Recurrent SARS-CoV-2 Infection Is Linked to the TLR7 rs179008 Variant and Related to Diminished Baseline T Cell Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Design and Sampling
2.3. Analysis of Single Nucleotide Polymorphisms
2.4. Characterization of Immune Response
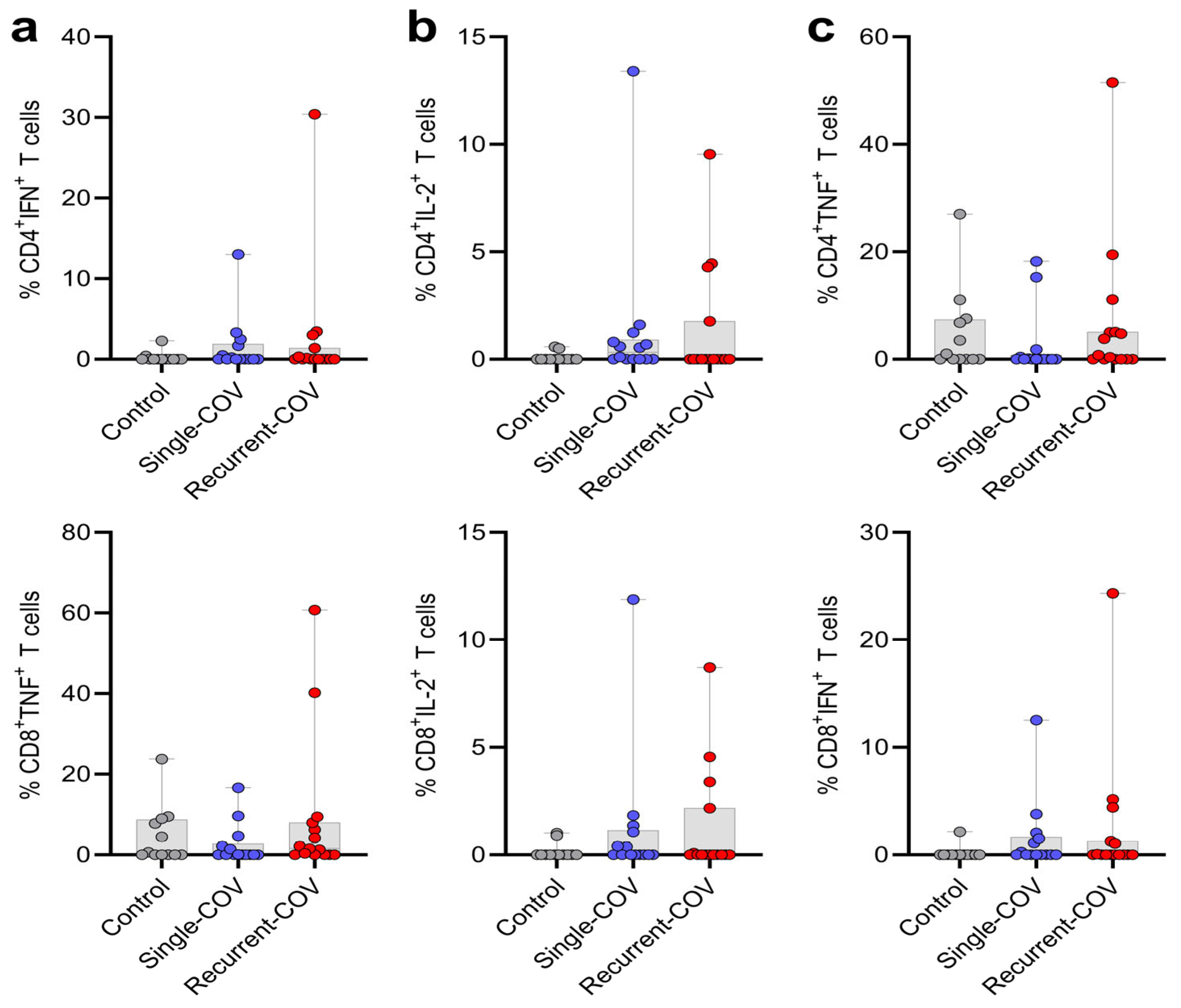
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Kim, S.; Barberia, L.; Ribeiro, A.F.; Gurzenda, S.; Ribeiro, K.B.; Abbott, E.; Blossom, J.; Rache, B.; Singer, B.H. Spatiotemporal Pattern of COVID-19 Spread in Brazil. Science 2021, 372, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Chvatal-Medina, M.; Mendez-Cortina, Y.; Patiño, P.J.; Velilla, P.A.; Rugeles, M.T. Antibody Responses in COVID-19: A Review. Front. Immunol. 2021, 12, 633184. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current Understanding of Its Pathophysiology, Clinical Presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The Pathogenesis and Treatment of the ‘Cytokine Storm’’ in COVID-19’. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Rha, M.S.; Shin, E.C. Activation or Exhaustion of CD8+ T Cells in Patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 2325–2333. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes with Therapeutic Implications. Science 2020, 8511, eabc8511. [Google Scholar] [CrossRef]
- Möhlendick, B.; Schönfelder, K.; Breuckmann, K.; Elsner, C.; Babel, N.; Balfanz, P.; Dahl, E.; Dreher, M.; Fistera, D.; Herbstreit, F.; et al. ACE2 Polymorphism and Susceptibility for SARS-CoV-2 Infection and Severity of COVID-19. Pharmacogenet Genom. 2021, 31, 165–171. [Google Scholar] [CrossRef]
- Jung, H.E.; Lee, H.K. Current Understanding of the Innate Control of Toll-like Receptors in Response to SARS-CoV-2 Infection. Viruses 2021, 13, 2132. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Y.; Wang, H.; Gao, Z.; Wang, Y.; Fang, M.; Shi, S.; Zhang, P.; Wang, H.; Su, Y.; et al. Toll-Like Receptor Signaling in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Innate Immune Responses and the Potential Application Value of Toll-Like Receptor Immunomodulators in Patients with Coronavirus Disease 2019. Front. Microbiol. 2022, 13, 948770. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Sulistiana, R.; Ratnawati, M.; Fibriana, A.I.; Bahrudin, U.; Widyaningrum, D.; Aljunid, S.M. Recurrent SARS-CoV-2 RNA Positivity after COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 20692. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.N.O.; Caldas, G.C.; de Oliveira, F.A.; da Silva, A.M.; da Silva, J.S.; da Silva, R.L.L.; de Jesus, A.R.; Magalhães, L.S.; de Almeida, R.P. COVID-19 Recurrence Is Related to Disease-Early Profile T Cells While Detection of Anti-S1 IgG Is Related to Multifunctional T Cells. Med. Microbiol. Immunol. 2023, 212, 339–347. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.V.; Santos, K.S.; Apostolico, J.S.; Fernandes, E.R.; Almeida, R.R.; Levin, G.; Magawa, J.Y.; Nunes, J.P.S.; Bruni, M.; Yamamoto, M.M.; et al. Recurrence of COVID-19 Associated with Reduced T-Cell Responses in a Monozygotic Twin Pair. Open Biol. 2022, 12, 210240. [Google Scholar] [CrossRef]
- Adrielle dos Santos, L.; Filho, P.G.d.G.; Silva, A.M.F.; Santos, J.V.G.; Santos, D.S.; Aquino, M.M.; de Jesus, R.M.; Almeida, M.L.D.; da Silva, J.S.; Altmann, D.M.; et al. Recurrent COVID-19 Including Evidence of Reinfection and Enhanced Severity in Thirty Brazilian Healthcare Workers. J. Infect. 2021, 82, 399–406. [Google Scholar] [CrossRef]
- Santos, C.N.O.; Magalhães, L.S.; Fonseca, A.B.d.L.; Bispo, A.J.B.; Porto, R.L.S.; Alves, J.C.; dos Santos, C.A.; de Carvalho, J.V.; da Silva, A.M.; Teixeira, M.M.; et al. Association between Genetic Variants in TREM1, CXCL10, IL4, CXCL8 and TLR7 Genes with the Occurrence of Congenital Zika Syndrome and Severe Microcephaly. Sci. Rep. 2023, 13, 3466. [Google Scholar] [CrossRef]
- Franco, K.G.S.; de Amorim, F.J.R.; Santos, M.A.; Rollemberg, C.V.V.; de Oliveira, F.A.; França, A.V.C.; Santos, C.N.O.; Magalhães, L.S.; Cazzaniga, R.A.; de Lima, F.S.; et al. Association of IL-9, IL-10, and IL-17 Cytokines with Hepatic Fibrosis in Human Schistosoma mansoni Infection. Front. Immunol. 2021, 12, 779534. [Google Scholar] [CrossRef]
- Magalhães, L.S.; Melo, E.V.; Damascena, N.P.; Albuquerque, A.C.B.; Santos, C.N.O.; Rebouças, M.C.; Bezerra, M.D.O.; Louzada da Silva, R.; de Oliveira, F.A.; Santos, P.L.; et al. Use of N-Acetylcysteine as Treatment Adjuvant Regulates Immune Response in Visceral Leishmaniasis: Pilot Clinical Trial and in vitro Experiments. Front. Cell. Infect. Microbiol. 2022, 12, 1045668. [Google Scholar] [CrossRef]
- Bagno, F.F.; Sérgio, S.A.R.; Figueiredo, M.M.; Godoi, L.C.; Andrade, L.A.F.; Salazar, N.C.; Soares, C.P.; Aguiar, A.; Almeida, F.J.; da Silva, E.D.; et al. Development and Validation of an Enzyme-Linked Immunoassay Kit for Diagnosis and Surveillance of COVID-19. J. Clin. Virol. Plus 2022, 2, 100101. [Google Scholar] [CrossRef]
- Fardo, D.W.; Becker, K.D.; Bertram, L.; Tanzi, R.E.; Lange, C. Recovering Unused Information in Genome-Wide Association Studies: The Benefit of Analyzing SNPs out of Hardy-Weinberg Equilibrium. Eur. J. Hum. Genet. 2009, 17, 1676–1682. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Azar, P.; Mejía, J.E.; Cenac, C.; Shaiykova, A.; Youness, A.; Laffont, S.; Essat, A.; Izopet, J.; Passaes, C.; Müller-Trutwin, M.; et al. TLR7 Dosage Polymorphism Shapes Interferogenesis and HIV-1 Acute Viremia in Women. JCI Insight 2020, 5, 136047. [Google Scholar] [CrossRef] [PubMed]
- Naushad, S.M.; Mandadapu, G.; Ramaiah, M.J.; Almajhdi, F.N.; Hussain, T. The Role of TLR7 Agonists in Modulating COVID-19 Severity in Subjects with Loss-of-Function TLR7 Variants. Sci. Rep. 2023, 13, 13078. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.S.; Bentes, A.A.; Diniz, L.M.; Carvalho, S.H.; Kroon, E.G.; Campos, M.A. Association Between Single-Nucleotide Polymorphisms in Toll-like Receptor 3 (Tlr3), Tlr7, Tlr8 and Tirap Genes with Severe Symptoms in Children Presenting COVID-19. Viruses 2024, 17, 35. [Google Scholar] [CrossRef]
- Singh, H.; Samani, D.; Aggarwal, S. TLR7 Polymorphism (Rs179008 and Rs179009) in HIV-Infected Individual Naïve to ART. Mediat. Inflamm. 2020, 2020, 6702169. [Google Scholar] [CrossRef]
- Guéry, J.C. Sex Differences in Primary HIV Infection: Revisiting the Role of TLR7-Driven Type 1 IFN Production by Plasmacytoid Dendritic Cells in Women. Front. Immunol. 2021, 12, 729233. [Google Scholar] [CrossRef]
- Buschow, S.I.; Biesta, P.J.; Groothuismink, Z.M.A.; Erler, N.S.; Vanwolleghem, T.; Ho, E.; Najera, I.; Ait-Goughoulte, M.; de Knegt, R.J.; Boonstra, A.; et al. TLR7 Polymorphism, Sex and Chronic HBV Infection Influence Plasmacytoid DC Maturation by TLR7 Ligands. Antivir. Res. 2018, 157, 27–37. [Google Scholar] [CrossRef]
- Santana, E.G.M.; Ferreira, F.d.S.; Brito, W.R.d.S.; Lopes, F.T.; de Lima, A.C.R.; Neto, G.d.S.P.; Amoras, E.d.S.G.; Lima, S.S.; da Costa, C.A.; Souza, M.S.; et al. TLR7 Rs179008 (A/T) and TLR7 Rs3853839 (C/G) Polymorphisms Are Associated with Variations in IFN-α Levels in HTLV-1 Infection. Front. Immunol. 2024, 15, 1462352. [Google Scholar] [CrossRef]
- Askar, E.; Ramadori, G.; Mihm, S. Toll-like Receptor 7 Rs179008/Gln11Leu Gene Variants in Chronic Hepatitis C Virus Infection. J. Med. Virol. 2010, 82, 1859–1868. [Google Scholar] [CrossRef]
- Rubtsova, K.; Rubtsov, A.V.; Halemano, K.; Li, S.X.; Kappler, J.W.; Santiago, M.L.; Marrack, P. T Cell Production of IFNy in Response to TLR7/IL-12 Stimulates Optimal B Cell Responses to Viruses. PLoS ONE 2016, 11, e0166322. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y.; Liu, J.; Huang, X.; Zhang, X.; Kirschning, C.; Xu, H.C.; Lang, P.A.; Dittmer, U.; Zhang, E.; et al. Toll-Like Receptor 7 Activation Enhances CD8+ T Cell Effector Functions by Promoting Cellular Glycolysis. Front. Immunol. 2019, 10, 2191. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Gautron, A.S.; De Marcken, M.; Keller, M.J.; Hafler, D.A. TLR7 Induces Anergy in Human CD4+ T Cells. Nat. Immunol. 2015, 16, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-Specific Recognition of Single-Stranded RNA via Till-like Receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Xing, J.; Chen, D.; Fang, C.; Mo, F.; Gong, Y.; Tan, Z.; Liang, G.; Xiao, W.; et al. TLR7 Modulates Extramedullary Splenic Erythropoiesis in P. Yoelii NSM-Infected Mice through the Regulation of Iron Metabolism of Macrophages with IFN-γ. Front. Immunol. 2023, 14, 1123074. [Google Scholar] [CrossRef]
- Wang, C.; Khatun, M.S.; Ellsworth, C.R.; Chen, Z.; Islamuddin, M.; Nisperuza Vidal, A.K.; Afaque Alam, M.; Liu, S.; Mccombs, J.E.; Maness, N.J.; et al. Deficiency of Tlr7 and Irf7 in Mice Increases the Severity of COVID-19 through the Reduced Interferon Production. Commun. Biol. 2024, 7, 1162. [Google Scholar] [CrossRef]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.Y.; Norris, C.M.; Oudit, G.Y. Sex Differences in COVID-19: Candidate Pathways, Genetics of ACE2, and Sex Hormones. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef]
- Fallerini, C.; Daga, S.; Mantovani, S.; Benetti, E.; Picchiotti, N.; Francisci, D.; Paciosi, F.; Schiaroli, E.; Baldassarri, M.; Fava, F.; et al. Association of Toll-like Receptor 7 Variants with Life-Threatening COVID-19 Disease in Males: Findings from a Nested Case-Control Study. eLife 2021, 10, e67569. [Google Scholar] [CrossRef]

| Model | Genotype | Control n (%) | Case n (%) | OR | Lower CI | Upper CI | p-Value | AIC |
|---|---|---|---|---|---|---|---|---|
| Codominant | A/A or A | 20 (76.9) | 13 (65) | 1.0 | 0.01663 | 59.8 | ||
| A/T | 2 (7.7) | 7 (35) | 5.38 | 0.96 | 30.06 | |||
| T/T or T | 4 (15.4) | 0 | 0.0 | 0.0 | 0.0 | |||
| Dominant | A/A or A | 20 (76.9) | 13 (65) | 1.0 | 0.3746 | 66.2 | ||
| A/T-T/T or T | 6 (23.1) | 7 (35) | 1.79 | 0.49 | 6.55 | |||
| Recessive | A/A or A-A/T | 22 (84.6) | 20 (100) | 1.0 | 0.1213 | 62.1 | ||
| T/T or T | 4 (15.4) | 0 | 0.0 | 0.0 | 0.0 | |||
| Overdominant | A/A or A-T/T or T | 24 (92.3) | 13 (65) | 1.0 | 0.01926 | 61.5 | ||
| A/T | 2 (7.7) | 7 (35) | 6.46 | 1.17 | 35.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.N.O.; dos Santos, P.L.; da Silva, A.M.; Magalhães, L.S. Recurrent SARS-CoV-2 Infection Is Linked to the TLR7 rs179008 Variant and Related to Diminished Baseline T Cell Immunity. Immuno 2025, 5, 17. https://doi.org/10.3390/immuno5020017
Santos CNO, dos Santos PL, da Silva AM, Magalhães LS. Recurrent SARS-CoV-2 Infection Is Linked to the TLR7 rs179008 Variant and Related to Diminished Baseline T Cell Immunity. Immuno. 2025; 5(2):17. https://doi.org/10.3390/immuno5020017
Chicago/Turabian StyleSantos, Camilla Natália Oliveira, Priscila Lima dos Santos, Angela Maria da Silva, and Lucas Sousa Magalhães. 2025. "Recurrent SARS-CoV-2 Infection Is Linked to the TLR7 rs179008 Variant and Related to Diminished Baseline T Cell Immunity" Immuno 5, no. 2: 17. https://doi.org/10.3390/immuno5020017
APA StyleSantos, C. N. O., dos Santos, P. L., da Silva, A. M., & Magalhães, L. S. (2025). Recurrent SARS-CoV-2 Infection Is Linked to the TLR7 rs179008 Variant and Related to Diminished Baseline T Cell Immunity. Immuno, 5(2), 17. https://doi.org/10.3390/immuno5020017





