about allergic reactions and toxicity. Further research is needed to unlock their full therapeutic potential. Additionally, preserving honeybee populations is essential, especially in the wake of colony collapse disorder, to sustain these valuable natural resources. Although not classified as medicine, honey is widely recognized for its preventive and therapeutic benefits. Its healing properties are closely linked to its chemical composition, botanical origin, and geographical source. Among its key bioactive components, phenolic compounds—particularly polyphenols—play a vital role in combating oxidative stress-related diseases, including cardiovascular and neurodegenerative disorders, inflammation, and cancer. It is important to note that many of the findings discussed in this review are based on in vitro and animal studies. While these models provide valuable insights into the mechanisms of action and therapeutic potential of honey and its bioactive compounds, their direct translation to human health outcomes remains a significant challenge. Differences in physiology, immune responses, and metabolism between species highlight the need for cautious interpretation. Therefore, further well-designed clinical trials are essential to confirm these effects in human populations. A more detailed analysis of the translational challenges and opportunities is beyond the scope of this review but represents an important topic for future investigation.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Plytycz, B.; Seljelid, R. From inflammation to sickness: Historical perspective. Arch. Immunol. Ther. Exp. 2003, 51, 105–109. [Google Scholar]
- Cucu, I. Signaling Pathways in Inflammation and Cardiovascular Diseases: An Update of Therapeutic Strategies. Immuno 2022, 2, 630–650. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Rana, M.N.; Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr. Diabetes Rep. 2022, 22, 27–37. [Google Scholar] [CrossRef]
- Shakoori, Z.; Salaseh, E.; Mehrabian, A.R.; Tehrani, D.M.; Dardashti, N.F.; Salmanpour, F. The amount of antioxidants in honey has a strong relationship with the plants selected by honey bees. Sci. Rep. 2024, 14, 351. [Google Scholar] [CrossRef]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a complementary medicine. Integr. Med. Insights 2017, 12, 1178633717702869. [Google Scholar] [CrossRef]
- Saad, B. History, present and prospect of greco-arab and islamic herbal medicine. In History, Present and Prospect of World Traditional Medicine; World Scientific Pub Co Inc.: Singapore, 2024; pp. 235–300. [Google Scholar]
- Arvia, R.; Biagi, M.; Baini, G.; Cappellucci, G.; Governa, P.; Balatri, S.; Zakrzewska, K. Hylotelephium telephium (L) H. Ohba leaves juice improves herpetic lesions: New findings from in vitro investigations. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Koo, T.H.; Zakaria, A.D.; Mustafa, M.Z. Honey clinical applications in complementary medicine: A critical review. J. Pharm. Pharmacogn. Res. 2024, 12, 1040–1055. [Google Scholar] [CrossRef]
- Naskar, A.; Chatterjee, K.; Roy, K.; Majie, A.; Nair, A.B.; Shinu, P.; Morsy, M.A.; Jain, N.; Pandey, M.; Gorain, B. Mechanistic Roles of Different Varieties of Honey on Wound Healing: Recent Update. J. Pharmacol. Pharmacother. 2024, 15, 5–18. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farich, B.; Masalha, M.; Egbaria, E.; Kmail, A.; El Ghouizi, A.; Ali, D.W.; Lyoussi, B.; Saad, B. Physicochemical properties, chemical composition, antioxidant properties, and antibacterial effects of four palestinian honey varieties. J. Pure Appl. Microbiol. 2024, 2024, 2315–2327. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-based medicinal formulations: A critical review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey microbiota, methods for determining the microbiological composition and the antimicrobial effect of honey–A review. Food Chem. X 2024, 23, 101524. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Izah, S.C. Honey as a Natural Antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef]
- Abu-Farich, B.; Hamarshi, H.; Masalha, M.; Kmail, A.; Aboulghazi, A.; El Ouassete, M.; Imtara, H.; Lyoussi, B.; Saad, B. Polyphenol contents, antibacterial and antioxidant effects of four Palestinian honey samples, and their anticancer effects on Human breast cancer cells. J. Pure Appl. Microbiol. 2024, 18, 1372–1385. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef]
- Zainuddin, A.N.; Mustakim, N.N.; Rosemanzailani, F.A.; Fadilah, N.I.; Maarof, M.; Fauzi, M.B. A comprehensive review of hon-ey-containing hydrogel for wound healing applications. Gels 2025, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.W.; Asif, M.; Ahmed, R.; Khan, A.S.; Raza, R. Pharmacology, nutrition value and therapeutic potential of honey: A review. J. Pharmacogn. Phytochem. 2024, 13, 40–47. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey bee products: Preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, R.A.; Mesaik, M.A. Anti-inflammatory effect of natural honey on bovine thrombin-induced oxidative burst in phagocytes. Phytother. Res. 2009, 23, 801–808. [Google Scholar] [CrossRef]
- Leong, A.G.; Herst, P.M.; Harper, J.L. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012, 18, 459–466. [Google Scholar] [CrossRef]
- Inoue, K.; Murayama, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Identification of phenolic compound in Manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J. Sci. Food Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Chepulis, L.M.; Francis, E. An initial investigation into the anti-inflammatory activity and antioxidant capacity of alphacyclodextrin-complexed Manuka honey. J. Complement. Integr. Med. 2012, 9, 25. [Google Scholar]
- Gasparrini, M.; Afrin, S.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Griswold, D.E.; Adams, J.L. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): Rationale for selective inhibition and progress to date. Med. Res. Rev. 1996, 16, 181–206. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Smith, T.J.; Ho, C.T.; August, D.A.; Yang, C.S. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem. Pharmacol. 2001, 62, 1175–1183. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE(2), TNF-alpha, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 109636. [Google Scholar] [CrossRef]
- Araujo, J.R.; Goncalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef]
- Habtemariam, S. Natural inhibitors of tumour necrosis factor-alpha production, secretion and function. Planta Med. 2000, 66, 303–313. [Google Scholar] [CrossRef]
- Scapagnini, G.; Foresti, R.; Calabrese, V.; Giuffrida Stella, A.M.; Green, C.J.; Motterlini, R. Caffeic acid phenethyl ester and curcumin: A novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002, 61, 554–561. [Google Scholar] [CrossRef]
- Masad, R.J.; Haneefa, S.M.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. The immunomodulatory effects of honey and associated flavonoids in cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef]
- Mihajlovic, D.; Rajkovic, I.; Chinou, I.; Colic, M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 2013, 5, 838–846. [Google Scholar] [CrossRef]
- Effects, A. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616–625. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Park, J.B.; Sung, W.J.; Kwon, Y.-C.; Park, K.-D.; Han, S.M.; et al. Bee venom inhibits Porphyromonas gingivalis lipopolysaccharides-induced pro-inflammatory cytokines through suppression of NF-kB and AP-1 signaling pathways. Molecules 2016, 21, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, Y.; Yan, Y.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Antioxidant and immunomodulatory activities in vitro of polysaccharides from bee collected pollen of Chinese wolfberry. Int. J. Biol. Macromol. 2020, 163, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Valverde, T.M.; Soares, B.N.; Nascimento, A.M.; Andrade, Â.L.; Sousa, L.R.; Vieira, P.M.; Santos, V.R.; Seibert, J.B.; Almeida, T.C.; Rodrigues, C.F.; et al. Anti-Inflammatory, antimicrobial, antioxidant and photoprotective investigation of red propolis extract as sunscreen formulation in polawax cream. Int. J. Mol. Sci. 2023, 24, 5112. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and Its Nutritional and Anti-Inflammatory Value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.-Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of Honey in Improving the Gut Microbial Balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: Physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. BioMed Res. Int. 2019, 2019, 4836378. [Google Scholar] [CrossRef]
- Yu, W.; Sun, F.; Xu, R.; Cui, M.; Liu, Y.; Xie, Q.; Guo, L.; Kong, C.; Li, X.; Guo, X.; et al. Chemical composition and anti-inflammatory activities of Castanopsis honey. Food Funct. 2023, 14, 250–261. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farich, B.; Masalha, M.; Hamarshi, H.; El Ghouizi, A.; Aboulghazi, A.; El Ouassete, M.; Weldali, D.; Lyoussi, B.; Saad, B. In vitro evaluation of Hyphaene thebaica honey as a multitarget therapeutic product. Microbes Immun. 2025, 2, 78–91. [Google Scholar] [CrossRef]
- Ao, X.; Yan, J.; Liu, S.; Chen, S.; Zou, L.; Yang, Y.; He, L.; Li, S.; Liu, A.; Zhao, K. Extraction, isolation and identification of four phenolic compounds from Pleioblastus amarus shoots and their antioxidant and anti-inflammatory properties in vitro. Food Chem. 2022, 374, 131743. [Google Scholar] [CrossRef] [PubMed]
- Boutoub, O.; El-Guendouz, S.; Manhita, A.; Dias, C.B.; Estevinho, L.M.; Paula, V.B.; Carlier, J.; Costa, M.C.; Rodrigues, B.; Raposo, S.; et al. Comparative study of the antioxidant and enzyme inhibitory activities of two types of Moroccan Euphorbia entire honey and their phenolic extracts. Foods 2021, 10, 1909. [Google Scholar] [CrossRef]
- Ooi, T.C.; Yaacob, M.; Rajab, N.F.; Shahar, S.; Sharif, R. The stingless bee honey protects against hydrogen peroxide-induced oxidative damage and lipopolysaccharide-induced inflammation in vitro. Saudi J. Biol. Sci. 2021, 28, 2987–2994. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Adenekan, O.T.; Soladoye, A.O. Effects of honey on inflammation and nitric oxide production in Wistar rats. J. Chin. Integr. Med. 2011, 9, 447–452. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurologia 2024, 39, 292–301. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Żak, N. Polyphenols as the Main Compounds Influencing the Antioxidant Effect of Honey—A Review. Int. J. Mol. Sci. 2024, 25, 10606. [Google Scholar] [CrossRef]
- Dzugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Hashim, K.N.; Chin, K.Y.; Ahmad, F. The mechanism of honey in reversing metabolic syndrome. Molecules 2021, 26, 808. [Google Scholar] [CrossRef] [PubMed]
- Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A comprehensive review of the effect of honey on human health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef]
- Ilia, G.; Simulescu, V.; Merghes, P.; Varan, N. The health benefits of honey as an energy source with antioxidant, antibacterial and antiseptic effects. Sci. Sports 2021, 36, 272-e1–272-e10. [Google Scholar] [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.P. Metabolic syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef]
- Saad, B. A review of the anti-obesity effects of wild edible plants in the mediterranean diet and their active compounds: From traditional uses to action mechanisms and therapeutic targets. Int. J. Mol. Sci. 2023, 24, 12641. [Google Scholar] [CrossRef]
- Saad, B. Management of obesity-related inflammatory and cardiovascular diseases by medicinal plants: From traditional uses to therapeutic targets. Biomedicines 2023, 11, 2204. [Google Scholar] [CrossRef]
- Saad, B. Prevention and treatment of obesity-related inflammatory diseases by edible and medicinal plants and their active compounds. Immuno 2022, 2, 609–629. [Google Scholar] [CrossRef]
- Haider, R.; Mehdi, A.; Das, G.K.; Ahmed, Z.; Zameer, S. Honey for Cardiovascular Diseases. J. Clin. Chem. 2024, 3. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bag, S.; Banerjee, P.; Chatterjee, J. Wound Healing Efficacy of Jamun Honey in Diabetic Mice Model through Reepithelialization, Collagen Deposition and Angiogenesis. J. Tradit. Complement. Med. 2020, 10, 529–543. [Google Scholar] [CrossRef]
- Eteraf-oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar]
- Stewart, J.A.; McGrane, O.L.; Wedmore, I.S. Wound Care in the Wilderness: Is There Evidence for Honey? Wilderness Environ. Med. 2014, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewski, M.; Spyrka, K.; Rojczyk, E.; Brela, J. Topical Application of Manuka Honey for the Treatment of Non-Healing Venous Leg Ulcers. Pharmaceuticals 2025, 18, 149. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M.; Gharehbagheri, A. Natural Honey: A New and Potent Anti-Angiogenic Agent in the Air-Pouch Model of Inflammation. Drug Res. 2013, 64, 530–536. [Google Scholar] [CrossRef]
- Barui, A.; Mandal, N.; Majumder, S.; Das, R.K.; Sengupta, S.; Banerjee, P.; Ray, A.K.; Roychaudhuri, C.; Chatterjee, J. Assessment of Molecular Events during in Vitro Re-Epithelialization under Honey-Alginate Matrix Ambience. Mater. Sci. Eng. C 2013, 33, 3418–3425. [Google Scholar] [CrossRef]
- Tonks, A.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kDa Component of Manuka Honey Stimulates Immune Cells via TLR4. J. Leukoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef]
- Kumar, P.; Rai, S.; Verma, S.K.; Prakash, P.S.; Chitara, D. Classification, mode of action and uses of various immunomodulators. In Immunomodulators and Human Health; Springer Nature: Singapore, 2022; pp. 3–38. [Google Scholar]
- Zebeaman, M.; Tadesse, M.G.; Bachheti, R.K.; Bachheti, A.; Gebeyhu, R.; Chaubey, K.K. Plants and plant-derived molecules as natural immunomodulators. BioMed Res. Int. 2023, 2023, 7711297. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. An updated review of functional ingredients of Manuka honey and their value-added innovations. Food Chem. 2024, 440, 138060. [Google Scholar] [CrossRef]
- Masad, R.J.; Nasser, R.A.; Bashir, G.; Mohamed, Y.A.; Al-Sbiei, A.; Al-Saafeen, B.H.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Characterization of immunomodulatory responses induced by manuka honey. Front. Immunol. 2022, 13, 1020574. [Google Scholar] [CrossRef]
- Masad, R.J.; Idriss, I.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Al-Marzooq, F.; Altahrawi, A.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Oral administration of Manuka honey induces IFNγ-dependent resistance to tumor growth that correlates with beneficial modulation of gut microbiota composition. Front. Immunol. 2024, 15, 1354297. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trac. Trends. Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Cao, Y.H.; Wang, Y.; Yuan, Q. Analysis of flavonoids and phenolic acid in propolis by capillary electrophoresis. Chromatographia 2004, 59, 135–140. [Google Scholar] [CrossRef]
- Awale, S.; Shrestha, S.P.; Tezuka, Y.; Ueda, J.; Matsushige, K.; Kadota, S. Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J. Nat. Prod. 2005, 68, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Campo Fernández, M.; Cuesta-Rubio, O.; Rosado Perez, A.; Montes De Oca Porto, R.; Márquez Hernández, I.; Piccinelli, A.L.; Rastrelli, L. GC-MS determination of isoflavonoids in seven red Cuban propolis samples. J. Agric. Food Chem. 2008, 56, 9927–9932. [Google Scholar] [CrossRef]
- Petrova, A.; Popova, M.; Kuzmanova, C.; Tsvetkova, I.; Naydenski, H.; Muli, E.; Bankova, V. New biologically active compounds from Kenyan propolis. Fitoterapia 2010, 81, 509–514. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends. Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Mo’zdzierz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; del Mar Aguilera-Luiz, M.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. B 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Gadge, A.S.; Shirsat, D.V.; Soumia, P.S.; Pote, C.L.; Pushpalatha, M.; Pandit, T.R.; Dutta, R.; Kumar, S.; Ramesh, S.V.; Mahajan, V.; et al. Physiochemical, biological, and therapeutic uses of stingless bee honey. Front. Sustain. Food Syst. 2024, 7, 1324385. [Google Scholar] [CrossRef]
- Iosageanu, A.; Stefan, L.M.; Craciunescu, O.; Cimpean, A. Anti-Inflammatory and Wound Healing Properties of Different Honey Varieties from Romania and Correlations to Their Composition. Life 2024, 14, 1187. [Google Scholar] [CrossRef]
- McLoone, P.; Tabys, D.; Yunussova, S.; Zhumbayeva, A.; Verrall, S.; Sungurtas, J.; Austin, C.; Allwood, J.W.; McDougall, G.J. Qualitative phytochemical analysis and in vitro investigation of the immunomodulatory properties of honeys produced in Kazakhstan. Nat. Prod. Res. 2023, 37, 996–1001. [Google Scholar] [CrossRef]
- Dekebo, A.; Geba, C.; Bisrat, D.; Jeong, J.B.; Jung, C. Wound Healing, Anti-inflammatory and anti-oxidant activities, and chemical composition of Korean propolis from different sources. Int. J. Mol. Sci. 2024, 25, 11352. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Ran, X. Efficacy and safety of honey dressings in the management of chronic wounds: An updated systematic review and meta-analysis. Nutrients 2024, 16, 2455. [Google Scholar] [CrossRef]
- Antonucci, L.; Locci, C.; Schettini, L.; Clemente, M.G.; Antonucci, R. Infant botulism: An underestimated threat. Infect. Dis. 2021, 53, 647–660. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Royal jelly: Biological action and health benefits. Int. J. Mol. Sci. 2024, 25, 6023. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Bulotta, R.M.; Biondi, V.; Passantino, A.; et al. Hive products: Composition, pharmacological properties, and therapeutic applications. Pharmaceuticals 2024, 17, 646. [Google Scholar] [CrossRef]
- Meo, S.A.; Ansari, M.J.; Sattar, K.; Chaudhary, H.U.; Hajjar, W.; Alasiri, S. Honey and diabetes mellitus: Obstacles and chal-lenges–road to be repaired. Saudi J. Biol. Sci. 2017, 24, 1030–1033. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research progress on therapeutic effect and mechanism of propolis on wound healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef]
- Balasubramaniam, A.K.; Elangovan, A.; Rahman, M.A.; Nayak, S.; Swain, D.; Babu, H.P.; Narasimhan, A.; Monga, V. Propolis: A comprehensive review on the nature’s polyphenolic wonder. Fitoterapia 2025, 183, 106526. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Sadek, K.M.; Shib, N.A.; Taher, E.S.; Rashed, F.; Shukry, M.; Atia, G.A.; Taymour, N.; El-Nablaway, M.; Ibrahim, A.M.; Ramadan, M.M.; et al. Harnessing the power of bee venom for therapeutic and regenerative medical applications: An updated review. Front. Pharmacol. 2024, 15, 1412245. [Google Scholar] [CrossRef]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef]
- Pareek, A.; Mehlawat, K.; Tripathi, K.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Apostolopoulos, V.; Chuturgoon, A. Melittin as a therapeutic agent for rheumatoid arthritis: Mechanistic insights, advanced delivery systems, and future perspectives. Front. Immunol. 2024, 15, 1510693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).