Abstract
A civil paternity investigation involving the parents of the deceased alleged father in order to establish a family relationship is presented. On the basis of the 23 autosomal short tandem repeat (aSTR) genotyping results, conclusive proof of paternity was not achieved, as the probability of paternity (W) was calculated to 0.99988. Additional genetic data of 17 classical and non-classical human leukocyte alleles (HLA) typing by next-generation sequencing (NGS) at a high-resolution level supported the hypothesis of grandpaternity over the hypothesis of coincidental paternal obligate allele (POA) sharing (total WaSTR&HLA = 0.9999998). The present study demonstrates the utility of 17 HLA genetic markers-typing in the solution of deficiency cases of disputed parentage.
1. Introduction
When an alleged father (AF) is not directly able to be tested, the father’s biological parents can perform a DNA grandparent test to help determine if he is the true father of a child. In such cases, the 23 aSTR loci analyzed may not provide adequate data to conclusively prove the relationship between grandparent–offspring. To overcome these problems, additional genetic markers in kinship parenthood testing are required [1,2].
2. Materials and Methods
A civil indirect relationship paternity case involving a putative father who had died in an automobile accident was assigned to our laboratory for testing. This case was requested by private individuals apart from the child in question; both of the deceased’s parents were submitted for testing to prove or disprove the suspected hypotheses. The mother of the child refused to provide a sample in order to be tested. Determination of paternity was attempted following a number of approaches to ensure the result was as accurate as possible, after obtaining informed consent for genetic studies from all participants prior to the sample collection. The genetic information of the pedigree is given in Figure 1.
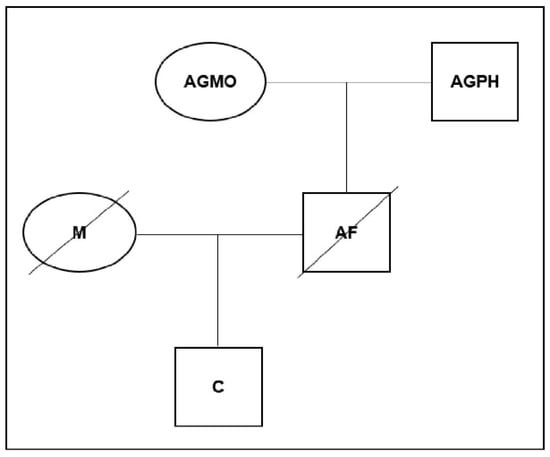
Figure 1.
Schematic pedigree for the kinship testing.
DNA was obtained from blood samples and/or buccal swabs from all individuals using the Maxwell® 16 Blood DNA Purification Kit and Maxwell® 16 Buccal Swab LEV DNA Kit, respectively (Maxwell Promega, Madison, WI, USA). In a first approach, all individuals were typed in the genetic marker system composed of the 16 autosomal STR loci, plus amelogenin for gender identification, applying the AmpFlSTR® NGM SElect™ PCR Amplification Kit (D10S1248, vWA, D16S539, D2S1338, D8S1179, D21S11, D18S51, D22S1045, D19S433, TH01, FGA, D2S441, D3S1358, D1S1656, D12S391, SE33) (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s instruction. The PowerPlex®16 HS System (Promega) was carried out, increasing the number of aSTR loci to 23 by 7 additional aSTR loci (Penta E, D5S818, D13S317, D7S820, CSF1PO, Penta D, TPOX). The amplified STR fragments were separated on an ABI 3130 Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). The data analysis and genotyping were automatically assigned by the GeneMapper ID-X Software v1.3 (Applied Biosystems). In order to increase the strength of the genetic evidence, further analysis of samples by HLA genotyping (17 classical HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, -DPA1, -DPB1, and non-classical HLA-F, -G, -H, -E genes, MICA and MICB) was performed using commercial locus-specific primers supplied by CareDX (AlloSeq Tx17 kit) on the Illumina MiSeq platform (Illumina, San Diego, CA, USA). Libraries were quantified by the Qubit 1X dsDNA High-Sensitivity Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The raw sequencing data (FASTQ files) were analyzed by the AlloSeq Assign analysis software Tx17.1 v1.0.3 (CareDX, Stockholm, Sweden) with references from the Immuno Polymorphism Database-ImMunoGeneTics project/HLA Database (IPD-IMGT/HLA database) v3.45.1.1 (https://www.ebi.ac.uk/ipd/imgt/hla/).
In this study, in order to establish the kinship relationship between the grandparents and the child in question, the statistical parameters of power of exclusion (PE), random grandparents not excluded (RGPNE), a random man not excluded (RMNE) and the probability of paternity (W) for aSTRs, were performed by the Buckleton values [3,4]. For HLA, the statistical parameter paternity index (PI) and probability of paternity (W) were performed by Essen-Möller values [5]. The PI is the likelihood ratio (LR), which is calculated by the mathematical formula LR = 1/p (where p is the frequency of HLA haplotype) assuming a 50% probability a priori. The posterior probability of paternity, denoted as W by Essen-Möller, is then calculated by the W = LR/(LR + 1) formula. The likelihood calculations were based upon allelic frequencies as estimated from Caucasoid (for aSTRs) [6] and Greek (for HLA) population databases, respectively [7].
3. Results and Discussion
A set of 25 aSTR loci is generally used for paternity/kinship analysis. However, the number of markers analyzed may be insufficient to conclusively prove the relationship between individuals on a second-degree kinship analysis. To overcome these problems, additional markers need to be examined, such as gonosomal X-STRs/Y-STRs, single nucleotide polymorphism (SNP) markers or human leukocyte antigens (HLA) [8,9,10].
In this case, the alleged grandparents (AGPs) were not excluded from biologic grandparentage as either one of the two, or both, AGPs harbor the paternal obligate alleles (POA). The possible STR genotype of the alleged father (AF) was deduced and the probability of paternity was calculated. The aSTR analysis of the results showed no exclusion of paternity, given the fact that the child in question had inherited all of the POA with a probability value of about 8671, which is weak data to support the hypothesis that the putative grandparents were the biological grandparents of the child. In particular, the combined PE was 0.9999999795, RGPNE = 1.48 × 10−4 (a reliable equation to determine the power of a genetic test to exclude a pair of individuals as grandparents; the cumulative RGPNE values <0.01 indicate that >99% of random pairs of individuals are excluded from grandparentage) [3,10]. The RMNE = 2.05 × 10−8, which yielded a 0.99988 probability of paternity. In this case, the single locus RGPNE values are higher than the RMNE values, illustrating the fact that it is more difficult to exclude pairs of individuals from grandparentage than to exclude single individuals from parentage. The total LR (8671) value also represents a likelihood that the genetic data support the hypothesis of grandpaternity over the hypothesis of coincidental POA-sharing (when the LR value is between 1000 and 10,000, the verbal equivalent is “strong support”). Table 1 and Table 2 indicate the analysis results of 16 aSTR loci of the AmpFlSTR® NGM SElect™ Kit (Applied Biosystems) and an additional 7 aSTR loci from the PowerPlex®16 HS System (Promega), respectively.

Table 1.
Genotypes and frequencies of the 16 aSTR alleles transmitted by the deceased father, applying the AmpFlSTR® NGM SElect™ PCR Amplification Kit (Applied Biosystems).

Table 2.
Genotypes and frequencies of the 7 additional aSTR alleles transmitted by the deceased father applying the PowerPlex®16 HS System (Promega).
So, further investigation was carried out by amplifying 17 classical and non-classical linked HLA loci located on the short arm of chromosome 6, inherited as an HLA haplotype in a Mendelian fashion from each parent, in order to check their paternity affiliation [11]. The HLA genotyping revealed no mismatches between the child and the alleged grandfather (AGPH), giving a paternity index of 500 and a probability of paternity equal to 0.9980 regarding 7 classical HLA-A, -B, -C, -DRB1, -DQA1, -DQB1, -DPB1 genes. (Table 3) The genetic markers HLA-DRB3, -DRB4, -DRB5, -DPA, -F, -G, -H, -E, MICA and MICB were not included in the statistical analysis as there are no allele frequency data in the Greek population. The HLA inherited haplotype from the AGPH was HLA-A*11:01:01, ~B*57:01:01, ~C*06:02:01, ~DRB1*03:01:01, ~DRB3*01:01:02, ~DQA1*05:01:01G, ~DQB1*02:01:01G, ~DPA1*01:03:01, ~DPB1*03:01:01G, ~F*01:01:02, ~G*01:01:03, ~H*02:07:01, ~E*01:03:02 and MICA 017, MICB 003:01. (Frequency of possible HLA haplotype ~A*11:01, ~B*57:01, ~C*06:02, ~DRB1*03:01, ~DQA1*05:01, ~DQB1*02:01, ~DPB1*03:01, transmitted by the father according to the Greek population database, f = 0.001) [7].

Table 3.
Genotypes of the HLA alleles transmitted by the deceased father by applying the AlloSeq Tx17 Kit (CareDX).
Combining the LR values obtained from the two systems (23 aSTR markers and 7 linked HLA loci), and assuming a 50% probability a priori, the arrived value of W was 0.9999998, which is sufficient for the verbal predicate “paternity practically proven”. Regarding W and CPI, there was a 99.99998% chance of excluding falsely the alleged father, because he was approximately 4,335,500 times as likely to be the father of the child in question as an unrelated Greek male.
In conclusion, conventional aSTR genotyping by 23 aSTR loci alone did not provide conclusive proof of paternity in this type of “indirect” paternity. In contrast, typing of the additional seven linked HLA loci gave a final probability (W) of 0.9999998, which provides “extremely strong support” for paternity. The present study demonstrates that testing for 17 classical and non-classical HLA loci by NGS greatly facilitates the solution of a deficiency case that would be difficult or time-consuming to solve by conventional aSTR markers-typing alone, as they contribute an additional power of exclusion for the identification of the parent–offspring relationship. The genetic evidence for true paternity based on two polymorphism systems (aSTR and HLA) can be considered reliable in this complex kinship analysis; it offers a high level of information, and also diminishes the possibility of false exclusion or minimizes the risk of wrong inclusion. Furthermore, before establishing a conclusion in a presumptive relationship between the parent and offspring, including the biological mother in civil paternity examinations involving the parents of the alleged father significantly strengthens the test results. Otherwise, in order to increase the validity of statistical evidence, additional genetic markers should be included [12,13,14].
Author Contributions
D.K. analyzed sequencing data and contributed to the writing–reviewing of the manuscript. A.T. contributed to the writing–reviewing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent for the genetic studies was obtained from all participants prior to the sample collection.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Zhou, Z.; Wang, L.; Quan, C.; Liu, Q.; Tang, Z.; Liu, L.; Liu, Y.; Wang, S. Pairwise kinship testing with a combination of STR and SNP loci. Forensic Sci. Int. Genet. 2020, 46, 102265. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, Q.; Ma, G.; Chen, Z.; Liu, Q.; Fu, L.; Cong, B.; Li, S. Utility of ForenSeq™ DNA Signature Prep Kit in the research of pairwise 2nd-degree kinship identification. Int. J. Legal Med. 2019, 133, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Scarpetta, M.A.; Staub, R.W.; Einum, D.D. Assessing exclusionary power of a paternity test involving a pair of alleged grandparents. Transfusion 2007, 47, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Trindade-Filho, A.; Ferreira, S.; Oliveira, S.F. Impact of a chromosome X STR Decaplex in deficiency paternity cases. Genet. Mol. Biol. 2013, 36, 507–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Egeland, T.; Mostad, P.F. Statistical Genetics and Genetical Statistics: A Forensic Perspective. Scand. J. Statist. 2002, 29, 297–307. [Google Scholar] [CrossRef]
- Steffen, C.R.; Coble, M.D.; Gettings, K.B.; Vallone, P.M. Corrigendum to ′U.S. Population Data for 29 Autosomal STR Loci′ [Forensic Sci. Int. Genet. 7 (2013) e82-e83]. Forensic Sci. Int. Genet. 2017, 31, e36–e40. [Google Scholar] [CrossRef] [PubMed]
- Dinou, A.; Chatzistamatiou, T.; Andreaki, M.; Michalopoulos, E.; Spyropoulou-Vlachou, M.; Stavropoulos-Giokas, C. High resolution analysis of the hellenic population’s HLA repertoire based on the hellenic cord blood bank inventory: Common alleles and haplotypes. HLA 2022. submitted. [Google Scholar]
- Kayser, M. Forensic use of Y-chromosome DNA: A general overview. Hum. Genet. 2017, 136, 621–635. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Peng, D.; Hao, B.; Wang, Z.; Huang, E.; Wu, R.; Sun, H. Improved pairwise kinship analysis using massively parallel sequencing. Forensic Sci. Int. Genet. 2019, 38, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.K.; Ren, Z.L.; Yang, Y.R.; Liu, Y.C.; Zhang, J.J.; Wu, H.J.; Li, Z.; Bo, X.C.; Wang, S.Q.; Yan, J.W.; et al. A 472-SNP panel for pairwise kinship testing of second-degree relatives. Forensic Sci. Int. Genet. 2018, 34, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.; Chabot-Richards, D. HLA testing in the molecular diagnostic laboratory. Virchows Arch. 2019, 474, 139–147. [Google Scholar] [CrossRef]
- Tao, R.; Xu, Q.; Wang, S.; Xia, R.; Yang, Q.; Chen, A.; Qu, Y.; Lv, Y.; Zhang, S.; Li, C. Pairwise kinship analysis of 17 pedigrees using massively parallel sequencing. Forensic Sci. Int. Genet. 2022, 57, 102647. [Google Scholar] [CrossRef] [PubMed]
- Apaga, D.L.T.; Dennis, S.E.; Salvador, J.M.; Calacal, G.C.; De Ungria, M.C.A. Comparison of Two massively parallel sequencing platforms using 83 single nucleotide polymorphisms for human identification. Sci. Rep. 2017, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Kaitholia, K.; Dash, H.R.; Shrivastava, P.; Kumawat, R.K.; Dixit, S.; Chaubey, G. Forensic characterization and genetic evaluation in the Central Indian population using 27 Y-STRs. Int. J. Legal Med. 2021, 135, 791–792. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).