Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma
Abstract
1. Introduction
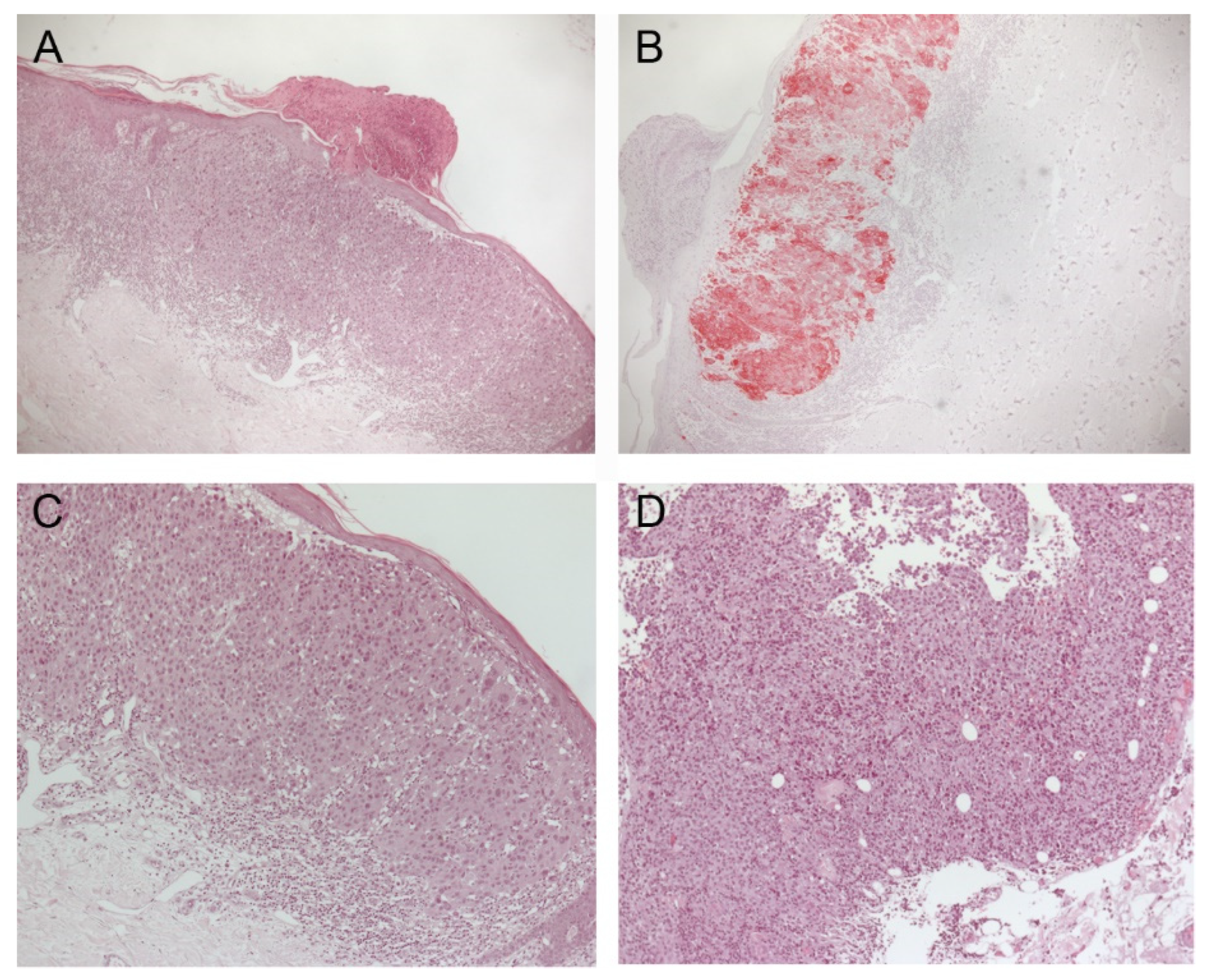
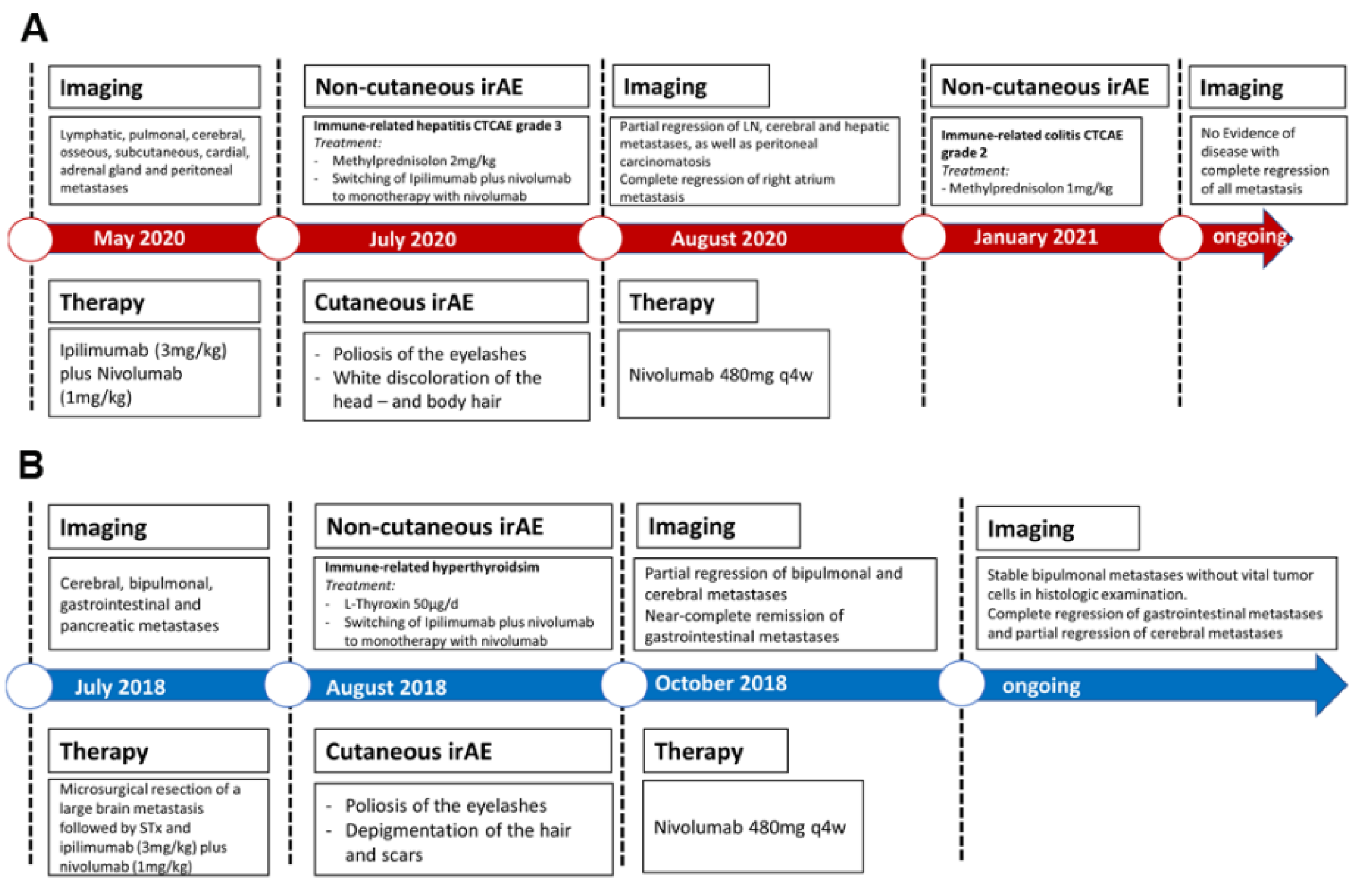
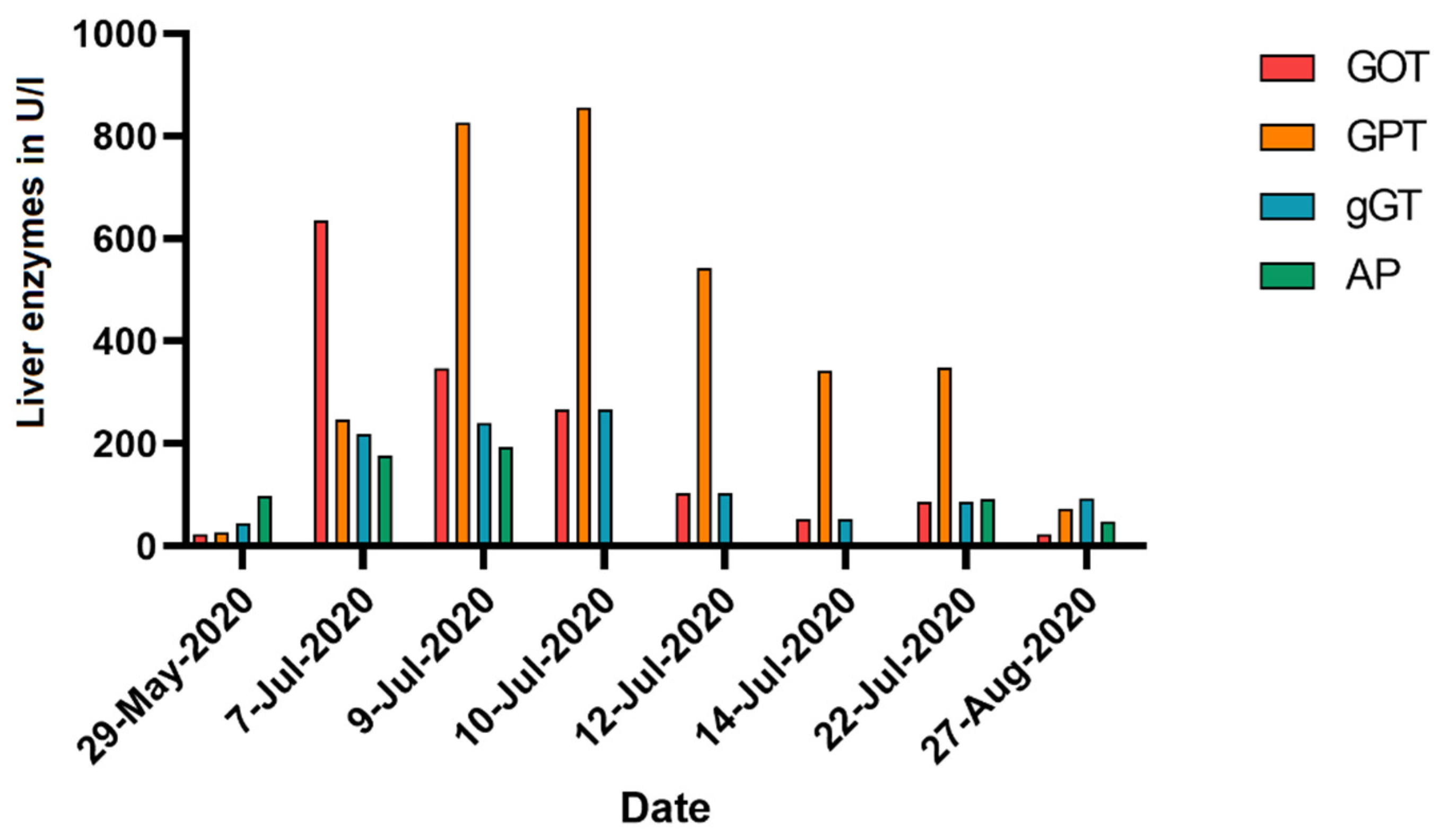
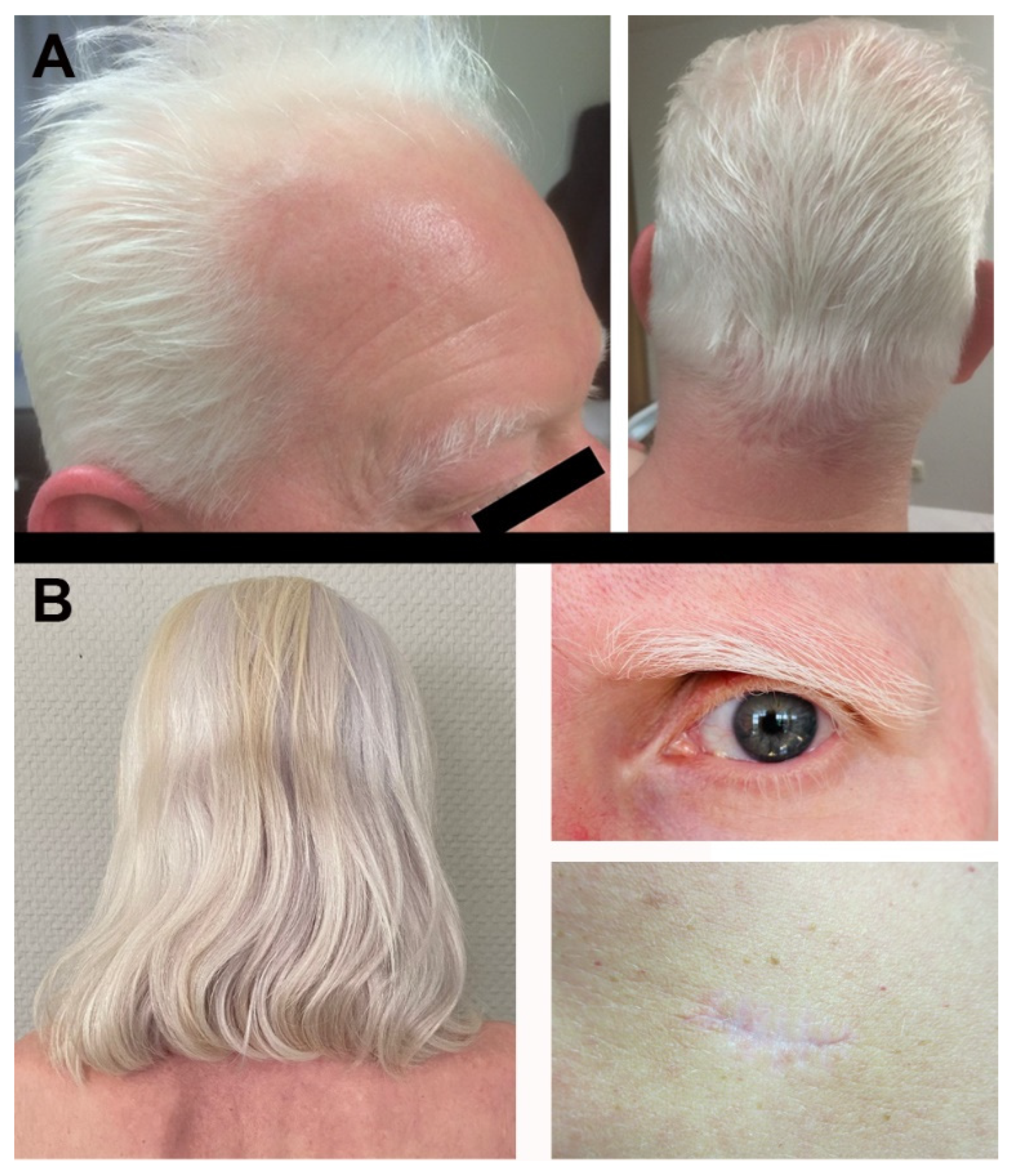
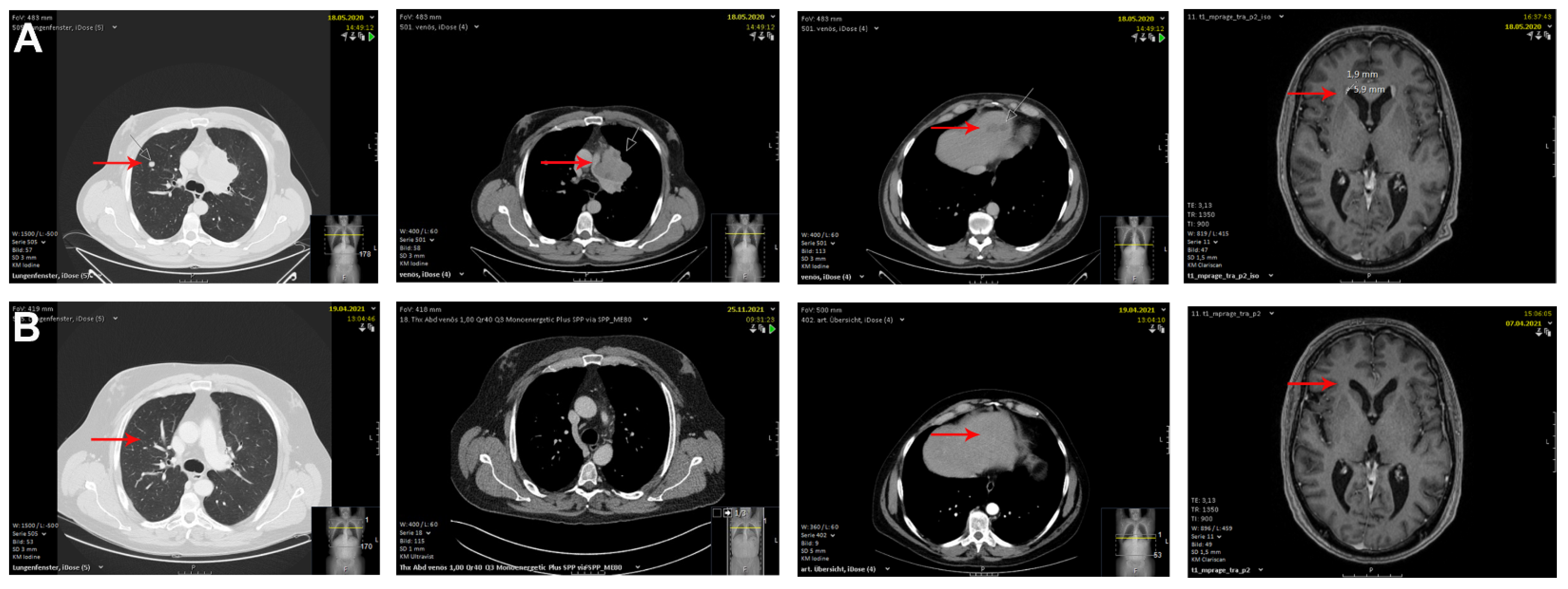
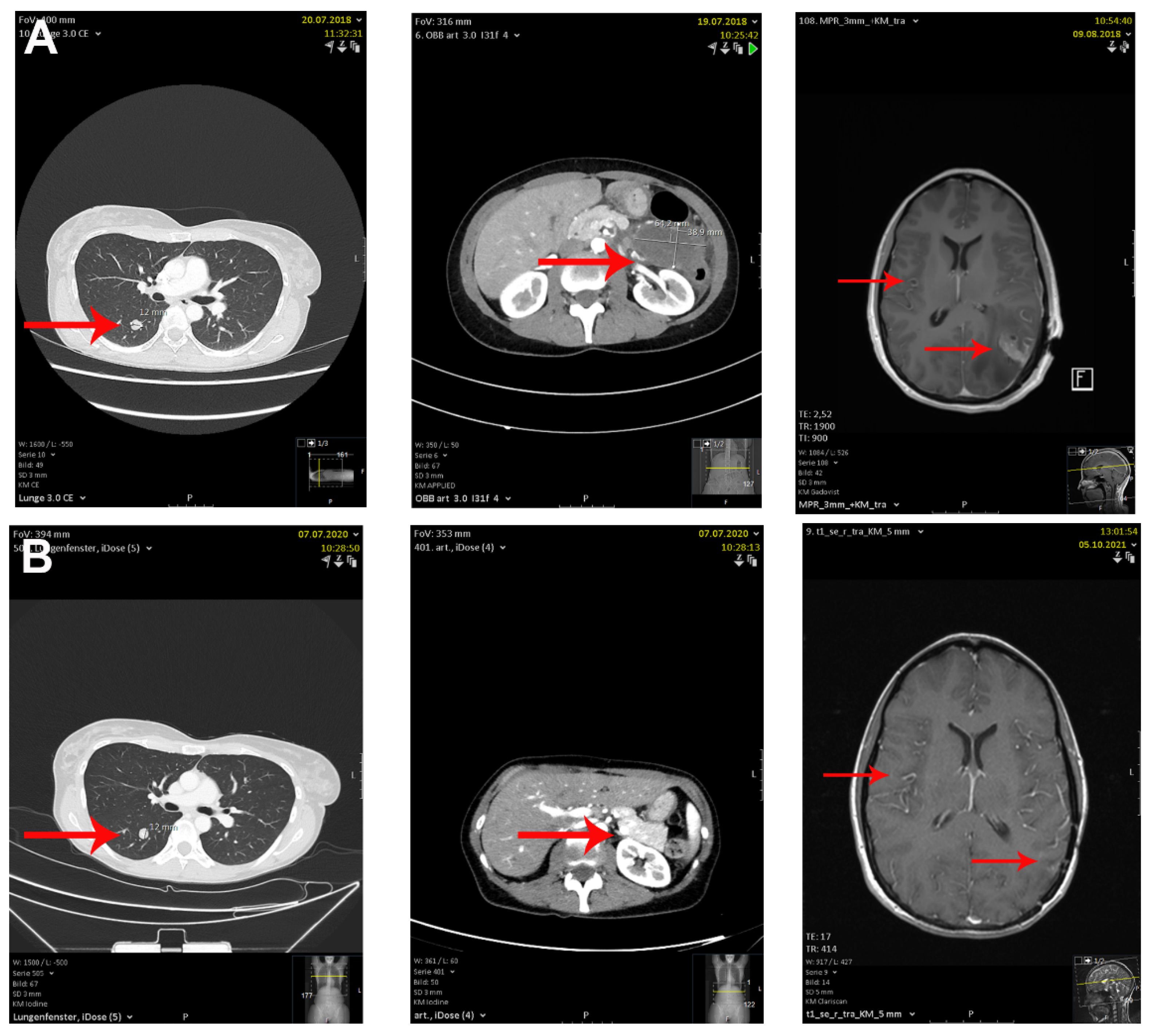
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Matull, J.; Livingstone, E.; Wetter, A.; Zimmer, L.; Zaremba, A.; Lahner, H.; Schadendorf, D.; Ugurel, S. Durable Complete Response in a Melanoma Patient with Unknown Primary, Associated with Sequential and Severe Multi-Organ Toxicity After a Single Dose of CTLA-4 Plus PD-1 Blockade: A Case Report. Front. Oncol. 2020, 10, 592609. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.J.; Carlino, M.S. Immune Checkpoint Inhibitor Toxicity. Curr. Oncol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Gault, A.; Anderson, A.E.; Plummer, R.; Stewart, C.; Pratt, A.G.; Rajan, N. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br. J. Dermatol. 2021, 185, 263–271. [Google Scholar] [CrossRef]
- Mandalà, M.; Larkin, J.M.G.; Ascierto, P.A.; Del Vecchio, M.; Gogas, H.; Cowey, C.L.; Arance Fernandez, A.N.A.M.; Dalle, S.; Schenker, M.; Grob, J.J.; et al. An analysis of nivolumab-mediated adverse events and association with clinical efficacy in resected stage III or IV melanoma (CheckMate 238). J. Clin. Oncol. 2019, 37, 9584. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Robert, C.; Hwu, W.J.; Hamid, O.; Ribas, A.; Weber, J.S.; Daud, A.I.; Hodi, F.S.; Wolchok, J.D.; Mitchell, T.C.; Hersey, P.; et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur. J. Cancer 2021, 144, 182–191. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival among Patients with Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2021. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Goppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Burzi, L.; Alessandrini, A.M.; Quaglino, P.; Piraccini, B.M.; Dika, E.; Ribero, S. Cutaneous Events Associated with Immunotherapy of Melanoma: A Review. J. Clin. Med. 2021, 10, 3047. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.H.; Alhumayed, M. Primary orbital melanoma with poliosis and a palpable mass. Arch. Ophthalmol. 2011, 129, 1382–1383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ediriwickrema, L.S.; Liu, C.Y.; Kikkawa, D.O.; Korn, B.S. Development of Poliosis Following Checkpoint Inhibitor Treatment for Cutaneous Melanoma. Ophthalmic Plast Reconstr. Surg. 2019, 35, e121–e122. [Google Scholar] [CrossRef]
- Failla, C.M.; Carbone, M.L.; Fortes, C.; Pagnanelli, G.; D’Atri, S. Melanoma and Vitiligo: In Good Company. Int. J. Mol. Sci. 2019, 20, 5731. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Hosoya, K.; Fujimoto, D.; Morimoto, T.; Kumagai, T.; Tamiya, A.; Taniguchi, Y.; Yokoyama, T.; Ishida, T.; Hirano, K.; Matsumoto, H.; et al. Association Between Early Immune-related Adverse Events and Clinical Outcomes in Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2020, 21, e315–e328. [Google Scholar] [CrossRef]
- Wolner, Z.J.; Marghoob, A.A.; Pulitzer, M.P.; Postow, M.A.; Marchetti, M.A. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br. J. Dermatol. 2018, 178, 265–269. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Levenig, C.; Berger, J. Vitiligo and cutaneous melanoma. A case study. Dermatologica 1991, 183, 239–245. [Google Scholar] [CrossRef]
- Blessing, K.; McLaren, K.M. Histological regression in primary cutaneous melanoma: Recognition, prevalence and significance. Histopathology 1992, 20, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Aung, P.P.; Nagarajan, P.; Prieto, V.G. Regression in primary cutaneous melanoma: Etiopathogenesis and clinical significance. Lab. Investig. 2017, 97, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Del Fiore, P.; Rastrelli, M.; Dall’Olmo, L.; Cavallin, F.; Cappellesso, R.; Vecchiato, A.; Buja, A.; Spina, R.; Parisi, A.; Mazzarotto, R.; et al. Melanoma of Unknown Primary: Evaluation of the Characteristics, Treatment Strategies, Prognostic Factors in a Monocentric Retrospective Study. Front. Oncol. 2021, 11, 627527. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Quach, H.T.; Dewan, A.K.; Davis, E.J.; Ancell, K.K.; Fan, R.; Ye, F.; Johnson, D.B. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects with Outcomes in Patients with Advanced Melanoma. JAMA Oncol. 2019, 5, 906–908. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Asami, Y.; Teramoto, Y.; Imamura, T.; Sato, S.; Maruyama, H.; Fujisawa, Y.; Matsuya, T.; Fujimoto, M.; et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo with Tumor Response in Patients with Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef]
- Amini Adle, M.; Chastagner, M.; Mansard, S.; Dalle, S. Image Gallery: Unilateral eyebrow depigmentation. Br. J. Dermatol. 2019, 180, e107. [Google Scholar] [CrossRef]
- Thomas, S.; Laino, A.; Sturm, R.; Nufer, K.; Lambie, D.; Shepherd, B.; Atkinson, V.; Adams, L.; Soyer, H.P.; Schaider, H. Focal regression of a primary melanoma, fading lentigines and poliosis in metastatic melanoma treated with anti-PD-1. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e176–e177. [Google Scholar] [CrossRef]
- Zarbo, A.; Belum, V.R.; Sibaud, V.; Oudard, S.; Postow, M.A.; Hsieh, J.J.; Motzer, R.J.; Busam, K.J.; Lacouture, M.E. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2017, 176, 1649–1652. [Google Scholar] [CrossRef]
- Gambichler, T.; Seifert, C.; Lehmann, M.; Lukas, C.; Scheel, C.; Susok, L. Concurrent Vogt-Koyanagi-Harada disease and impressive response to immune checkpoint blockade in metastatic melanoma. Immunotherapy 2020, 12, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Fusumae, T.; Kamiya, K.; Maekawa, T.; Komine, M.; Murata, S.; Inoda, S.; Takahashi, R.; Kawashima, H.; Ohtsuki, M. Vogt-Koyanagi-Harada disease-like uveitis induced by vemurafenib for metastatic cutaneous malignant melanoma. J. Dermatol. 2018, 45, e159–e160. [Google Scholar] [CrossRef] [PubMed]
| Author | Primary Melanoma | Age | Tumor Stage | ICI Agent | First Signs of Poliosis | Treatment Response | Other irAE | Ref. |
|---|---|---|---|---|---|---|---|---|
| Korn et al. | right eyelid | 52 years | IIIC | Ipilimumab + Nivolumab | 2 months upon initiation | complete remission | none | [15] |
| Gault et al. | unknown | unknown | IV | Ipilimumab | 5 years | complete remission | none | [5] |
| Wolner et al. | right lower back | 60 years | IV | Pembrolizumab | 4 months upon treatment initiation | partial response | none | [19] |
| Zarbo et al. | unknown | 65 years | IV | Ipilimumab + Nivolumab | 3 months upon initiation | partial response | alopecia, hepatitis, hypothyroidism | [30] |
| Thomas et al. | right shoulder | unknown | IV | Pembrolizumab | 5 months upon initiation | complete response | non-segmental vitiligo | [29] |
| Haist et al. | unknown and upper back | 48 years and 53 years | IV | Ipilimumab + Nivolumab | After 2 and 3 cycles of cICB | complete response | hypothyroidism, vitiligo, hepatitis and colitis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haist, M.; Stege, H.; Maikranz, V.; Halley Blanco, M.; Grabbe, S.; Loquai, C. Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno 2022, 2, 307-316. https://doi.org/10.3390/immuno2020020
Haist M, Stege H, Maikranz V, Halley Blanco M, Grabbe S, Loquai C. Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno. 2022; 2(2):307-316. https://doi.org/10.3390/immuno2020020
Chicago/Turabian StyleHaist, Maximilian, Henner Stege, Verena Maikranz, Maria Halley Blanco, Stephan Grabbe, and Carmen Loquai. 2022. "Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma" Immuno 2, no. 2: 307-316. https://doi.org/10.3390/immuno2020020
APA StyleHaist, M., Stege, H., Maikranz, V., Halley Blanco, M., Grabbe, S., & Loquai, C. (2022). Poliosis Is Associated with Response to Checkpoint-Inhibitor Therapy: A Case Report of Two Patients with Multifocal Metastatic Melanoma. Immuno, 2(2), 307-316. https://doi.org/10.3390/immuno2020020







