The New Anatomy of Neuroimmunology
Abstract
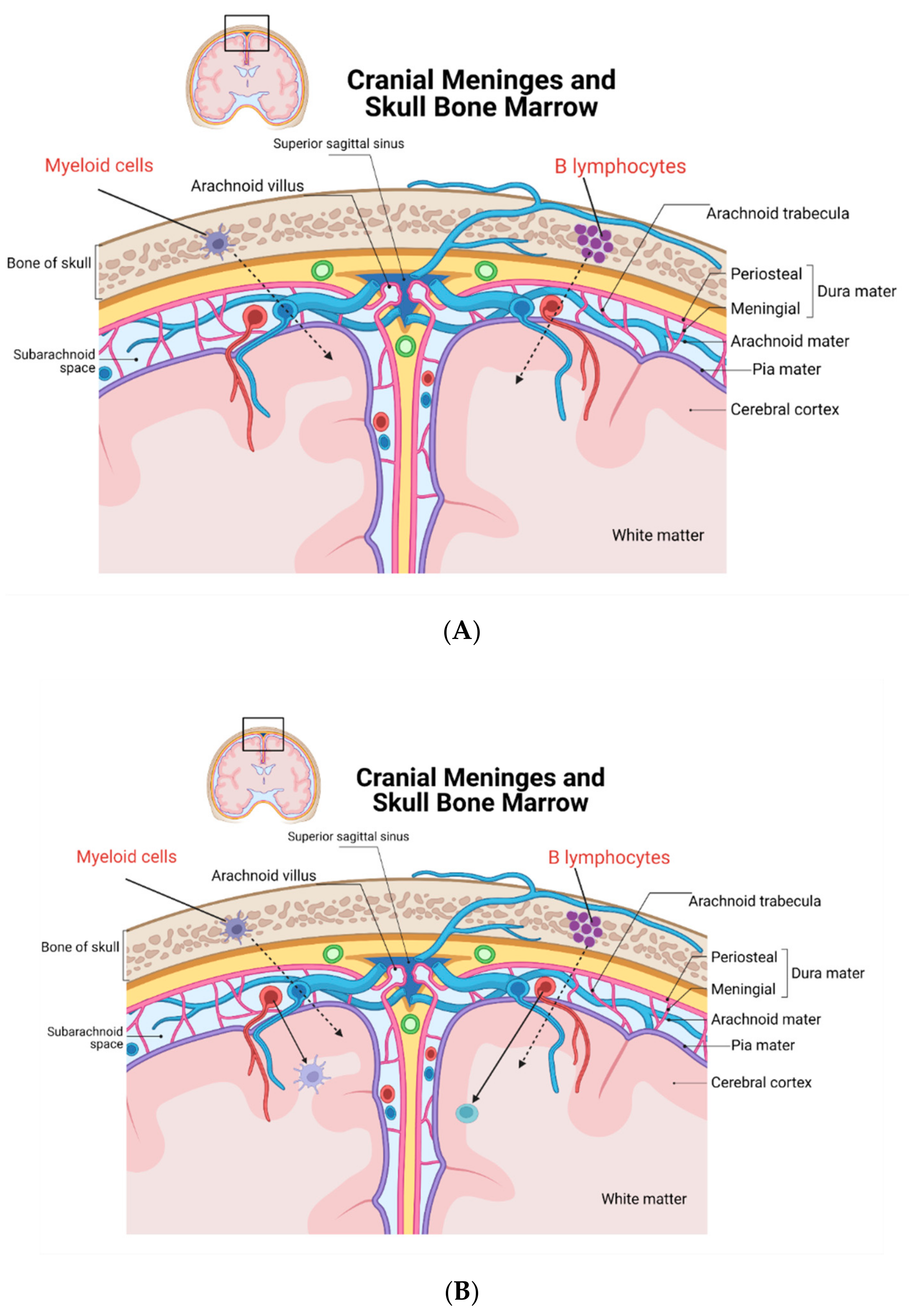
:1. Introduction
2. New Discoveries on CNS Leukocytes and Their Origin
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–871. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Prinz, M. Origin of microglia: Current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015, 1617, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavares, G.A.; Louveau, A. Meningeal Lymphatics: An Immune Gateway for the Central Nervous System. Cells 2021, 10, 3385. [Google Scholar] [CrossRef] [PubMed]
- Yankova, G.; Bogomyakova, O.; Tulupov, A. The glymphatic system and meningeal lymphatics of the brain: New understanding of brain clearance. Rev. Neurosci. 2021, 32, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sandrone, S.; Moreno-Zambrano, D.; Kipnis, J.; van Gijn, J. A (delayed) history of the brain lymphatic system. Nat. Med. 2019, 25, 538–540. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Absinta, M.; Ha, S.-K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017, 6, e29738. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Mundt, S.; Keller, A.; Greter, M. The dural sinus hub: More than just a brain drain. Cell 2021, 184, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Drieu, A.; Mamuladze, T.; Alves de Lima, K.; Dykstra, T.; Wall, M.; Papadopoulos, Z.; Kanamori, M.; Salvador, A.F.; Baker, W.; et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021, 184, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.; Frodermann, V.; Courties, G.; Rohde, D.; Sun, Y.; Vandoorne, K.; Wojtkiewicz, G.R.; Santos Masson, G.; Vinegoni, C.; Kim, J.; et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 2018, 21, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Brioschi, S.; Wang, W.-L.; Peng, V.; Wang, M.; Shchukina, I.; Greenberg, Z.J.; Bando, J.K.; Jaeger, N.; Czepielewski, R.S.; Swain, A.; et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 2021, 373, eabf9277. [Google Scholar] [CrossRef]
- Schafflick, D.; Wolbert, J.; Heming, M.; Thomas, C.; Hartlehnert, M.; Börsch, A.-L.; Ricci, A.; Martín-Salamanca, S.; Li, X.; Lu, I.-N.; et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 2021, 24, 1225–1234. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Xu, D.; Huang, C.; Xing, R.; He, D.; Xu, H. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity 2021, 54, 2784–2794. [Google Scholar] [CrossRef]
- Arneth, B.M. Impact of B cells to the pathophysiology of multiple sclerosis. J. Neuroinflamm. 2019, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally Expanded B Cells in Multiple Sclerosis Bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Kettenmann, H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 2019, 104, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Latour, L.L.; McGavern, D.B. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat. Immunol. 2018, 19, 442–452. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solaro, C.; Barratt, D.; Vaccarezza, M. The New Anatomy of Neuroimmunology. Immuno 2022, 2, 255-259. https://doi.org/10.3390/immuno2010016
Solaro C, Barratt D, Vaccarezza M. The New Anatomy of Neuroimmunology. Immuno. 2022; 2(1):255-259. https://doi.org/10.3390/immuno2010016
Chicago/Turabian StyleSolaro, Claudio, David Barratt, and Mauro Vaccarezza. 2022. "The New Anatomy of Neuroimmunology" Immuno 2, no. 1: 255-259. https://doi.org/10.3390/immuno2010016
APA StyleSolaro, C., Barratt, D., & Vaccarezza, M. (2022). The New Anatomy of Neuroimmunology. Immuno, 2(1), 255-259. https://doi.org/10.3390/immuno2010016





