Abstract
Leishmaniasis is a zoonotic and vector-borne infectious disease that is caused by the genus Leishmania belonging to the trypanosomatid family. The protozoan parasite has a digenetic life cycle involving a mammalian host and an insect vector. Leishmaniasisis is a worldwide public health problem falling under the neglected tropical disease category, with over 90 endemic countries, and approximately 1 million new cases and 20,000 deaths annually. Leishmania infection can progress toward the development of species–specific pathologic disorders, ranging in severity from self-healing cutaneous lesions to disseminating muco-cutaneous and fatal visceral manifestations. The severity and the outcome of leishmaniasis is determined by the parasite’s antigenic epitope characteristics, the vector physiology, and most importantly, the immune response and immune status of the host. This review examines the nature of host–pathogen interaction in leishmaniasis, innate and adaptive immune responses, and various strategies that have been employed for vaccine development.
1. Introduction
Leishmaniasis is a protozoan parasitic disease caused by over 20 Leishmania species; it is among the major neglected vector-borne tropical diseases with outbreak and considerable mortality. Leishmania is a dimorphic obligate intracellular protozoan parasite with two distinct and alternating developmental stages: the promastigote form, which is the flagellated and motile stage residing in the midgut of Sandfly vectors, and the amastigote, non-motile form that resides within the mononuclear phagocytes in the mammalian host. All species of Leishmania are transmitted by the female Phlebotomine Sandflies, which infect a range of mammals including humans, rodents and canids [1]. There are over 90 sandfly species that are known to transmit Leishmania parasites.
Leishmaniasis has three main forms: visceral, cutaneous and mucocutaneous. Nearly 95% cases of visceral leishmaniasis (VL), also known as kala-azar, if left untreated, become fatal. Visceral, cutaneous (CL) or mucocutaneous leishmaniasis (MCL) are endemic in Algeria and countries in East Africa, where outbreaks of VL occur frequently [2]. In addition, Post-kala-azar dermal leishmaniasis (PKDL) also exists, mainly in East Africa and the Indian subcontinent. PKDL is a dermal manifestation of VL, developing in 5–10% of kala-azar patients after 2–3 years of treatment who are also considered a potential source of Leishmania infection. As estimated by the WHO, between 50,000 and 90,000 new cases of VL and between 600,000 and 1 million new cases of CL occur worldwide annually, where different species of Leishmania cause varied clinical manifestations, from self-healing cutaneous lesions to life-threatening VL (https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 30 November 2021)). VL has a broad range of clinical manifestations, primarily affecting the lymphoreticular system, leading to lymphadenopathy, hepatomegaly and splenomegaly, pancytopenia, hypergammaglobulinaemia, and renal disease [3]. CL represents a small localised erythematous nodule developing at the site of the sandfly bite, which gradually develops into an ulcerative condition, rarely penetrating into the subcutaneous tissue [4]. MCL is a highly disfiguring and life-threatening condition occurring in patients with a history of CL with mucosal involvement after 1–5 years of healing [5,6]. Hematogenous or lymphatic dissemination of the parasite in MCL affects the mucosal surfaces of the mouth, nose, and pharynx.
Leishmaniasis can be subclinical (inapparent), localized (skin lesions), and/or disseminated (cutaneous, mucosal, or visceral). There are several factors that determine the clinical presentation and outcome of the disease, such as the parasite and host factors, inflammatory responses, parasite-escape mechanisms as well as the host immunocompetence [7,8]. This review will focus on the mechanisms of leishmanial pathogenesis, host immune responses against the parasite, and the current status of the vaccine development.
2. Life Cycle of Leishmania
Leishmania transmission occurs primarily via the bite of infected female sandflies belonging to the genus Phlebotomous (Old World) or Lutzomyia (New World). Leishmania species have their individual characteristics but they share a similar digenetic life involving a mammalian host and an insect vector. However, in the case of VL in India, and CL caused by Leishmania tropica, instead of sandflies, human beings are incidental hosts of infection (human anthroponosis), and other mammals act as reservoir hosts. Through blood transfusion, sharing needles and due to transplacental spread or organ transplantation, VL can be initiated by amastigotes [9,10]. The chances of transmitting infection by sub-clinically infected humans are feeble [11]. Canids, especially dogs, act as a reservoir of the parasite in most cases; cats, rats, hares, opossums, foxes and other wild animals may also serve as sylvatic reservoirs [12,13,14,15,16]. Environmental factors such as climate change involving alterations in temperature, deforestation, natural disasters and poor socio-economic factors, also contribute to the spread of this disease. Due to geographical overlap, malaria–VL co-infections occur in large populations; it is common in East African countries where malaria and VL are co-endemic [17,18]. Drug resistance, organ transplantation and immunosuppression by HIV-1 contribute to the spread of Leishmaniasis [19]. VL patients co-infected with HIV-1 may also be important reservoirs for sustained Leishmania transmission [20].
Leishmania parasite exists in two forms: Promastigote, a flagellated form that is found in sandflies, and Amastigote, a non-flagellated tissue form that can survive within the phagosomes of macrophage in mammalian hosts. An infected female sandfly (Phlebotomus and Lutzomyia sp.) injects the promastigotes while biting a mammalian host which then enter into the phagocytic cells. Sandflies are relatively weak, prefer moist and dark areas to rest and become active during evening and thus, prefer the bloodmeal at or after dusk. Within the phagocytic cells, promastigotes transform into round non-flagellated amastigotes. The replicative amastigotes then enter into the sandfly while feeding on an infected host.
2.1. Sandfly-Specific Stages of the Parasite
Amastigotes transform into infective metacyclic promastigotes inside the sandfly gut [21], which at a subsequent bloodmeal, are regurgitated [22] and injected back into the mammalian skin to complete the life cycle (Figure 1). This is the only established route of Leishmanial infection [23]; the amastigote forms are present in the skin and are not usually found in the peripheral circulation.
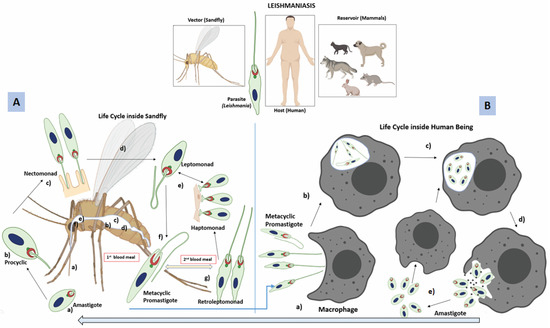
Figure 1.
Digenetic life cycle of Leishmania: (A) Inside the sandfly, amastigotes undergo several non-infective stages before they finally differentiate into metacyclic promastigotes; (a) amastigotes enter into the sandfly (vector), while it bites an infected mammal (host), (b) amastigotes transform into replicative procyclic promastigotes (flagellated form) inside the fly abdominal midgut, (c) Procyclic stage transforms into an elongated nectomonad promastigote that attaches to the microvilli of the abdominal midgut through its flagella, (d) nectomonad then differentiates into a replicative form (leptomonad promastigote), and migrates towards the thoracic midgut, (e) leptomonad promastigotes can differentiate into either heptomonad promastigotes, or (f) metacyclic promastigotes. The haptomonad promastigotes can attach to the stomodeal valve, (g) during the second blood meal, a new replicative stage, known as retroleptomonad promastigote, exists due to reverse metacyclogenesis, where they multiply rapidly and differentiate into metacyclic promastogotes enhancing sandfly infectivity. (B) The metacyclic promastigote belongs to the infective stage that is transmitted to the mammalian host when an infected sandfly bites; (a) metacyclic promastigotes are taken up by phagocytic cells such as macrophages and neutrophils present in the skin, (b) promastigotes are internalised into phagosomes (later, it transforms into a parasitophorus vacuole), (c) promastigotes change into amastigotes inside the parasitophorus vacuole and proliferate, (d) amastigotes burst out from the phagocytes, (e) amastigotes can enter into another life cycle inside the sandfly when taken with the blood meal, or can re-infect fresh phagocytes.
The amastigotes, while inside the sandfly, undergoe transformation via several non-infective stages; first, it becomes a procyclic promastigote, which after few days, differentiates into a strong, elongated motile nectomonad promastigote; this is followed by its transformation into shorter replicative forms, known as leptomonad promastigotes [24], allowing flies to regurgitate parasites into the skin before it takes the second blood meal. The infection is amplified in the vector’s gut [24]; sometimes, a small number of the nectomonad/leptomonad promastigotes also differentiate into haptomonad promastigotes [25]. Finally, they develop into infective metacyclic promastigotes (metacyclogenesis). These promastigotes, while inside the sandfly gut, secrete filamentous proteophosphoglycan (fPPG), which condenses into the promastigote secretory gel (PSG), helping in its transmission [26]. Usually, it takes about 7 to 14 days for a transmissible infection to develop within the vector [27]. Up to 76% of infected bites contain enriched doses of metacyclic promastigotes (80 to 100%). L. mexicana and L. infantum bites contain less than 75% metacyclic promastigotes [26]. PSG production is vital in creating a blocked fly as it compels the infected sandfly to go for successive blood meals [27] because metacyclic promastigotes are capable of de-differentiation inside the sandfly into retroleptomonad promasigotes (via reverse metacyclogenesis), increasing parasite load in its next blood meal (Figure 1). Rogers et al. have shown that a single sandfly can ingest around 3200 amastigotes in a 1.6 mL bloodmeal, and within 5 days, the parasite population grows to approximately 35,000 per fly [28]. By the 10th day, the parasite number falls to 1000, probably due to exhaustion of the nutrient, or the immune response mounted by the sandfly [29].
2.2. Stages Inside the Mammalian Phagocytes
Metacyclic promastigotes of Leishmania are highly motile cells, capable of migrating through a collagen matrix [30]. They can invade beyond the bite site either directly or through infected phagocytic cells, fostering the ability of the parasite to establish the disease from CL to VL. The metacyclic promastigotes interact with phagocytic cells such as macrophages through its active motile flagellum [31]. Inside the macrophage, the elongated promastigote transforms into a spherical body with a shorter flagellum. The growth rate of the amastigote is slower than the promastigote, possibly to reduce metabolic load and proliferate and survive for a longer time, thus enhancing the infectivity. The amastigote protects itself from the surrounding acidic medium containing proteases by keeping the flagellar pocket neck closed [29]. The amastigotes proliferate inside the phagosome and then get out through cell rupture to infect other phagocytic cells.
3. Host Immune Response
There are several important factors that contribute to a successful transmissible infection, such as the species of the parasite, the vector combination, PSG production, and the host immune response against the parasite. The first crucial event is the initial contact and stable interaction of highly polarized and motile promastigotes with mammalian host cells for their efficient phagocytosis. Macrophages act as the primary host cells for Leishmania; however, monocytes, dendritic cells (DCs), and neutrophils are also infected and have important roles in the immunopathology of Leishmaniasis.
3.1. Innate Immune Response
Promastigote uptake is a receptor-mediated process where it binds avidly to macrophages and other phagocytic cells, but poorly to lymphocytes. The cell surface components of Leishmania, which change in various parasitic forms (promastigote to amastigote), are important determinants of parasite virulence. Promastigotes are covered in a thick glycocalyx composed of a variety of extensively branched, glycosylphosphatidylinositol (GPI)-anchored glycoconjugates, including lipophosphoglycan (LPG), metaloprotease glycoprotein (gp) 63, and members of a secreted family of heavily glycosylated proteins [32]. Amastigotes are covered by a densely packed glycocalyx mainly composed of glycoinositolphospholipids and glycosphingolipids [32], LPG, gp63 and Proteophoglycan (PPG) are the major determinants in initiating phagocytosis and for intracellular survival of L. major [33,34]. LPG of L. donovani is also capable of blocking LPS-mediated expression of E-selectin, intercellular adhesion molecule–1, and vascular cell adhesion molecule–1 on endothelial cells [35]. Thus, Leishmania LPG exerts its inhibitory effect possibly by affecting the endothelial cell functions and gene expression that are required for inflammation.
3.1.1. Role of Neutrophils and Salivary Components of Sandfly during Early Immune Response
During the early phase of CL, neutrophils and eosinophils increase in number, which is followed by an increasing phagocytic response by macrophages. Neutrophils predominate at the inoculation site within first few hours of infection, they take up promastigotes, and serve as a vehicle for subsequent infection of monocyte-derived or tissue-resident macrophages [36]. Neutrophils and eosinophils possess leishmanicidal activity that restrains parasite progression [37]. Neutrophils could enhance killing of L. braziliensis in infected macrophages through upregulation of TNF-α and ROS [38]. Activated neutrophils are capable of killing promastigotes via the neutrophil trap (NET) [39] and also through oxygen metabolites generated during phagocytosis-induced respiratory burst [40]. In NET associated with promastigotes, DNA, elastase, and histones are detected; histones likely exhibit the leishmanicidal activity [41].
While injecting the promastigotes into the host skin, the sandfly also salivates and delivers certain salivary proteins that are chemotactic and inducer of inflammatory response facilitating Leishmania infection [42]. The saliva also contains vector gut microbiota that activates inflammasome-derived IL-1β production and aids in neutrophil recruitment [43]. The saliva of Lutzomyia longipalpis contains a female-specific secreted endonuclease called Lundep capable of hydrolysing DNA, thus negating the effect of neutrophil NETosis, in order to protect the promastigotes [44]. A 45 kDa neutrophil chemotactic salivary protein acts through a G-protein-coupled receptor, which enhances lesion pathology and increases the parasite burden in mice upon co-infection with Leishmania parasites [45]. Co-infection with helminths such as Filaria may modulate the immune response to Phlebotomus duboscqi (Pd) saliva; repeated exposure to Pd saliva polarizes human monocyte function towards a tolerized phenotype while co-infection with filaria favors a Leishmania-promoting Th2/regulatory immune response [46].
Neutrophils appear to restrain parasite progression [36] by secreting cytokines and chemokines, releasing its granular contents, triggering pattern recognition signals, and by interacting directly with other inflammatory and resident cells [47,48,49]. Leishmania promastigotes release a soluble chemotactic factor (LCF), as confirmed in the supernatants of L. major, L. aethiopica, and L. donovani parasites cultures, which aids in neutrophil recruitment. This induces IL-8 secretion by neutrophils, thus, amplifying neutrophil recruitment. Often, gut microbes present in the saliva of the sandfly are egested into host skin along with Leishmania parasites. The egested microbes trigger the inflammasome to produce significantly higher levels of IL-1β compared to levels induced by parasites alone. Removing gut microbiota, or blocking IL-1β before transmission, abolishes neutrophil recruitment and impairs Leishmania dissemination [43]. IL-17A-producing Innate Lymphoid Cells (ILCs), which are RORγt+, are involved in the microbiota-driven immunopathology in CL. Pateints infected with L. braziliensis were found often with dominant Staphylococcus dysbiosis, and this probably can influence IL-17 synthesis. The lesion size in mice infected with L. major was also found to be increased due to Staphylococcus colonization [50]. Thus, investigating the mechanistic aspects of immunomodulation due to sandfly microbiota can offer insight into the immunopathology of early infection in Leishmaniasis.
There is also evidence to suggest the survival of Leishmania promastigotes following their rapid uptake by neutrophils. Under an experimental condition, inhibiting neutrophilic infiltration increased host resistance to infection [49]. L. major and L. donovani promastigotes were observed to be surviving in human neutrophils which correlated with a lack of respiratory burst. Inhibition of IP-10 (gamma interferon-inducible protein 10) production by neutrophils in presence of Leishmania can inhibit recruitment and activation of NK cells and Th1 cells (Figure 2). Thus, by infecting neutrophils, promastigotes find an escape from neutrophil-mediated defense mechanisms [51]. IL-17 seems important for neutrophil recruitment during the development of Leishmania-induced human and murine lesions. CD4+ T cells and neutrophils produce increased amounts of IL-17 in L. major infected BALB/c mice [52,53]. However, promastigotes also somewhat contribute to programmed cell death of neutrophils [50], which are cleared via phagocytosis by macrophages and DCs. Infected neutrophils often deliver viable promastigotes to macrophages during their phagocytosis [54,55].
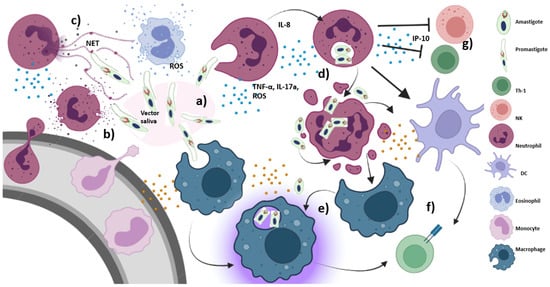
Figure 2.
Early immune response triggered by Leishmania: (a) within minutes of infection, promastigotes are rapidly taken up by phagocytic cells, including neutrophils and macrophages, (b) promastigotes are injected into the host skin along with certain vector-derived salivary proteins as well as vector salivary microbiota, which exhibit neutrophil chemotactic properties and trigger inflammasome formation. IL-8 secretion by neutrophils also amplifies further neutrophil recruitment. Monocyte and eosinophil infiltration also occur, (c) neutrophils and eosinophils possibly show leishmanicidal activity that controls parasite proliferation. Activated neutrophils are capable of killing promastigotes via NET and ROS generation, (d) Leishmania is capable of accelerating neutrophil apoptosis, which are then phagocytosed by macrophages and dendritic cells along with the amastigotes, (e) Amastigotes released from apoptotic neutrophils can infect uninfected macrophages where they are internalized into phagosomes, divide many times by binary fission, eventually getting released by rupturing the macrophages, (f) activated macrophages and dendritic cells present the processed parasitic antigens to T cells for adaptive immune response, (g) IP-10 secretion by neutrophils inhibits recruitment and activation of NK cells and Th1 cells.
3.1.2. Macrophage as a Cellular Host of Leishmania
In leishmaniasis, amongst the myeloid host cells, macrophages play quite a significant role: one, as a replicative niche during the acute phase of infection, and two, as anti-leishmanial effector, immunoregulatory, and permissive host cells for long-term survival of the parasite [36,56]. During CL, infected macrophages remain near the sandfly bite. Other forms of leishmaniasis develop when infected macrophages migrate away from the sandfly bite site; VL involves dissemination throughout the body, especially to the liver and spleen via infected macrophges.
Parasitophorous Vacuoles (PV) as a Safe Haven for Leishmania
Uptake of promastigotes by macrophage involves complement receptors and PAMPs such as LPG or gp63 [33,57]. Among the Leishmania promastigote surface molecules, glycoinositol phospholipids (GIPLs) are most abundant and are actively expressed on promastigotes as well as amastigotes [58,59,60]. The promastigotes bind to the complement receptor 1 (CR1), CR3, fibronectin receptor, and the mannose-fucose receptor on the surface of macrophages (Figure 3) [61]. Various opsonins such as C3b/iC3b, mannan-binding lectin (MBL), and galectins can bind LPG, gp63, and PPG present on the surface of L. major promastigotes [33,34,62,63,64]. TLR-2 and TLR-9 play opposite roles in host response to Leishmania infection; TLR-2 is involved in parasite survival in macrophages upon activation by LPG, whereas TLR-9 seems to promote a host-protective response [65]. The best studied Leishmania protease is gp63, which is capable of activating host phosphatases such as PTP1B, PTP-PEST, TCPTP, and SH-1. Upon activation, SHP-1 downregulates the macrophage response to IFN-γ by interacting with JAK-2, which further modulates TLR and lectin-mediated pathways [66]. However, Leishmania phosphatases are equally relevant for the development of the parasite and its survival. LmPRL-1, one of the phosphatases that promotes virulence during macrophage infection, is secreted within exosomes and localized in the PV membrane. During metacyclogenesis, LmPRL-1 is constantly expressed by promastigotes that seems to help the intracellular replication of the parasite in primary mouse macrophages [66]. LPG supports survival of Leishmania within human peripheral blood macrophages by suppressing oxidative burst [67]. Murine peritoneal macrophages, stimulated with L. braziliensis LPG, show higher TNF-α, IL-1β, IL-6 and NO production than L. infantum. Furthermore, L. braziliensis LPG activates NF-κB [68]. Induction of TGF-β by murine peritoneal macrophages following infection with L. amazonensis has been reported. TGF-β treated mice develop large, non-healing lesions, thus overcoming the inherent resistance to leishmaniasis in C57BL/6 (Th1) mice [69].
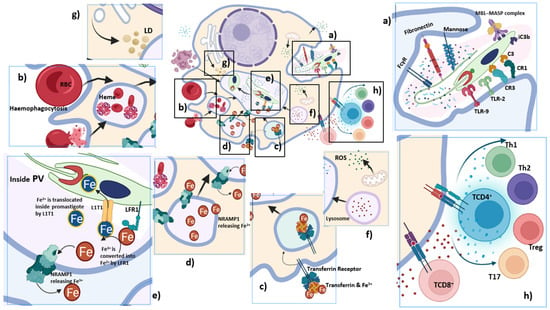
Figure 3.
Macrophage as the final destination of Leishmania: Once promastigote interacts with the macrophage, it gets internalized inside the phagosome. The phagosome fuses with lysosomes and endosomes; modifications within the membrane and the lumen of the phagosome transforms it into a hydrolytic compartment with privileged environment for Leishmania, termed as parasitophorous vacuoles (PV). Inside PV, promastigotes differentiate into amastigotes; (a) Uptake of promastigotes by macrophages is a receptor-mediated phagocytosis, where the PAMPs of the Leishmania parasite, LPG and gp63, interact with complement receptors (CR1, CR3), Fc receptor, fibronectin receptor, mannose receptor, TLR-2, and TLR-9. Leishmania can fix C3, and induce its subsequent cleavage to iC3b. The MBL-MASP complex can also bind to the surface of promastigotes likely via mannose residues, enhancing parasite uptake by macrophages; (b) Leishmania lack in heme synthesis machinery; hence, it induces infected macrophages to become hyperactive and triggers hemophagocytosis that takes place at a higher rate for the supply of heme; LHR1 helps in the transport of iron; (c) Leishmania activates iron regulatory proteins to stimulate expression of transferrin receptors for iron uptake. Transferrin, taken up by Leishmania-infected macrophages, is delivered to PV. Transferrin endocytosed by amastigotes is degraded within them, since the availability of transferrin promotes amastigote multiplication; (d) Nramp1, a membrane protein present on late endosomes and lysosomes of macrophages, fuses with PV and translocates Fe2+ from the vesicles into the cytosol. Amastigotes seek continuous iron sources to compete with the host; (e) Ferric iron reductase (LFR1) and ferrous iron transporter (LITI) are mediators for iron acquisition and their expression in Leishmania is upregulated while inside the PV to compete with the host for iron. LFR1 reduces the insoluble Fe3+ to soluble Fe2+ and L1T1 transports Fe2+ inside the PV; (f) Lysosomal vesicles fuse with the PV containing the parasite, but Leishmania survives without being degraded by the lysosomal enzymes. The delay in the recruitment of LAMP-1 induced by Leishmania also dampens the interactive activity of phagosomes with the lysosomal vesicles inside macrophages. Macrophage activates ROS generation for killing promastigotes and amastigotes. However, during Leishmania infection, ROS generation is significantly reduced via upregulation of Heme-oxygenase-1 (HO-1); (g) Lipid droplets (LDs) rich in triacylglycerols and sterol esters from ER are released into the cytoplasm and are taken up by PV. Since Leishmania cannot synthesize cholesterol, host LDs, low density lipoproteins (LDL), high density lipoproteins (HDL) and lipoprotein lipase compensate for its energy and lipid requirements; (h) Th1, Th2, Th17 and Treg cells play an important role in leishmaniasis. Impairment of CD8+ T cell proliferation/function as well as anergy facilitates survival of Leishmania.
Once the contact has been made between the promastigote and the macrophage, it is internalized inside the phagosome. The phagosome surrounding the ingested promastigote fuses with lysosomes and endosomes modify both the membrane and the lumen of the phagosome [70]. These changes transform phagosome into a hydrolytic compartment with a privileged environment for Leishmania, termed as parasitophorous vacuoles (PV), an endocytic organelle. The actin-based flagellar motility plays an important role in the phagocytic uptake, taking the promastigote smoothly towards the cell centre inside a PV. The flagellum exhibits active beating, reorienting itself towards the plasma membrane and even protruding out of the membrane. At the end of the intracellular flagellar oscillation, they are drawn close to the host cell nucleus as observed, in real time and at high-resolution time-lapse microscopy, during the encounter between hamster-derived virulent metacyclic-enriched L. donovani promastigotes and primary bone-marrow-derived macrophages (BMMs) [71]. This continuous flagellar movement leads to plasma membrane damage, favouring lysosomal exocytosis. The flagellum also acts as a sensory organ being often in contact with the PV membrane to sense the longevity of the macrophages by assessing its metabolites [72,73]. This allows the amastigotes to decide whether to proliferate inside the macrophage or not.
Leishmania species not only survive inside the PV but also multiply without being degraded by the lysosomal enzymes [74]. Phagosomes containing promastigotes poorly interact with endosomes as well as lysosomes and delay the recruitment of LAMP-1. Insertion of LPG into the phagosome membrane destabilizes its lipid microdomains hampering the microbicidal activity in J774 macrophages [75]. This event leads to the exclusion of the membrane fusion regulator, synaptotagmin V (Syt V), which dampens recruitment of the V-ATPase and interferes with the phagosome acidification [76]. Hamster peritoneal macrophages infected with L. donovani promastigotes show a decrease in the lysosomal enzymes such as p-galactosidase, N-acetyl-β-D-glucosaminidase, and α-mannosidase [77]. The pinocytic rate of macrophages increases following Leishmania infection, resulting in the expression of leishmanial antigens on their surfaces [70]. PV of mouse bone marrow derived macrophage infected with L. amazonensis WHOM/BR/75 Josefa strain consisted of host plasma membrane, phagosomal organelles, and parasite-derived [78] proteases, phosphatases, and cytosolic expression of Rab7, LAMP1, and LAMP2 under highly acidic pH [79,80]. Promastigote transforms into amastigote inside the PV and its intracellular metabolism requires a neutral pH. An increase in temperature and a decrease in pH is believed to trigger differentiation of promastigote into amastigote [81]. In the case of L. amazonensis, iron uptake and generation of hydrogen peroxide are the major inducers of parasite differentiation [82,83]. The densely packed glycocalyx comprising glycoinositolphospholipids and glycosphingolipids protects the amastigote from the acidic pH as low as 4.0 [84]. Autophagosomes have also been shown to fuse with Leishmania PVs [85]. Two distinct types of PVs have been observed inside the infected macrophages, one with multiple amastigotes, as in L. amazonensis, whereas in the case of L. major, PV can have a single amastigote parasite [86,87].
Upon infection of mouse macrophages with Leishmania, ROS generation is significantly reduced, through the upregulation of antioxidant enzyme, Heme-oxygenase-1 (HO-1). An anti-inflammatory condition is created due to the release of carbon monoxide (CO) by HO-1 during heme degradation. CO then inhibits TLR-4 association with MyD88 and TRIF. This further inactivates NF-κB- and IRF3-dependent proinflammatory cytokine production, making the environment conducive for the parasite [88]. Nitric Oxide Synthase (iNOS, NOS2) induced NO production is important for the elimination of Leishmania [36]. iNOS gene knock-out mice become more susceptible to L. major infection; macrophages derived from iNOS deficient mice are unable to eliminate L. major in vitro [89,90].
Leishmania-Induced Hemophagocytosis and Iron Uptake
During erythropoiesis, red pulp macrophages in the spleen, Kupffer cells in the liver and central nurse macrophages in the bone marrow ensure a coordinated metabolism of iron. Macrophages can recycle up to 95% of the iron found in the body while maintaining erythropoiesis through hemophagocytosis, a process where erythrocytes and leukocytes are consumed (phagocytosed) in the bone marrow, liver, or spleen by macrophages or histiocytes. Spleen and liver macrophages phagocytose senescent or injured blood cells, providing continuous delivery of recycled iron under steady-state conditions and during anaemic stress frequency [91]. Trypanosomatid parasites are incapable of producing heme, hence, they infect macrophages for heme acquisition. Following infection (e.g., Salmonella, Mycobacterium, Babesia, and Leishmania), hemophagocytosis by macrophages occurs at a high rate [91,92,93,94,95]. Hematophagocytosis has been observed in the bone marrow of infected VL patients recovering from amphotericin B and sodium stibogluconate treatment [96,97,98,99,100,101].
Hemophagocytosis by heavily infected macrophages in the spleen is the possible cause of anemia in VL. IFN-γ and TNF-α are capable of inducing hemophagocytosis associated with infection by Salmonella, Epstein-Barr virus, lymphocytic choriomeningitic virus, cytomegalovirus and Trypanosoma brucei [102,103,104,105,106]. L. donovani infected macrophages show significant level of hemophagocytosis; uninfected macrophages rarely show hemophagocytosis, suggesting that Leishmania is capable of modulating macrophage function directly [107]. Phagocytosis of RBCs also allows nutrient availability for the pathogen, helping in their growth and survival. Down-regulation of Signal regulatory protein α (SIRPα) was observed in L. donovani-infected RAW264.7 cells, as well as in MGCs in the spleen of mice infected with L. donovani [107]. CD47-SIRPα signaling is the known mechanism for limiting hemophagocytosis, which recognises RBCs and gives the phagocytosis-inhibitory signals. L. donovani infection thus induces the downregulation of SIRPα in macrophages and disrupts the CD47-SIRPα interaction resulting in hemophagocytosis.
Slc11a1 (formerly Nramp1; Natural resistance-associated macrophage protein) is a membrane protein of late endosomes and lysosomes of macrophages, which translocates Fe2+ from the vesicles into the cytosol [108]. This is immediately recruited to the membrane of microbe-containing phagosomes so that Leishmania amastigotes need to compete with the host for iron. This is recognized as a host susceptibility gene for infections with L. donovani and L. infantum [109]. Nramp1 is downregulated by ubiquitin-proteasome degradation pathway in case of Leishmania infection [110]. Hepcidin, the iron-regulatory peptide hormone, induces the signalling for downregulation of Nramp1. Thus, when Nramp1 degradation is blocked using proteasome inhibitor, or by transcriptional agonist of hepcidin, it leads to depletion of phagolysosomal iron pool [110]. Leishmania can also induce the host cell to internalize iron. L. donovani activates iron regulatory proteins (IRPs), which further stimulate expression of transferrin receptors [111]. PVs of L. amazonensis infected macrophages fuse with transferrin-containing endosomes to maintain a constant supply of iron for the proliferating amastigotes [112]. There are three mediators for iron acquisition that are upregulated in Leishmaniasis; ferric iron reductase (LFR1), ferrous iron transporter (LITI) and heme transporter (LHR1). LFR1 reduces the insoluble Fe3+ to soluble Fe2+ and L1T1 transports Fe2+ inside the PV. Leishmania expresses LHR1 and LIT1 to uptake iron within macrophages (Figure 3) [81]. Macrophages infected with L. donovani promasigotes as well as amastigotes show inhibition of NAD(P)H oxidase assembly at the phagosome membrane [113].
Lipid Uptake by Leishmania
For an intracellular parasite like Leishmania, lipid metabolism is of considerable importance to fulfil its need of lipids. A significant increase in cholesterol content and moderate decrease in Phospholipid (PL) content has been observed during the differentiation of promastigotes into amastigotes. A decline in the membrane and serum cholesterol levels [114] in the VL patient was found to be inversely proportional to the parasitic load in the spleen [115,116]. An increase in Phosphatidylserine (PS) in amastigotes may also suggest its role in the pathogenicity of leishmaniasis [117]. Leishmania can degrade CerPCho (sphingomyelin) from host cells possibly to enhance its virulence [118]. Lipid droplets (LD), rich in triacylglycerols and sterol esters, are derived from the accumulation of newly formed lipids within ER. These LDs bud off from ER and are released into the cytoplasm [119]. Leishmania cannot synthesize cholesterol, and thus, host LDs, low density lipoproteins (LDL), high density lipoproteins (HDL) and lipoprotein lipase, compensate for its energy and lipid requirement. Leishmania is capable of capturing cholesterol from LDL through its specific LDL binding sites. L. amazonensis has both HDL and LDL binding sites and can scavenge cholesterol from plasma. Host cholesterol has been identified on the membrane extensions of L. amazonensis [120]. In L. donovani, COX-2-mediated PGE2 release is involved in downregulation of microbiocidal activity of macrophages [121]. Leishmania vector saliva can modulate eicosanoids metabolism and LD formation in the host cells [122,123]. The LD formation in macrophages infected with L. major has also been documented; LDs are observed inside PV as well as in the parasite cytoplasm. [124].
Cytokine Induced Macrophage Activation
Macrophages are indispensable for parasite survival, replication and differentiation; at the same time, they are also the major effector cells responsible for elimination of the parasites. For Leishmania, the macrophage is the final host cell for its proliferation. There are two functionally distinct macrophage phenotypes: M1, a classically activated pro-inflammatory subtype with microbicidal properties, and M2 that is an alternatively activated anti-inflammatory subtype that is associated with the resolution of inflammation. Th1 cell-mediated production of IFN-γ, TNF-α, and GM-CSF polarizes macrophages to M1 phenotype, whereas Th2 cell-mediated production of IL-4, IL-10, IL-13, TGF-β, and M-CSF polarizes macrophages to M2 phenotype [125]. M1 macrophages are characterized by a high production of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-12, IL-18, IL-23, and Type 1 IFN [126]. Restriction of parasite growth has been associated with the induction of robust Th1 responses and activation of iNOS-expressing M1 macrophage [127]. During Leishmania infection, mTOR (mammalian target of rapamycin) pathway plays an important role in regulating M2 polarization. It increases expression of M2 markers such as arginase-1, IL-10, TGF-β, CD206, and CD163, while causing reduction in the expression of M1 macrophage parameters such as ROS, NO, iNOS, NOX-1, IL-12, IL-1β, and TNF-α. This M2 polarization induced by Leishmania aids in the survival of parasite inside the host [128]. Reciprocal changes in histone lysine methylation/demethylation of M (LPS + IFN-γ)/M(IL-10) genes is one of the factors that direct macrophage polarization for its successful establishment within the host [129].
L. donovani infection stimulates TNF-α production by macrophages but abolishes IL-1β generation in vitro and in vivo [130,131,132,133]; in the case of infection with L major, IL-1β generation by macrophages is enhanced [134]. Expression of TNF-α in response to LPS is also inhibited in L. donovani-infected macrophages [130]. Cells of the mouse pouch exudate recruited in response to Leishmania infection seem to express RANTES, MIP-1α, MIP-1β, MIP-2, IP-10, MCP-1 and TCA-3 [135], suggesting recruitment of a mixed immune cell population including neutrophils, monocytes, macrophages, and eosinophils.
Active leishmaniasis in humans is associated with an increased level of serum IL-10 as well as increased IL-10 mRNA expression in tissue lesions [136,137]. IL-10 production represents a shift from a pro-inflammatory and protective immune response to a regulatory immune response, causing uncontrolled disease progression. Infected macrophages are activated by IFN-γ and TNF-α to kill intracellular amastigotes via the L-arginine nitric oxide pathway [138,139]. Leishmania-specific lymphoproliferative unresponsiveness and lower production of IFN-γ upon Leishmania antigen stimulation lead to susceptivity towards development of clinical VL [140,141]. Th1 polarization leads to immunosuppressive response [137]; thus, negating IFN-γ can be vital for Leishmania survival.
3.1.3. Dual Role of Complement
The complement system acts as a first line of host immune defense that is activated by a proteolytic cascade to eliminate the invading pathogens through the membrane attack complex (MAC) formation and opsonisation. Complement receptors are the primary mediators of parasitic adhesion; in the presence of complement, Leishmania can bind efficiently with macrophages. Complement plays a diabolically opposite role: complement-mediated lysis by MAC leads to the elimination of parasites, whereas opsonization by C3b/iC3b enhances phagocytic activity that aids in the internalization and eventual survival of the parasite. Opsonization with C3 leads to enhancement of binding and phagocytosis of L. major promastigotes by macrophages. L. enrietti and L. tropica activate the complement alternative pathway leading to iC3b deposition on its surface, and hence, enhanced uptake of promastigotes via complement receptors [142].
The cutaneous species of Leishmania (L. major, L. mexicana, L. mexicana amazonensis, L. braziliensis guyanens, L. enriettii and L. tropica) are susceptible to lysis by normal serum through activation of the alternative pathway on the promastigote surface [142,143]. For visceral strains such as L. chagasi promastigotes, complement seems to be a vehicle to escape from the inoculation site to viscera [144]. Complement plays an important role in controlling the CL lesions caused by L. amazonensis. Complement-dependent adhesion of Leishmania is mediated by leukocyte integrin Mac-1 and CR1 [145]. Both metacyclic and logarithmic-phase promastigotes of human L. major are capable of binding to Mac-1 or CR1. Mac-1 is also the predominant receptor mediating the internalization of complement-opsonized metacyclic L. major promastigotes by monocyte-derived macrophages [146]. Promastigotes of L. donovani activate the complement classical pathway when opsonized by host natural antibodies and deposit C3 on the parasite surface [146]. C3-coated promastigotes bind to RBC as well as mononuclear phagocytes through CR1 and CR3 [147], leading to enhanced phagocytosis. Promastigotes of L. braziliensis can bind to the MBL–MASP complex likely via mannose, activate the complement lectin pathway, and thus, enhance parasite uptake by phagocytes [148]. The binding of MBL, CL-11, ficolin -1, and ficolin-3, but not ficolin-2, was observed on the surface of live metacyclic promastigotes, highlighting the possible role MBL and ficolins play in the initial innate immune response to L. infantum [149]. The complement regulatory protein, factor H, from human serum and factor H-like proteins from dog serum, were found to bind to L. infantum and inactivate C3b in the presence of factor I [150].
3.1.4. Role of Dendritic Cell
DCs are capable of recognizing PAMPs expressed by Leishmania via PRRs leading to their activation, which can greatly influence innate and adaptive immune responses in order to promote parasite eradication. During infection with L. braziliensis, TLR-2 deficiency (TLR2−/− mice) resulted in activation of DC with increased IL-12 p40 production and decreased IL-10 production. This induced reduction in lesion size in TLR2−/− mice, with limiting L. braziliensis infection. Deficiency of MyD88 inhibited IL-12 p40 by Leishmania-infected DCs, which resulted in lower levels of DC activation, impairing the protective immunity [151]. In the case of L. mexicana, TLR-2 of monocyte derived DCs interacts with LPG to enhance MHC class II and CD86 expression [152]. DC/TLR-9 interaction enhances neutrophil recruitment during L. infantum infection in mice [153]. Bone marrow-derived DCs via intracellular TLR-9 interaction produce IL-12, which further enhances cytotoxicity of NK cells and IFN-γ expression [154].
Cell surface DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) mediates efficient internalization of L. mexicana promastigotes in vitro [155]. DCs stimulated with excreted-secreted antigens (ESA) of L. major and L. donovani show reduction in the expression of DC-SIGN [155]. Parasitic uptake by DCs leads to their enhanced migration to lymph node for antigen presentation to T cells. Leishmania have evolved strategies to interfere with DC migration, by inhibiting expression of CCR7 through IL-10 production [156]. In animal models, CCR2 gene knock-out [157] as well as deficiency in CCL19 and CCL21 [156] caused reduced DC migration to secondary lymphoid organs for antigen presentation.
DCs produce IL-12, and promote Th1 differentiation, characterized by IFN-γ production by Th1 cells, which leads to parasite elimination. Epidermal Langerhans cells (LCs), observed near the inoculation site, internalise L. major amastigotes more than promastigotes. The parasite uptake sets off protective Th1 immunity, which is associated with upregulation of MHC-class I and class-II antigens, costimulatory molecules such as CD40, CD54, CD80, and CD86, and IL-12 p40 production [158]. However, infection with L. amazonensis can alter DC functions, favouring parasite survival. Amastigote infection is incapable of inducing CD40-dependent IL-12 production [159]. L. infantum infected DCs express less costimulatory molecules and higher levels of IL-10 [160]. Thus, Leishmania is also capable of downregulating Th1 mediated adaptive immune response as one of its pathogenic mechanisms.
3.2. Adaptive Immune Response
The course of Leishmania infection and its resolution greatly depends on efficient establishment of cell mediated immune response. T cell response correlates with recovery from human leishmaniasis as well as resistance to it. CD4+ T cell response seems to correlate with lesion development, while activation of CD4+ and/or CD8+ T cells appears to be essential for the healing process [161]. Th1, Th2, Th17, and Treg subsets play important roles in leishmaniasis [162,163]. There is some evidence of antibody-mediated protection; however, cell-mediated immune response is principally instrumental in providing protection against leishmaniasis [164,165,166].
3.2.1. Immunomodulation by T Helper Subtypes
Th1 and Th2 Polarization
The Th1 immune response not only plays a critical role in protecting the host against primary infection but also provides lifelong immunity to reinfection [167,168]. A protective immune response against CL due to L. major, L. mexicana, or L. amazonensis, as well as VL caused by L. donovani or L. infantum, relies on the establishment of the pro-inflammatory T cell profile [163]. CD4+ Th1 cells induce macrophage-driven proinflammatory response which secrete TNF-α, IL-1β, IL-6, IL-12, IL-18, and IL-23 cytokines, induce ROS generation, and enhance phagocytosis [126,169]; together, they are very effective in generating protective immunity against L. donovani [170]. In murine experimental CL, protective immunity appears to be dependent on IFN-γ producing CD4+ Th1 and CD8+ Tc1 cells. Whether CD4+ and CD8+ T-cell responses are involved in resistance and/or cure of VL depends on their efficient memory responses, influenced by IFN-γ, IL-2, and TNF-α. CD4+TNF-α+IFN-γ+, CD4+IL-2+TNF-α+IFN-γ+, CD4+TNF-α+, and CD4+IFN-γ+ T cells increase throughout the treatment. CD8+IL-2+TNF-α+IFN-γ+ and CD8+TNF-α+IFN-γ T cell counts also increase, contributing to the healing process [171]. Th2 response is correlated with an anti-inflammatory phenotype producing IL-4, IL-13, IL-10, and TGF-β cytokines. IL-4, IL-10, and TGF-β modulate Th1 responses by dampening macrophage activation, and hence, aggravate the disease [172,173]. Th2 response can also induce IL-21-mediated downregulation of iNOS, TNF-α, and TLR-4, allowing the Leishmania parasite to proliferate [174,175]. Poudel et al. (2020) have shown that IL-4 promotes CL pathology by promoting Th2 immune response as well as via pathogenic CD8+ T cell responses [176]. IL-10 is an important regulatory cytokine and its sources have been identified as CD4+/CD25+ T cells (Th2), CD4+/CD25−/FoxP3+ regulatory T cells (Tregs) and CD4+/CD25−/FoxP3− T cells (Th1) [177,178,179]. IL-10 is capable of inhibiting phagocytosis, and thus, it contributes to the growth and spread of Leishmania [180,181]. IL-10 acts as an immunosuppressive factor in VL and facilitates the spread of Leishmania parasites. IL-10 secretion by T cells can influence immune activation at the early stage of infection, which renders BALB/c mice susceptible to an uncontrolled L. major infection [182]. IL-27 was found to have pleiotropic effects on Th1, Th2, and Th17 cells during L. major infection; it exhibits a regulatory role in balancing between protective immunity and pathogenesis during leishmaniasis. Thus, Leishmania infection is characterized by a mixed Th1/Th2 response, where a dominant Th1 response provides resistance, whereas a dominant Th2 response confers susceptibility.
T Regulatory and T17 Cells
T regulatory cells (Tregs) play a fundamental role in the infection and persistence of Leishmania. In the case of L. donovani and L. major infection, Tregs lead to disease exacerbation and prevent immune-mediated parasite clearance and disease reactivation [183,184]. Tregs are also beneficial in resolving a hyper-inflammatory state and lead to disease remediation in case of L. amazonensis [185]. In the murine model of CL, Tregs act as a major suppressor of T effector cells. There is an accumulation of IL-10 producing Tregs (CD4+CD25+FoxP3+) at the CL lesions as well as in VL patients with persistence of parasite load and reactivation of the pathology [137,184]. Tregs isolated from bone marrow produce IL-10 and inhibit effector T cell activation. Drug unresponsive patients exhibit higher levels of IL-10, indicating its role in immunosuppression in VL patients [186]. Anderson et al. have suggested CD4+CD25−Foxp3−Th1 cells as the dominant player in IL-10–mediated immune suppression in chronic CL induced by L. major; in the absence of Foxp3+ cells, expansion of antigen-induced CD4+ T effector cells takes place optimally [179,187]. PBMCs from CL patients, when challenged with Leishmania antigens, show upregulation of the activation markers CD25 and CD69 and expression of costimulatory molecule, CD86 [188]. Adoptive transfer of Tregs from naive mice can halt disease progression, reduce the parasite burden, and downregulate production of IL-10, IL-13, IL-17, and IFN-γ in a mouse model of chronic L. (Viannia) panamensis infection. Impaired Treg cell response during L. (Viannia) panamensis infection has also been reported [189]. However, the Treg-mediated immune suppression among human patients still needs validation.
Th17 cells can play a role in balancing the pro- and anti-inflammatory responses in experimental models as well as in patients with Leishmania infection [162,163] Elevated levels of IL-17, suggesting activation of Th17 cells, have been observed in patients with CL and MCL, due to L. major [53,190], L. braziliensis [191], L. tropica [192], L. panamensis [191], L. guyanensis, L. amazonensis, and L. naiffi [193] infections. IL-17 also acts as a crucial modulator of adaptive immunity against Leishmania by initiating neutrophil recruitment [162]. At the early phase of VL infection, a strong IL-17 response was observed which progressively reduced to basal level during chronic VL, due to suppression of Th17 cell proliferation by Tregs (CD4+CD25+ T cells). TGF-β and IL-35, derived from CD4+CD25+ T cells, are the key mediators for the downregulation of IL-17 during chronic VL [194]. A recent experiment in BALB/c mice with L. donovani infection showed that low levels of Th17 cytokines, IL-17, IL-22 and IL-23, and elevated levels of IL-6, IL-1β and TGF-β were associated with active infection. Amphotericin B treatment restored production of IL-17 and IL-22, suggesting Th17 cytokines are possibly associated with protection against VL infection [195].
3.2.2. CD8 T Cells
In human CL, CD8+ T cells are capable of aggravating disease pathogenesis as well providing protection to the host [196,197]. Joshi et al. have shown impairment of CD8+ T cell function facilitating survival of L. donovani; chronic infection in the mouse model leads to proliferation of defective/anergic CD8+ T cells [198]. The expressions of negative regulators of T cell activation, Cytotoxic T lymphocytes antigen 4 (CTLA-4) and programmed death protein 1 (PD1) are elevated in VL patients, together with upregulated IL-10 mRNA expression, CD94, CD158a, and CD158b [199]. T cells from VL patients stained more positive for Fas and Annexin-V in comparison to post-treatment or healthy controls [200]. CD8+ T cells secreting IL-10 have been noted in PKDL as well as with L. guanyensis infection [201,202]. In the lesions from PKDL patients, CTLA-4 mRNA expression was higher in the case of pre-treatment compared to post-treatment or controls [203]. B7-H1 (290-amino-acid type I transmembrane glycoprotein belonging to B7-CD28 family) preferentially co-stimulates IL-10 production in resting T cells; PD-1/B7-H1 interaction mediates the inhibition of activated T cell response [204]. Blockade of B7-H1 thus is possibly a way to enhance T cell response and inhibit L. donovani infection [198,199,200,201,202,203,204,205,206].
3.2.3. Defects in Antigen Presentation to T Cells
Leishmania infection-induced defects in antigen presentation has been observed. It inhibits induction of MHC class I and II, as observed in case of L. donovani [131], or brings about defects in peptide loading while expressing normal level of MHC class II in L. amazonensis infection [207,208]. Defective antigen loading has also been observed in L. major infected macrophages [209]. Reduction of MHC class I-restricted antigen presentation upon infection with L donovani parasites has been observed in murine studies. [131,210]
Subsequent to the binding of T-cell receptor (TCR) to the MHC II-peptide complex on the antigen presenting cells (APCs), binding of CD28 or CD40L on T cells to costimulatory molecules such as those of the B7 family or CD40 provides a key element for the exquisite control of T cell activation. Leishmania can interfere with macrophage costimulatory signals. L. donovani infection blocks LPS-mediated B7-1 expression in infected macrophages [211,212]. In the case of infection with L. amazonensis, disruption of CD40/CD40L ligation results in enhanced susceptibility [213] through inhibition of iNOS expression [213,214] and IL-12 production [215] by infected macrophages. Macrophages infected with L. major also show defects in CD40 signalling in a p38-dependent manner [216]. Interaction between CD28 on T cells and B7 molecule is a major costimulatory signal for T cell activation on murine peritoneal macrophages; its expression is also decreased on the surface of L. donovani-infected BALB/c macrophages [212]. L. chagasi infection downregulates CD11b expression in monocytes, diminishes CD54 and HLA-DR expression in infected monocytes, and IFN-γ stimulated HLA-DR and HLA-ABC expression in infected macrophages. There is a negative correlation between CD54 and CD86 expression in both monocytes and macrophages, possibly leading to anergy [210]. Infected macrophages with L. donovani demonstrate profound effects on the self-peptide repertoire presented by MHC I molecules, as evident through changes in antigen processing such as in the composition of proteasomes, altered protein expression and turn-over in different cellular compartments [217].
4. Vaccine Strategies
Antimonial drug-based treatment does not confer significant protection as it is toxic to the host and sometimes fail to achieve recovery due to antimicrobial resistance [218,219]. A range of pre-clinical models using murine, canine, and hamsters have been developed to assess the candidate vaccines to prevent VL and CL. Currently, there is no licenced vaccine for human leishmaniasis although several vaccines are undergoing clinical trials. Recently, two new strains of L. major- MHOH/IL/2019/MRC-01 (L. major MRC-01) and MHOH/IL/2019/MRC-02 (L. major MRC-02) were found in Israel; L. major MRC-02 strain was selected as a new vaccine candidate for CL [220].
4.1. First-Generation Vaccine
The first-ever vaccine against Leishmaniasis, developed in the early 1940s, called ‘Leishmanilization’, has been used for over 60 years [221]. The technique involves injecting live virulent parasites in healthy individuals usually in an inconspicuous area of the body (face or limbs), to protect the recipient from subsequent disfiguring natural infection [222]. This approach was later developed and live virulent L. major promastigotes were harvested and used in large-scale vaccination trials. In 1967, it began in Uzbekistan where a mixture of live and killed promastigotes were used. During the 1970s in Iran and in the 1980s in Israel, Leishmanization (LZ) was initiated, but subsequently discontinued [223]. In Iran, in preliminary trials, ~80% protection was achieved; however, the practice of LZ was discontinued due to major complications including non-healing skin lesions, exacerbation of skin diseases, loss of infectivity of the parasites upon repeated subculturing and the potential impact of immunosuppression [224,225]. LZ got replaced by First generation vaccine based on live attenuated, fractionated Leishmania antigen and killed parasites, offering a safer vaccine.
4.1.1. Live Attenuated Vaccine
Attenuated parasites that are infectious but not pathogenic have major advantages as live vaccines since they can mimic the natural course of infection [226]. Extensive research has been carried out to test the efficacy of attenuated live vaccines in canine and mice models with L. mexicana and L. major for CL, and L. infantum and L. donovani for VL [227]. Razi Vaccine and Serum Institute, Iran conducted phase I and II studies on the safety and immunogenicity of different doses of inactivated L. major promastigotes with or without BCG. BCG was used as an adjuvant in a few versions of the Venezuelan, Ecuadorian and Iranian candidate vaccines to enhance the cell-mediated immunity. A trivalent preparation consisting of L. brazilensis, L. guyanensis and L. amazonensis antigens was evaluated in Ecuador. The autoclaved L. major preparation mixed with BCG adjuvant was used in several field trials in Iran and Sudan, which was later replaced by a formulation involving precipitation of the autoclaved L. major in aluminium hydroxide (alum-ALM). Alum-ALM mixed with BCG showed significantly higher ability to convert the leishmanin skin test [228].
Live attenuated parasites can be classified into two groups depending on the attenuation techniques. Undefined attenuation can be carried out through irradiation or by chemical mutagenesis with or without overt selection; for example, in vitro selection with the aminoglycoside antibiotic gentamicin was used to attenuate L. major, L. mexicana and L. infantum [229]. Defined attenuation involves specific mutagenesis to derive mutant parasites through targeted gene knock-out, where the parasites are unlikely to recover the deleted genes. Several gene targets have been selected for defined attenuated Leishmania vaccines, such as dhfr, lpg2, cpa, cpb, Ufm1, p27, SIR2, BT1, HSP70, centrin, and the paraflagellar rod-2 locus [227,230,231,232]. However, genetically modified vaccines showed varying degree of stability and protection in animal models [227,233]. L. major dihydrofolate reductase-thymidylate synthase (DHFR-TS) gene knock-out was the first to be tested against virulent L. amazonensis and L. major infections as a potential vaccine [234,235] in susceptible and resistant murine models, but failed to confer protective immunity in rhesus monkey [236]. L. mexicana lacking cysteine proteinase genes, cpa and cpb, was found to be protective in murine models [237]. The cpa/cpb-deficient L. mexicana exhibited significantly lower levels of Th2-associated cytokines such as IL-10 and TGF-β than the wild types in the primary lesion of hamsters [238].
Centrin is a calcium-binding cytoskeletal protein required for centrosome duplication and segregation in higher eukaryotes. Attenuated L. donovani strain with centrin gene deletion (Ldcen−/−) when used in BALB/c mice showed growth arrest in amastigotes but promastigotes were unaffected [239]. Ldcen−/− parasites also induced high antibody titre when compared with commercially available vaccine against Canine VL [240]. A single dose (1 × 107 Ldcen−/−) without any adjuvant elicited strong CD4+ and CD8+ T cell activation along with Th1 predominant immune response and considerable reduction in bone marrow L. infantum load [241].
Co-cultured CD4+ T cells and macrophages from dogs, immunized with Ldcen−/− and challenged with L. infantum, exhibit high microbicidal activity [242]. Ldcen−/− vaccination offered significant protection only in young mice against L. donovani which correlated with increased Ig2a antibody, lymphoproliferative response, and significant NO production. However, it failed to induce an adaptive immune response in aged mice [243]. Ldcen−/2W− parasite vaccination also showed efficacy in asymptomatic infection against VL. When C57Bl/6 mice were infected with 103 parasites (i.v.) of wild L. donovani expressing LL0 epitope (bacterial exotoxin listeriolysin-o of L. monocytogenes) and after 3 weeks, they were immunized with Ldcen−/− expressing 2W epitope, the result showed comparable CD4+ T cell proliferation and CD4+ memory cell response (Tcm) in both asymptomatic and naive animals that received Ldcen−/− immunization. They also showed reduction in splenic parasite burden [244]. Intradermal immunization with Ldcen−/−, combined with an adjuvant salivary protein LJM19 from sand fly vector, conferred long-lasting protection against VL in hamsters in a 9-months study period [245]. Banerjee et al. showed that Ldcen−/− parasites induced IL-23-dependent IL-17 production in a murine model of VL [246]. In various CL endemic areas in Iran, the centrin gene in L. infantum expressed more highly in exponential growth phase than in stationary phase, highlighting its essential role in parasite proliferation, and hence, be a promising candidate for developing a genetically modified live attenuated vaccine [247].
L. donovani parasites lacking amastigote specific protein p27 (Ldp27−/−) are safe as an immunogen. Twenty week following virulent challenge (3 × 106 stationary phase Ldp27−/−), a significant reduction in parasite burden, induction of pro-inflammatory cytokines responses, and increased leishmanial activity in association with NO were observed. Thus, Ldp27−/− vaccination is capable of offering long-term protection in BALB/c mice [248]. A genetically modified live attenuated L. major (MRH0/IR/75/ER) strain lacking the p27 gene has been evaluated for its immunogenicity. BALB/c mice were inoculated subcutaneously (s.c.) with 3 × 106 stationary phase Lmp27−/− mutant promastigotes which induced Th1 response, along with no skin lesion and low parasitic burden in liver and spleen [249]. L. major p27 gene knock-out (Lmp27−/−) strain also elicited its protective immunity against homologous (L. major) and heterologous (L. infantum) infections [250]. IFN-γ and IgG2a levels were increased in both immunized groups, while the IFN-γ/IL-4 and IgG2a/IgG1 ratios showed a polarization towards a Th1 response [250].
Li∆HSP70-II attenuated vaccine includes L. infantum deletion mutant, lacking both HSP70-II alleles (ΔHSP70-II). BALB/c VL mice model, vaccinated subcutaneously with 107 Li∆HSP70-II stationary promastigotes in the right footpad, showed a reduction in liver damage and rapid parasite-specific IFN-γ production by CD4+ and CD8+ T cells; however, this vaccine failed to control the chronic phase of the disease [251]. Li∆HSP70-II vaccinated BALB/c mice controlled the progression of CL caused by L. amazonensis infection, with enhanced IFN-γ and systemic Th2 mediated humoral response, and reduction of IL-10 secretion that favours the IFN-γ to activate leishmanicidal activity by macrophages [252].
4.1.2. Killed Parasite Vaccines
In the late 1930s, the pioneering work of Brazilian scientists demonstrated the efficacy of killed parasites as therapeutic as well as prophylactic vaccines against CL and VL [253]. However, no first-generation killed vaccine has shown sufficient efficacy as a prophylactic vaccine [254]. A whole-cell vaccine approach using L. infantam and L. chagasi promastigotes, treated with amotosalen (S-59), a synthetic psoralen, and with low UV irradiation, resulted in permanent covalent DNA cross-linking within parasites. These vaccines were called Killed but Metabolically Active (KBMA). Mice immunized with KBMA stationary-phase promastigotes (3 times at 2-week intervals) were capable of activating macrophages producing NO [255]. Whole-cell killed recombinant L. tropica stress-inducible protein (LFST-II) conferred better protection from whole-cell killed soluble L. tropica antigen (SLA). It showed higher delayed type hypersensitivity (DTH) response and IFN-γ production [256]. L. tropica has also been used to construct DNA vaccine recently based on its ribosomal L5 gene, which exhibited up-regulated Th1 response following leishmanial infection [257].
4.2. 2nd Generation Vaccination
Second-generation vaccines are based on recombinant/synthetic antigens/peptides, recombinant bacteria/virus expressing antigens, or genetically modified Leishmania species, and native fractions purified from parasites [230,258]. Several Leishmania proteins have been identified as vaciine candidates based on their abundance and surface localizations.
4.2.1. gp63
gp63 is a major immunodominant 63-kDa glycoprotein expressed on Leishmania promastigotes that greatly influences host cell signaling mechanisms and aids in survival inside macrophages [259]. Liposomal formulations have been used as a potent adjuvant for gp63-based vaccine. gp63, purified from L. donovani and formulated in cationic distearoylphatidylcholine (DSPC) liposomes, induced protective response against VL in BALB/c mice; animals were intraperitoneally immunized with 3 doses at 2 weeks’ interval (2.5 mg of gp63 entrapped in liposome). Mice showed IFN-γ augmentation and down-regulation of IL-4 [260]. Monophosphoryl lipid-trehalosedicorynomycolate (MPL-TDM)- based liposomes containing gp63 elicited high IgG2a and IFN-γ at three weeks; early immune response was mixed Th1/Th2 along with IL-12 and increased NO from DCs [261]. HLA-A2 restricted peptide derived from L. mexican or L. major gp63, and HLA-DR1 restricted peptide derived from L. major gp63, showed immunogenicity when tested in HHD-II mice (HLA-A0201 transgenic mice containing human HLA-A0201 gene and mouse H2-Kb gene, for MHC Class I in BALB/c mice) and FVB/N-DR1 mice (transgenic mice having HLA-DR1 gene inserted) for MHC Class II, respectively [262,263]. Another vaccine formulation containing HLA-DR1 or HLA-AA2 peptides derived from L. major gp63 with an oily adjuvant (MontanideTM), or both the peptides associated with adjuvant, were able to generate a strong IgG response against L. infantum and cellular response for up to 211 days after the last vaccination for VL without presenting any toxicity in immunized animals [264].
BALB/c mice vaccinated with amastin-gp63 (15 µg) showed the best result among three of the epitope-based vaccines (KMP-11-gp63, Amastin-Gp63, and Amastin-KMP-11). At the 12th week post-infection, parasite reduction rates of KMP-11-gp63, Amastin-gp63, and Amastin-KMP-11 groups were 88.42%, 91.01%, and 89.38%, respectively. These epitope-based vaccines are rich with HLA-DR1, HLA-A2, HLA-A24 restricted pepides. Amastin-gp63 contained a largest number of HLA-A2, HLA-A24, HLA-DR1, and H2-d epitopes, and exhibited better protective efficacy. IgG1 and IgG2 isotype levels were also high post immunization, suggesting that the vaccines induced a dominant Th2 response [265]. Another multi-epitope vaccine approach, using gp63 along with LPG3, Thiol-specific antioxidant (TSA), LmSti1 antigens from L. major, showed poor antigenicity (0.92%) [266].
4.2.2. Leishmania Homolog for Receptors of Activated C Kinase (LACK)
L. infantum Activated protein kinase A receptor analogue (LACK) antigen has been found to confer protection against L. major in BALB/c mice when administered along with IL-12 before infection [267]. Recombinant Lactobacillus lactis, secreting IL-12 and expressing LACK antigen, was administered subcutaneously in mice (0.5 × 109 live bacteria) fortnightly. The delay in footpad swelling with significant reduction in parasite burden in immunized mice was seen; immunization induced Ag-specific CD4+ Th1 and CD8+ CTL response [268]. Oral immunization using live L. lactis co-expressing LACK and IL-12 elicited protective Th1 response against L. major infection in BALB/c mice [269].
L. tarentulae-LACK/KMP-11/EGFP in stationary phase along with CpG oligonucleotide (ODN) adjuvant was tested in mouse left footpad (2 × 107 L. tarentulae–LACK/KMP-11/EGFP parasites plus 20 µg CpG ODN), which showed significant reduction in parasite load [270]. When L. major soluble antigen (SLA) or recombinant LACK (rLACK) antigen were delivered subcutaneously together with cholera vaccine to BALB/c mice, a Th1 type response was observed with increased IFN-γ production; however, an exacerbation of infection occurred following challenge with L. major [271]. Liposomes consisting of 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) encapsulating SLA with polyethelene glycol (PEG) failed to confer protection in the murine model of Leishmania infection [272]. However, immunostimulatory cationic lipids formed from DOTAP, 1,2-dioleoylsn Glycero-3-phosphoethanolamine (DOPE), and cholesterol encapsulating single-gene expression plasmid of pcLACK showed protection against CL [273].
4.2.3. Kinetoplastid Membrane Protein-11 (KMP-11)
KMP-11 is found in both promastigotes and amastigotes of Leishmania. KMP-11 transfected macrophages derived from bone marrow of BALB/c mice and allogenic DCs derived from the bone marrow of C57BL/6 mice, elicited significantly strong CD8+ CTL response [274]. Ex-vivo, KMP-11 pulsed bone marrow-derived DCs and CpG oligonucleotide also induced protection in a murine model of VL by reducing parasite load in visceral organs. This reflected in restored lymphoproliferative responses and modulation of parasite-specific Th1 and Th17 responses, isotope switching towards IgG2a, and enhanced production of IFN-γ. The vaccinated group also showed downregulation of IL-10 and TGF-β, which were upregulated in control mice group, promoting persistence of infection [275]. KMP-11 delivered through DCs elicited protective cellular immunity through pro-inflammatory cytokines (IFN-γ, IL-12, TNF-α), followed by upregulated P38-MAPK signaling [276]. KMP-11, when encapsulated in polyglycolide-co-L-lactide (PGLA) nanoparticles, also reduced the parasitic burden via induction of macrophage-driven pro-inflammatory immune response [277].
In a recent report, eukaryotic subunit vaccine comprising of recombinant LACK and KMP-11 antigen along with CpG ODN adjuvant showed a more effective immune response in BALB/c mice than prokaryotic subunit vaccine by shifting the immune response towards Th1 response [278].
4.2.4. Fucose Mannose Ligand (FML) and Monophosphoryl Lipid A (MPL-A)
The Fucose-mannose ligand (FML) glycoprotein of L. donovani combined with saponin induced 92–97% protection against zoonotic VL [279]. FML pulsed DCs also confer Th1 mediated protection against the lethal infection with L. infantum [280].
In recent years, adjuvant compounds derived from plants have been used to improve the immunogenicity of the vaccine. Glycyrrhizin (GL) is a natural triterpenoid saponin having immunomodulatory activities. When LPS stimulated murine peritoneal macrophages were treated with FML along with various GL concentrations and infected with L. infantum, the result showed enhanced production of NO, TNF-α and IL-12p70 and reduction of IL-10 levels in comparison with FML treatment alone [281]. Recently, Chimera A formulated with Monophosphoryl lipid A (MPL-A) was also used to vaccinate BALB/c mice, followed by i.v. 1 × 107 promastigotes of L. infantum challenge. Brito et al. designed Chimera A by mapping T cell epitopes of known L. infantum antigens [282]. This chimera A formulated with MPL-A stimulated Th1 response with IFN-γ, INF-α, IL-2 expression and decreased IL-4 and IL-10 expression along with reduced splenic parasitic load [283].
4.2.5. Hypothetical, In Silico Protein Constructs and Other Proteins
A vaccine combining two L. braziliensis protein, hypothetical (LbHyp) and the eukaryotic initiation factor 5a (EiF5a), was able to induce heterologous protection against VL [284]. The immunogenicity of another Leishmania-specific hypothetical protein, LiHyT, along with saponin was evaluated in BALB/c mice; the vaccinated mice showed higher levels of IFN-γ, IL-12, and GM-CSF and good titres of LiHyT- and parasite-specific IgG2a antibodies [285]. The recombinant enolase associated with saponin induced high levels of IFN-γ and IL-12 in a murine model along with low levels of IL-4, IL-10, and IgG1 antibody [286]. A peptide-based vaccine has also been constructed based on in silico modelling with 6 HTL, 18 CTL, and 25 B-cell epitopes from three hypothetical membrane proteins of L. donovani. An adjuvant was added at the N-terminus to enhance its immunogenicity. The vaccine construct was found to be non-toxic, non-allergic, and thermally stable. Molecular docking confirmed its binding with TLR-2 [287]. Recently, another fusion vaccine was constructed using immunoinformatics, targeting KMP-11 gene, and B-type flagellin (Fli-C) as an adjuvant; it was found to be a potent stimulator of the cellular and humoral immune response against VL [288].
4.2.6. Vaccines against Canine Leishmaniasis
Immune regulation of L. infantum, the causative agent of most canine leishmaniasis (CanL), requires a balance between the pro-inflammatory CD4+ Th1 cells, which controls parasite replication, and Tregs that induce immunosuppressive/regulatory response that is required to dampen aggravated inflammation. The control of infection by L. infantum (syn. L. chagasi) in dogs is needed to stop the spread of zoonotic VL [289]. Four vaccines are available for the prevention of CanL: Leishmune® and Leish-Tec® in Brazil, and CaniLeish® and LetiFend® in Europe [290].
Leishmune vaccine is the first licensed vaccine against canine visceral leishmaniasis (CVL) registered in Brazil in 2004. It is a FML glycoprotein preparation enriched with L. donovani promastigotes, with the adjuvant Quillaja saponaria saponin, and has undergone phase I, II, and III filed trials [291]. In phase III field trials, the vaccine demonstrated 92% to 95% protection against CVL in the vaccinated group [292]. However, in 2014, the Leishmune® vaccine production and marketing license were withdrawn by the Brazilian Ministry of Agriculture as the company failed to comply with the technical regulation approved in the Interministerial Normative Instruction 31 (IN-31/2007) [293]. Thus, currently, there are three vaccines available against CanL in the market; (1) Leish-Tec® from Ceva Animal Health, Brazil, (2) CaniLeish® from Virbac Santé Animale, France, and (3) Letifend® from Laboratorios Leti, Spain. Their reported efficacies vary between 68.4% and 80% [290,294].
Leish-Tec® comprises a recombinant protein A2 from L. donovani amastigotes and saponin as vaccine adjuvant. It was licensed in Brazil in 2007 and is currently the only authorized CanL vaccine in the country [290]. Leish-Tec® induced protective immunity in beagle dogs against a high dose intravenous infection of L. chagasi; however, it provided only partial protection [295]. Vaccination of sub-clinical dogs with LeishTec® was found to be safe with a mild and site-specific adverse event of 3.06% [296,297]. A study conducted in dogs under field conditions suggested that about 43% of Leish-Tec® recipients developed disease over time [298].
CaniLeish (LiESP/QA-21) is the first European CanL vaccine, released in 2011. It is composed of purified excreted–secreted proteins of L. infantum (LiESP), together with Quilaja saponaria saponin (QA-21) as an adjuvant. LiESP/QA-21 increased significantly IFN-γ secretion and IgG2 production in vaccinated dogs, concomitant with induction of iNOS pathway and production of NO derivatives [299]. Dogs vaccinated with LiESP/QA-21 or CaniLeish® developed Th1 immune response within 3 weeks. In a one-year randomized controlled CaniLeish® vaccine field trial in the CanL Mediterranean endemic area with a heterogeneous canine population, no infection was observed during the first-year [300]; however, the vaccine failed to maintain L. infantum specific IFN-γ at 9 months. Thus, the field trial did not support the previously reported efficacy to prevent active L. infantum infection in dogs from endemic areas and naturally exposed parasites [301].
In 2016, LetiFend® (Laboratorios LETI, Spain) was licensed for veterinary use in Europe [302]. The active component of LetiFend® is recombinant protein Q formed by five antigenic fragments from four different L. infantum proteins (ribosomal proteins LiP2a, LiP2b and LiP0 and the histone H2A), without any adjuvant. Its efficacy was evaluated in different breeds of dogs in the endemic area of Spain and France in randomized, double-blind, placebo-controlled, and field trials. The vaccine reduced the risk of developing CanL following natural infection with L. infantum [303,304].
Use of salivary proteins has recently gained much attention for preparing a vaccine against CVL. A vaccine comprising L. braziliensis promastigote protein, sand fly gland extract (SGE) and saponin adjuvant was evaluated in a dog model. The vaccine exhibited strong antigenicity and induced SGE-specific humoral immune response. Increase in the circulating CD21+ B-cells and CD5+ T-cells was observed [305]. Intradermal challenge with L. infantum plus salivary gland extract in dogs immunized with a vaccine composed of L. braziliensis antigens plus saponin as an adjuvant (LBSap vaccine) was found to elevate total anti-Leishmania IgG as well as IgG1 and IgG2 isotypes in vaccinated dogs. The number of circulating B cells (CD21+) as well as CD4+ and CD8+ T lymphocytes were also high and exhibited a high level of IFN-γ and low IL-10 and TGF-β1 expression in spleen with reduced parasite load [306].
LiESA-MDP is another second-generation dog vaccine candidate, based on a 54 kDa protein derived from L. infantum. Holzmuller et al. showed that a combination of LiESAp and muramyl dipeptide (MDP) induced total protection against experimental CL in 18 healthy Beagle dogs when challenged with 108 metacyclic L. infantum promastigotes [307]. The efficacy of LiESAP-MDP was evaluated in a randomized field trial in a large population of dogs in an endemic area of France; dogs were vaccinated with 100 µg lipophilized LiESAP antigen and 200 µg MDP. After 2 years, the vaccinated dogs showed 92% efficacy, as evident from the detection of Leishmanial DNA in bone marrow aspirates. Enhancement of NO mediated anti-leishmanial activity of canine macrophages were observed with higher IFN-γ production by Th1 cells [308,309].
Another heterologus therapeutic vaccine composed of L. braziliensis with MPL adjuvant (LBMPL) was tested in dogs naturally infected by L. infantum. Animals developed strong antigen-specific lymphoproliferation mainly of CD4+ T cells and CD8+ T cells with high TNF-α and low IL-10 levels. It also blocked transmission of parasites to sandflies in LBMPL-vaccinated dogs [310,311].
Recently, phase I and phase II clinical trial in dogs were performed to evaluate the toxicity of LBSap vaccine (comprising L. braziliansis antigen and saponin), in comparison to Leishmune® and Leish-tech®. The results showed an increase in CD21+ B lymphocytes in case of the Leishmune® group and CD14+ monocytes in LBSap and Leishmune® groups. In the LBSap group, an increase in both IFN-γ producing subpopulations of T cells was observed; in the Leish-Tec® group, an increase in IFN-γ producing CD8+ lymphocytes were observed. The three vaccines, Leishmune®, LiESP/QA-21, and LBSap, did not induce adverse reactions and prevented CL [292].
4.3. Third-Generation Vaccine
Third-generation vaccines are DNA vaccines that provide an extremely flexible method of vaccination for inducing stronger Th1 immune response. gp63, LACK and ORFF are the most extensively studied DNA vaccine candidates against VL [312]. DNA vaccines are advantageous as they are relatively simple to produce and administer, stable, and often very immunogenic than their recombinant protein counterparts [313]. DNA vaccines are able to induce both humoral and cellular immune responses, which can be further tailored through vector modifications, or by incorporation of immunomodulators such as CpG motifs, GM-CSF, Flt3 ligands, and/or cytokine genes [314,315]. The concept of heterologous prime boosting has been employed successfully against Leishmania [316]. The first DNA vaccine had the gp63 gene expressed in S. typhimurium, which stimulated the CD4+ Th1 subset without any detectable DTH response [317]. The level of protection was enhanced by adding gp46 and cysteine proteinase b (CPb) along with gp63 [318]. The use of aluminum phosphate [319] and plasmid encoding CD40L (as adjuvants) further increased the vaccine efficiency [320].
Multi-epitope vaccine comprising gp63, KMP-11, and amastin from Leishmania was used as a prime-boost strategy against VL, where immune response was primed with DNA vaccine, then boosted with protein antigen/vector [321]. NH36 DNA, a main component of FML, together with aluminium phosphate, induced a strong Th1 response providing protection in mice against L. chagasi, L. mexicana and L. amazonensis [319,322,322]. Plasmid DNA encoding TSA and LmSTI1 used alone or in tandem, protected mice against L. major infection by inducing specific CD4+ Th1 responses without requiring any adjuvant [323]. Plasmid cocktails encoding LmSTI1, TSA, and LACK induced full protection against L. major infection [324]. Constructs pCMV3ISS-TSA and pCMV3ISS-LmSTI1 induced a comparable level of protection in mice infected with a highly virulent strain MHOM/TN/95/GLC94 of L. major (Zymodeme MON-25; isolated from a Tunisian patient). Administration of combination plasmids containing LACKp24, TSA, LmsTI1 and CPa genes yielded the strongest protective effects [325]. Immunization of dogs with multicomponent DNA vaccine, which contained genes for L. donovani H1, H2A, H2B, H3, H4, p36 (LACK), PSA-2, TSA, STI1, and ARP-1, resulted in antigen-induced lymphoproliferative and IFN-γ (but not IL-4) response [326].
Leishmania histones are excellent immunogens for the host immune system. Plasmids containing L. infantum nucleosomal histones (H2A, H2B, H3, H4) (dose 25 µg per leg) have been tested in a murine model of L. major infection, which developed a strong Th1 response against CL [327,328]. The intradermal vaccination increased IFN-γ production, in addition to CCXC29, CXCL10, and CC12 [329]. Hamsters immunized with recombinant histone proteins of L. donovani (rLdh2-4) provided protection against VL (107amastigotes) [330]. BALB/c mice, immunized with plasmids expressing H2A, H2B, H3, and H4 histone proteins, and challenged with L. braziliensis, had lesion development prevented [331].
A recombinant lentiviral vaccine expressing a KMP11-Hydrophilic acylated surface protein B (HASPB) fusion protein of L. infantum induced IFN-γ and IL-4, and a specific antibody response against L. infantum, reducing the parasite burden [332]. A DNA vaccine comprising KMP11-HASPB genes of L. major induced a strong immune response in BALB/c mice and reduced the parasitic burden in spleen and lymph [333]. A pEGFP-N1-KMP11-gp96 DNA vaccine also induced a robust Th1 response and protected against L. major (MRHO/IR/75/ER) [334].
Leishmania sp. obtains heme from HbR-mediated endocytosis and is an important glycolytic enzyme in the parasite. Thus, HbR-full length-DNA (HbR-FL) and HbR-N-terminal extracellular domain-DNA (HbR-N-DNA) have been tested in BALB/c mice and hamsters for protection against the AG83 strain of L. donovani; a total reduction in splenic and hepatic parasite burden matched with full protection against VL [335]. Another vaccine, LEISHDNAVAX, comprising plasmids coding for five leishmania antigenic determinants with T cell restricted epitopes (KMP-11, TSA, CPA, CPB and P74), showed protection in mice against L. donovani infection [336].
Recombinant vaccine containing LEISH-F2 and LEISH-F3+, when administered with a TLR4 antagonist, has been shown to induce CD4+ mediated T cell immune response in animal models and provide therapeutic efficacy in CL. RNA vaccines expressing F2 and F3+ sequences have also been found to enhance CD4+ and CD8+ T cell responses, protecting mice against L. donovani infection [337]. A DNA vaccine using LmxMBA gene lacking the transmembrane regions of L. mexicana, when immunized prophylactically, reduced the size of the lesion and parasitic load in the mouse footpad, compared to the control groups [338].
LACK, when administered as a DNA vaccine, is able to protect against L. major and L. infantum in mice, and against L. infantum in Beagle dogs [339,340,341,342,343]. A combination construct containing LACK + Native thiol-specific antioxidant (TSA) increased survival upto 32 weeks following challenge with 2 × 106 L. major promastigotes, compared to the control mice that died within 11 weeks [344]. The protective effect correlated well with strong IFN-γ response and reduced levels of IL-4 [345]. A multivalent vaccine containing LeIF, LACK and TSA genes against CL elicited stronger Th1 immune response, and delayed progression of lesion size [346]. Another multivalent vaccine comprising the most immunogenic regions of L. major (MRHO/IR/75/ER) including TSA, LmST11, LACK and KMP11 was tested in BALB/c mice with or without vector containing IL-12. Mice immunized with lL-12 + multiple antigens constructs showed Th1 polarisation, and hence, reduced parasite burden [347]. However, the protective effect of a prime-boost vaccine using LACK construct in Beagle dogs against L. infantum was suppressed by IL-12 administration [348].
ChAd63-KH is a replication-defective simian adenoviral vector expressing a novel synthetic gene KH (a self-cleaving polyprotein) comprising KMP-11 and HASPB. In the phase I human trial, it induced IFN-γ production significantly and DC activation, in addition to eliciting CD8+ T cells [349]. The phase IIa safety and immunogenicity trial was conducted in Sudanese patients with persistent PKDL. ChAd63-KH vaccine showed minimal adverse reactions and induced potent innate and cell-mediated immune responses [350].
L. infantum pyridoxal kinase (PK) recombinant protein with saponin, or DNA vaccine, significantly reduced the parasite load in spleen, liver, draining lymph nodes and bone marrow of the BALB/c mice challenged with L. infantum. The protective Th1 response was characterized by high levels of IFN-γ, IL-12, GM-CSF and specific IgG2a antibodies, whereas IL-4, IL-10 and parasite-specific IgG1 antibodies were lowered [351].
As an innovative strategy, an immunomodulatory drug, sirolimus (SIR), was also used to boost a prophylactic DNA vaccine against leishmaniasis. Syrian hamsters were treated with SIR along a DNA vaccine consisting of four plasmids (LACK, TRYP, PAPLE22 and KMPII). Although the DNA vaccine on its own efficiently reduced the burden of parasites in the skin and lymph nodes, SIR offered enhanced protection [352].
5. Conclusions
Early inflammatory events inducing macrophage dysfunction and T-cell dependent immune response direct the species-specific pathogenesis in Leishmaniasis. The intracellular strategies by the parasite and the subtlities of the host–pathogen stand off that allow the parasite to reside indefinitely inside the macrophage, make the cure challenging. Controlling Leishmaniasis through vaccination is also a challenge. Although a number of vaccine candidates in the form of plasmid coding genes, defined subunits, live or killed or fractionated forms have been evaluated, only a small number of them have proven promising. Several attenuated and protein-based vaccines have shown encouraging results in offering protection against different species of Leishmania in animal models, but their potential to revert back to virulent parasite is fraught with risk. Use of nano-carriers for Leishmania vaccine containing combination vaccines with adjuvants also seems promising; however, the cure from Leishmaniasis is still a dream.
Author Contributions
Conceptualization, H.Y and U.K.; writing—original draft preparation, H.Y. and A.A.; writing—review and editing, U.K., M.N.A.-A. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Syamal Roy acknowledges the Indian Council of Medical Research, Government of India for his Emeritus position. Illustrations were created using the Biorender tool by Hadida Yasmin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ashford, R.W. Leishmaniasis reservoirs and their significance in control. Clin. Dermatol. 1996, 14, 523–532. [Google Scholar] [CrossRef]
- Weekly Epidemiological Report. 2020. Available online: http://www.who.int/wer (accessed on 10 January 2022).
- Herwaltd, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Bailey, M.S.; Lockwood, D.N. Cutaneous leishmaniasis. Clin. Dermatol. 2007, 25, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Weigle, K.; Saravia, N.G. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous Leishmania-sis. Clin. Dermatol. 1996, 14, 433–450. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Gregory, D.J.; Sladek, R.; Olivier, M.; Matlashewski, G. Comparison of the effects of Leishmania major or Leishmania do-novani infection on macrophage gene expression. Infect. Immun. 2008, 76, 1186–1192. [Google Scholar] [CrossRef]
- Cruz, I.; Morales, M.A.; Noguer, I.; Rodríguez, A.; Alvar, J. Leishmania in discarded syringes from intravenous drug users. Lancet 2002, 359, 1124–1125. [Google Scholar] [CrossRef]
- Pagliano, P.; Carannante, N.; Rossi, M.; Gramiccia, M.; Gradoni, L.; Faella, F.S.; Gaeta, G.B. Visceral leishmaniasis in pregnancy: A case series and a systematic review of the literature. J. Antimicrob. Chemother. 2005, 55, 229–233. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Gomes, R.B.B.; Silva, M.R.B.; Garcez, L.M.; Ramos, P.K.; Santos, R.S.; Shaw, J.J.; David, J.R.; Maguire, J.H. Competence of the Human Host as a Reservoir forLeishmania chagasi. J. Infect. Dis. 2000, 182, 997–1000. [Google Scholar] [CrossRef]
- Chitimia, L.; Muñoz-García, C.; Sánchez-Velasco, D.; Lizana, V.; del Río, L.; Murcia, L.; Fisa, R.; Riera, C.; Giménez-Font, P.; Jiménez-Montalbán, P.; et al. Cryptic Leishmaniosis by Leishmania infantum, a feature of canines only? A study of natural infection in wild rabbits, humans and dogs in southeastern Spain. Vet. Parasitol. 2011, 181, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Dipineto, L.; Manna, L.; Baiano, A.; Gala, M.; Fioretti, A.; Gravino, A.E.; Menna, L.F. Presence of Leishmania infantum in Red Foxes (Vulpes vulpes) in Southern Italy. J. Wildl. Dis. 2007, 43, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, M.K.; Andreadou, M.; Leontides, L.; Kasabalis, D.; Mylonakis, M.; Koutinas, A.F.; Rallis, T.; Ikonomopoulos, J.; Saridomichelakis, M.N. Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet. Parasitol. 2014, 202, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.S.; Ferreira, E.; Lima, A.C.V.M.d.R.; Tonelli, G.B.; Rêgo, F.D.; Paglia, A.P.; Andrade-Filho, J.D.; Paz, G.F.; Gontijo, C.M.F. Detection of Leishmania spp in silvatic mammals and isolation of Leishmania (Viannia) braziliensis from Rattus rattus in an endemic area for leishmaniasis in Minas Gerais State, Brazil. PLoS ONE 2017, 12, e0187704. [Google Scholar] [CrossRef]
- Singh, N.; Mishra, J.; Singh, R.; Singh, S. Animal Reservoirs of Visceral Leishmaniasis in India. J. Parasitol. 2013, 99, 64–67. [Google Scholar] [CrossRef]
- Oryan, A.; Akbari, M. Worldwide risk factors in leishmaniasis. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef]
- Cloots, K.; Marino, P.; Burza, S.; Gill, N.; Boelaert, M.; Hasker, E. Visceral Leishmaniasis-HIV Coinfection as a Predictor of Increased Leishmania Transmission at the Village Level in Bihar, India. Front. Cell. Infect. Microbiol. 2021, 11, 604117. [Google Scholar] [CrossRef]
- Aschale, Y.; Ayehu, A.; Worku, L.; Tesfa, H.; Birhanie, M.; Lemma, W. Malaria-visceral leishmaniasis co-infection and associated factors among migrant laborers in West Armachiho district, North West Ethiopia: Community based cross-sectional study. BMC Infect. Dis. 2019, 19, 239. [Google Scholar] [CrossRef]
- Shen, S.-S.; Qu, X.-Y.; Zhang, W.-Z.; Li, J.; Lv, Z.-Y. Infection against infection: Parasite antagonism against parasites, viruses and bacteria. Infect. Dis. Poverty 2019, 8, 49. [Google Scholar] [CrossRef]
- Bates, P.A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef]
- Rogers, M.E.; Ilg, T.; Nikolaev, A.V.; Ferguson, M.; Bates, P.A. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 2004, 430, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.; Rogers, M. New Insights into the Developmental Biology and Transmission Mechanisms of Leishmania. Curr. Mol. Med. 2004, 4, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Gossage, S.M.; Rogers, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: Implications for understanding the life cycle. Int. J. Parasitol. 2003, 33, 1027–1034. [Google Scholar] [CrossRef]
- Killick-Kendrick, R.; Molyneux, D.H.; Ashford, R.W. Leishmania in phlebotomid sandflies. I. Modifications of the flagellum associated with attachment to the mid-gut and oesophageal valve of the sandfly. Proc. R Soc. Lond. B Biol. Sci. 1974, 187, 409–419. [Google Scholar]
- Giraud, E.; Martin, O.; Yakob, L.; Rogers, M. Quantifying Leishmania Metacyclic Promastigotes from Individual Sandfly Bites Reveals the Efficiency of Vector Transmission. Commun. Biol. 2019, 2, 84. [Google Scholar] [CrossRef]
- Bates, P.A. Revising Leishmania’s life cycle. Nat. Microbiol. 2018, 3, 529–530. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Sunter, J.; Gull, K. Shape, form, function and Leishmania pathogenicity: From textbook descriptions to biological understanding. Open Biol. 2017, 7, 170165. [Google Scholar] [CrossRef]
- Rittig, M.G.; Schröppel, K.; Seack, K.H.; Sander, U.; N’Diaye, E.N.; Maridonneau-Parini, I.; Solbach, W.; Bogdan, C. Coiling phag-ocytosis of trypanosomatids and fungal cells. Infect. Immun. 1998, 66, 4331–4339. [Google Scholar] [CrossRef]
- Petropolis, D.B.; Rodrigues, J.C.; Viana, N.B.; Pontes, B.; Pereira, C.F.; Silva-Filho, F.C. Leishmania amazonensis promastigotes in 3D Collagen I culture: An in vitro physiological environment for the study of extracellular matrix and host cell interactions. PeerJ 2014, 2, e317. [Google Scholar] [CrossRef]
- Dedet, J.P.; Pratlong, F.; Lanotte, G.; Ravel, C. Cutaneous leishmaniasis. The parasite. Clin. Dermatol. 1999, 17, 261–268. [Google Scholar] [CrossRef]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Naderer, T.; McConville, M.J. The Leishmania-macrophage interaction: A metabolic perspective. Cell. Microbiol. 2008, 10, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.K.; Bovis, L.; Matura, R.; Zhu, B.; He, S.; Lum, H.; Turco, S.J.; Ho, J.L. Leishmania lipophosphoglycan reduces monocyte transendothelial migration: Modulation of cell adhesion molecules, intercellular junctional proteins, and chemoattractants. J. Immunol. 1998, 160, 1857–1865. [Google Scholar] [PubMed]
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef]
- Novais, F.O.; Santiago, R.C.; Báfica, A.; Khouri, R.; Afonso, L.; Borges, V.; Brodskyn, C.; Netto, M.B.; Barral, A.; De Oliveira, C.I. Neutrophils and Macrophages Cooperate in Host Resistance against Leishmania braziliensis Infection. J. Immunol. 2009, 183, 8088–8098. [Google Scholar] [CrossRef]
- de Souza Carmo, E.V.; Katz, S.; Barbiéri, C.L. Neutrophils reduce the parasite burden in Leishmania (Leishmania) amazonensis-infected macrophages. PLoS ONE 2010, 3, e13815. [Google Scholar]
- Pearson, R.D.; Steigbigel, R.T. Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J. Immunol. 1981, 127, 1438–1443. [Google Scholar]
- Guimaraes-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceicao-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Titus, R.G.; Ribeiro, J.M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 1988, 239, 1306–1308. [Google Scholar] [CrossRef]
- Dey, R.; Joshi, A.B.; Oliveira, F.; Pereira, L.; Guimarães-Costa, A.B.; Serafim, T.D.; de Castro, W.; Coutinho-Abreu, I.V.; Bhattacharya, P.; Townsend, S.; et al. Gut Microbes Egested during Bites of Infected Sand Flies Augment Severity of Leishmaniasis via Inflammasome-Derived IL-1β. Cell Host Microbe 2018, 23, 134.e6–143.e6. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.C.; Oliveira, F.; Debrabant, A.; Valenzuela, J.G.; Ribeiro, J.M.; Calvo, E. Lundep, a sand fly salivary endonuclease in-creases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 2014, 10, e1003923. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Costa, A.B.; Shannon, J.P.; Waclawiak, I.; Oliveira, J.; Meneses, C.; de Castro, W.; Wen, X.; Brzostowski, J.; Serafim, T.D.; Andersen, J.F.; et al. A sand fly salivary protein acts as a neutrophil chemo-attractant. Nat. Commun. 2021, 12, 3213. [Google Scholar] [CrossRef] [PubMed]
- Sangare, M.; Coulibaly, Y.I.; Huda, N.; Vidal, S.; Tariq, S.; Coulibaly, M.E.; Coulibaly, S.Y.; Soumaoro, L.; Dicko, I.; Traore, B.; et al. Individuals co-exposed to sand fly saliva and filarial parasites exhibit altered monocyte function. PLoS Negl. Trop. Dis. 2021, 15, e0009448. [Google Scholar] [CrossRef]
- Charmoy, M.; Megnekou, R.; Allenbach, C.; Zweifel, C.; Perez, C.; Monnat, K.; Breton, M.; Ronet, C.; Launois, P.; Tacchini-Cottier, F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukoc. Biol. 2007, 82, 288–299. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Moniz-de-Souza, M.C.; Alexandre-Moreira, M.S.; Dias, W.B.; Lopes, M.F.; Nunes, M.P.; Lungarella, G.; DosReis, G.A. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 2007, 179, 3988–3994. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Sacks, D. The influence of early neutrophil-Leishmania interactions on the host immune response to infec-tion. Front. Cell. Infect. Microbiol. 2012, 2, 59. [Google Scholar] [CrossRef]
- Singh, T.P.; Carvalho, A.M.; Sacramento, L.A.; Grice, E.A.; Scott, P. Microbiota instruct IL-17A-producing innate lymphoid cells to promote skin inflammation in cutaneous leishmaniasis. PLOS Pathog. 2021, 17, e1009693. [Google Scholar] [CrossRef]
- van Zandbergen, G.; Hermann, N.; Laufs, H.; Solbach, M.D.W.; Laskay, T. Leishmania Promastigotes Release a Granulocyte Chemotactic Factor and Induce Interleukin-8 Release but Inhibit Gamma Interferon-Inducible Protein 10 Production by Neutrophil Granulocytes. Infect. Immun. 2002, 70, 4177–4184. [Google Scholar] [CrossRef]
- Kostka, S.L.; Dinges, S.; Griewank, K.; Iwakura, Y.; Udey, M.C.; Von Stebut, E. IL-17 Promotes Progression of Cutaneous Leishmaniasis in Susceptible Mice. J. Immunol. 2009, 182, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, V.S.; Santos, C.S.; Cardoso, C.R.D.B.; De Andrade, J.; Dos-Santos, W.L.; Clarêncio, J.; Silva, J.S.; Borges, V.; Netto, M.B.; Brodskyn, C.I.; et al. Human mucosal leishmaniasis: Neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur. J. Immunol. 2010, 40, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- van Zandbergen, G.; Klinger, M.; Mueller, A.; Dannenberg, S.; Gebert, A.; Solbach, W.; Laskay, T. Cutting Edge: Neutrophil Granulocyte Serves as a Vector for Leishmania Entry into Macrophages. J. Immunol. 2004, 173, 6521–6525. [Google Scholar] [CrossRef] [PubMed]
- Gueirard, P.; Laplante, A.; Rondeau, C.; Milon, G.; Desjardins, M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell. Microbiol. 2008, 10, 100–111. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef]
- Beverley, S.M.; Turco, S.J. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998, 6, 135–140. [Google Scholar] [CrossRef]
- Ferguson, M. The surface glycoconjugates of trypanosomatid parasites. Philos. Trans. R Soc. Lond. B Biol. Sci. 1997, 352, 1295–1302. [Google Scholar] [CrossRef]
- McConville, M.J.; Ralton, J.E. Developmentally regulated changes in the cell surface architecture of Leishmania parasites. Behring Inst. Mitt. 1997, 99, 34–43. [Google Scholar]
- Ferguson, M.A. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 1999, 112, 2799–2809. [Google Scholar] [CrossRef]
- Kane, M.M.; Mosser, D.M. Leishmania parasites and their ploys to disrupt macrophage activation. Curr. Opin. Hematol. 2000, 7, 26–31. [Google Scholar] [CrossRef]
- Joshi, P.B.; Kelly, B.L.; Kamhawi, S.; Sacks, D.L.; McMaster, W.R. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 2002, 120, 33–40. [Google Scholar] [CrossRef]
- Pelletier, I.; Hashidate, T.; Urashima, T.; Nishi, N.; Nakamura, T.; Futai, M.; Arata, Y.; Kasai, K.; Hirashima, M.; Hirabayashi, J.; et al. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: Possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 2003, 278, 22223–22230. [Google Scholar] [CrossRef] [PubMed]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A Role for Insect Galectins in Parasite Survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Soulat, D.; Bogdan, C. Function of Macrophage and Parasite Phosphatases in Leishmaniasis. Front. Immunol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pandey, S.P.; Jha, M.K.; Chandel, H.S.; Saha, B. Leishmania expressed lipophosphoglycan interacts with Toll-like receptor (TLR)-2 to decrease TLR-9 expression and reduce anti-leishmanial responses. Clin. Exp. Immunol. 2013, 172, 403–409. [Google Scholar] [CrossRef]
- Frankenburg, S.; Leibovici, V.; Mansbach, N.; Turco, S.J.; Rosen, G. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J. Immunol. 1990, 145, 4284–4289. [Google Scholar]
- Ibraim, I.C.; de Assis, R.R.; Pessoa, N.L.; Campos, M.A.; Melo, M.N.; Turco, S.J.; Soares, R.P. Two biochemically distinct lipophosphoglycans from Leishmania braziliensis and Leishmania infantum trigger different innate immune responses in murine macrophages. Parasites Vectors 2013, 6, 54. [Google Scholar] [CrossRef]
- Barral-Netto, M.; Barral, A.; Brownell, C.E.; Skeiky, Y.A.; Ellingsworth, L.R.; Twardzik, D.R.; Reed, S.G. Transforming growth factor-beta in leishmanial infection: A parasite escape mechanism. Science 1992, 257, 545–548. [Google Scholar] [CrossRef]
- Chang, K.; Fong, D. Cell Biology of Host-Parasite Membrane Interactions in Leishmaniasis. Ciba Found. Symp. 1983, 99, 113–137. [Google Scholar] [CrossRef]
- Forestier, C.-L.; Machu, C.; Loussert, C.; Pescher, P.; Späth, G.F. Imaging Host Cell-Leishmania Interaction Dynamics Implicates Parasite Motility, Lysosome Recruitment, and Host Cell Wounding in the Infection Process. Cell Host Microbe 2011, 9, 319–330. [Google Scholar] [CrossRef]
- Gluenz, E.; Ginger, M.L.; McKean, P.G. Flagellum assembly and function during the Leishmania life cycle. Curr. Opin. Microbiol. 2010, 13, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gluenz, E.; Höög, J.L.; Smith, A.E.; Dawe, H.R.; Shaw, M.K.; Gull, K. Beyond 9+0: Noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010, 24, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Burchmore, R.J.; Barrett, M. Life in vacuoles—Nutrient acquisition by Leishmania amastigotes. Int. J. Parasitol. 2001, 31, 1311–1320. [Google Scholar] [CrossRef]
- Dermine, J.-F.; Goyette, G.; Houde, M.; Turco, S.J.; Desjardins, M. Leishmania donovani lipophosphoglycan disrupts phagosome microdomains in J774 macrophages. Cell. Microbiol. 2005, 7, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Vinet, A.F.; Fukuda, M.; Turco, S.J.; Descoteaux, A. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog. 2009, 5, e1000628. [Google Scholar] [CrossRef]
- Chakraborty, P.; Das, P.K. Suppression of macrophage lysosomal enzymes after Leishmania donovani infection. Biochem. Med. Metab. Biol. 1989, 41, 46–55. [Google Scholar] [CrossRef]
- Henriques, C.; Atella, G.C.; Bonilha, V.L.; de Souza, W. Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitol. Res. 2003, 89, 123–133. [Google Scholar]
- Courret, N.; Fréhel, C.; Gouhier, N.; Pouchelet, M.; Prina, E.; Roux, P.; Antoine, J.C. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites. J. Cell Sci. 2002, 115, 2303–2316. [Google Scholar] [CrossRef]
- Körner, U.; Fuss, V.; Steigerwald, J.; Moll, H. Biogenesis of Leishmania major -Harboring Vacuoles in Murine Dendritic Cells. Infect. Immun. 2006, 74, 1305–1312. [Google Scholar] [CrossRef]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the macrophage: A multifaceted interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Mittra, B.; Andrews, N.W. IRONy OF FATE: Role of iron-mediated ROS in Leishmania differentiation. Trends Parasitol. 2013, 29, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mittra, B.; Cortez, M.; Haydock, A.; Ramasamy, G.; Myler, P.; Andrews, N. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 2013, 210, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.A.; Baatz, J.E.; Kreishman, G.P.; Mukkada, A.J. pH homeostasis in Leishmania donovani amastigotes and promastigotes. Proc. Natl. Acad. Sci. USA 1988, 85, 7602–7606. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Schlesinger, P.H.; Steinberg, T.H.; Mangel, W.F.; Kobayashi, T.; Russell, D.G. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 1999, 112, 681–693. [Google Scholar] [CrossRef]
- Real, F.; Mortara, R.A.; Rabinovitch, M. Fusion between Leishmania amazonensis and Leishmania major Parasitophorous Vacuoles: Live Imaging of Coinfected Macrophages. PLoS Negl. Trop. Dis. 2010, 4, e905. [Google Scholar] [CrossRef]
- Chang, K.-P.; Dwyer, D. Leishmania Donovani. Hamster macrophage interactions in vitro: Cell entry, intracellular survival, and multiplication of amastigotes. J. Exp. Med. 1978, 147, 515–530. [Google Scholar] [CrossRef]
- Saha, S.; Basu, M.; Guin, S.; Gupta, P.; Mitterstiller, A.-M.; Weiss, G.; Jana, K.; Ukil, A. Leishmania donovani Exploits Macrophage Heme Oxygenase-1 To Neutralize Oxidative Burst and TLR Signaling–Dependent Host Defense. J. Immunol. 2018, 202, 827–840. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Charles, I.G.; Smith, A.C.; Ure, J.; Feng, G.-J.; Huang, F.-P.; Xu, D.; Muller, W.; Moncada, S.; Liew, F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375, 408–411. [Google Scholar] [CrossRef]
- Murray, H.W.; Nathan, C.F. Macrophage Microbicidal Mechanisms In Vivo: Reactive Nitrogen versus Oxygen Intermediates in the Killing of Intracellular Visceral Leishmania donovani. J. Exp. Med. 1999, 189, 741–746. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Mallory, F.B. A histological study of typhoid fever. J. Exp. Med. 1898, 3, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kaarthigeyan, K.; Aparna, V.; Srinivas, S. Tuberculosis associated hemophagocytic syndrome in infancy. Indian Pediatr. 2008, 45, 593–595. [Google Scholar] [PubMed]
- Slovut, D.P.; Benedetti, E.; Matas, A.J. Babesiosis and hemophagocytic syndrome in an asplenic renal transplant recipient. Transplantation 1996, 62, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Granert, C.; Elinder, G.; Ost, A.; Henter, J.I. Kala-azar in a one-year-old Swedish child. Diagnostic difficulties because of active hemophagocytosis. Acta Paediatr. 1993, 82, 794–796. [Google Scholar] [CrossRef]
- Fathalla, M.; Hashim, J.; Alkindy, H.; Wali, Y. Cerebrospinal fluid involvement in a case of visceral leishmaniasis associated with hemophagocytic lymphohistiocytosis. Sultan Qaboos Univ. Med. J. 2007, 7, 253–256. [Google Scholar]
- Kilani, B.; Ammari, L.; Kanoun, F.; Ben Chaabane, T.; Abdellatif, S.; Chaker, E. Hemophagocytic syndrome associated with visceral leishmaniasis. Int. J. Infect. Dis. 2006, 10, 85–86. [Google Scholar] [CrossRef][Green Version]
- Ozyurek, E.; Özçay, F.; Yilmaz, B.; Özbek, N. Hemophagocytic lymphohistiocytosis associated with visceral Leishmaniasis: A Case Report. Pediatr. Hematol. Oncol. 2005, 22, 409–414. [Google Scholar] [CrossRef]
- Gagnaire, M.-H.; Galambrun, C.; Stéphan, J.L. Hemophagocytic Syndrome: A Misleading Complication of Visceral Leishmaniasis in Children—A Series of 12 Cases. Pediatrics 2000, 106, e58. [Google Scholar] [CrossRef]
- Marom, D.; Offer, I.; Tamary, H.; Jaffe, C.L.; Garty, B.Z. Hemophagocytic Lymphohistocytosis associated with Visceral Leishmaniasis. J. Pediatr. Hematol. Oncol. 2001, 18, 65–70. [Google Scholar] [CrossRef]
- Bode, S.; Bogdan, C.; Beutel, K.; Behnisch, W.; Greiner, J.; Henning, S.; Jorch, N.; Jankofsky, M.; Jakob, M.; Schmid, I.; et al. Hemophagocytic Lymphohistiocytosis in Imported Pediatric Visceral Leishmaniasis in a Nonendemic Area. J. Pediatr. 2014, 165, 147.e1–153.e1. [Google Scholar] [CrossRef]
- Pilonieta, M.C.; Moreland, S.M.; English, C.N.; Detweiler, C.S. Salmonella enterica infection stimulates macrophages to hemophagocytose. mBio 2014, 5, e02211. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.E.; McCoy, M.W.; Pilonieta, M.C.; Nix, R.N.; Detweiler, C.S. Chronic Murine Typhoid Fever Is a Natural Model of Secondary Hemophagocytic Lymphohistiocytosis. PLoS ONE 2010, 5, e9441. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Hildeman, D.; Kappler, J.; Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004, 104, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Brisse, E.; Imbrechts, M.; Put, K.; Avau, A.; Mitera, T.; Berghmans, N.; Rutgeerts, O.; Waer, M.; Ninivaggi, M.; Kelchtermans, H.; et al. Mouse Cytomegalovirus Infection in BALB/c Mice Resembles Virus-Associated Secondary Hemophagocytic Lymphohistiocytosis and Shows a Pathogenesis Distinct from Primary Hemophagocytic Lymphohistiocytosis. J. Immunol. 2016, 196, 3124–3134. [Google Scholar] [CrossRef]
- Cnops, J.; De Trez, C.; Stijlemans, B.; Keirsse, J.; Kauffmann, F.; Barkhuizen, M.; Keeton, R.; Boon, L.; Brombacher, F.; Magez, S. NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia. PLoS Pathog. 2015, 11, e1004964. [Google Scholar] [CrossRef]
- Morimoto, A.; Omachi, S.; Osada, Y.; Chambers, J.K.; Uchida, K.; Sanjoba, C.; Matsumoto, Y.; Goto, Y. Hemophagocytosis in Experimental Visceral Leishmaniasis by Leishmania donovani. PLoS Negl. Trop. Dis. 2016, 10, e0004505. [Google Scholar] [CrossRef]
- Marquis, J.F.; Gros, P. Intracellular Leishmania: Your iron or mine? Trends Microbiol. 2007, 15, 93–95. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Goswami, T.; Evans, C.A.; Sibthorpe, D.; Papo, N.; White, J.K.; Searle, S.; Miller, E.N.; Peacock, C.S.; Mohammed, H.; et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001, 3, 773–784. [Google Scholar] [CrossRef]
- Banerjee, S.; Datta, R. Leishmania infection triggers hepcidin-mediated proteasomal degradation of Nramp1 to increase phagolysosomal iron availability. Cell Microbiol. 2020, 22, e13253. [Google Scholar] [CrossRef]
- Das, N.K.; Biswas, S.; Solanki, S.; Mukhopadhyay, C.K. Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cell Microbiol. 2009, 11, 83–94. [Google Scholar] [CrossRef]
- Borges, V.M.; Vannier-Santos, M.A.; De Souza, W. Subverted transferrin trafficking in Leishmania-infected macrophages. Parasitol Res. 1998, 84, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Ball, W.B.; Gupta, P.; Giri, J.; Ukil, A.; Das, P.K. Leishmania donovani Prevents Oxidative Burst-mediated Apoptosis of Host Macrophages through Selective Induction of Suppressors of Cytokine Signaling (SOCS) Proteins. J. Biol. Chem. 2014, 289, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Guha, R.; Das, S.; Roy, S. Liposomal cholesterol delivery activates the macrophage innate immune arm to facilitate intracellular Leishmania donovani killing. Infect. Immun. 2014, 82, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Lal, C.S.; Pandey, K.; Das, V.N.R.; Das, P.; Roychoudhury, K.; Roy, S. Human visceral leishmaniasis: Decrease in serum cholesterol as a function of splenic parasite load. Ann. Trop. Med. Parasitol. 2011, 105, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, H.B.-B.; Guichard, M.; Lawton, P.; Delton, I.; Azzouz-Maache, S. Changes in Lipid and Fatty Acid Composition During Intramacrophagic Transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids 2017, 52, 433–441. [Google Scholar] [CrossRef]
- Zhang, K.; Beverley, S.M. Phospholipid and sphingolipid metabolism in Leishmania. Mol. Biochem. Parasitol. 2010, 170, 55–64. [Google Scholar] [CrossRef]
- Zhang, O.; Wilson, M.C.; Xu, W.; Hsu, F.F.; Turk, J.; Kuhlmann, F.M.; Wang, Y.; Soong, L.; Key, P.; Beverley, S.M.; et al. Deg-radation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathog. 2009, 5, e1000692. [Google Scholar] [CrossRef]
- Vallochi, A.L.; Teixeira, L.; Oliveira, K.D.S.; Maya-Monteiro, C.M.; Bozza, P.T. Lipid Droplet, a Key Player in Host-Parasite In-teractions. Front. Immunol. 2018, 9, 1022. [Google Scholar] [CrossRef]
- De Cicco, N.N.; Pereira, M.G.; Corrêa, J.R.; Andrade-Neto, V.V.; Saraiva, F.B.; Chagas-Lima, A.C.; Gondim, K.C.; Torres-Santos, E.C.; Folly, E.; Saraiva, E.M.; et al. LDL uptake by Leishmania amazonensis: Involvement of membrane lipid microdomains. Exp. Parasitol. 2012, 130, 330–340. [Google Scholar] [CrossRef]
- Das, P.; De, T.; Chakraborti, T. Leishmania donovani secretory serine protease alters macrophage inflammatory response via COX-2 mediated PGE-2 production. Indian J. Biochem. Biophys. 2014, 51, 5542–5551. [Google Scholar]
- Carregaro, V.; Valenzuela, J.G.; Cunha, T.M.; Verri, W.A., Jr.; Grespan, R.; Matsumura, G.; Ribeiro, J.M.; Elnaiem, D.E.; Silva, J.S.; Cunha, F.Q. Phlebotomine salivas inhibit immune inflammation-induced neutrophil migration via an autocrine DC-derived PGE 2/IL-10 sequential pathway. J. Leukoc. Biol. 2008, 84, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Santos, T.; Prates, D.B.; Andrade, B.B.; Nascimento, D.O.; Clarêncio, J.; Entringer, P.F.; Carneiro, A.B.; Silva-Neto, M.A.C.; Miranda, J.C.; Brodskyn, C.I.; et al. Lutzomyia longipalpis Saliva Triggers Lipid Body Formation and Prostaglandin E2 Production in Murine Macrophages. PLoS Negl. Trop. Dis. 2010, 4, e873. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, S.; Rabhi, I.; Trentin, B.; Piquemal, D.; Regnault, B.; Goyard, S.; Lang, T.; Descoteaux, A.; Enninga, J.; Guizani-Tabbane, L. Lipid droplet formation, their localization and dynamics during Leishmania major macrophage infection. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.I.; Muxel, S.M.; Zampieri, R.A.; Müller, K.E.; Nerland, A.H.; Floeter-Winter, L.M. Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles. Sci. Rep. 2019, 9, 19841. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef]
- Kumar, A.; Das, S.; Mandal, A.; Verma, S.; Abhishek, K.; Kumar, A.; Kumar, V.; Ghosh, A.K.; Das, P. Leishmania infection activates host mTOR for its survival by M2 macrophage polarization. Parasite Immunol. 2018, 40, e12586. [Google Scholar] [CrossRef]
- Parmar, N.; Chandrakar, P.; Kar, S. Leishmania donovani Subverts Host Immune Response by Epigenetic Reprogramming of Macrophage M(Lipopolysaccharides + IFN-γ)/M(IL-10) Polarization. J. Immunol. 2020, 204, 2762–2778. [Google Scholar] [CrossRef]
- Descoteaux, A.; Matlashewski, G. c-fos and TNF gene expression in Leishmania donovani–infected macrophages. Mol. Cell. Biol. 1989, 9, 5223–5227. [Google Scholar]
- Reiner, N.E.; Ng, W.; McMaster, W.R. Parasite-accessory cell-interactions in murine leishmaniasis 2. Leishmania donovani suppresses macrophage expression of class-I and class-II major histocompatibility complex gene-products. J. Immunol. 1987, 138, 1926–1932. [Google Scholar]
- Olivier, M.; Tanner, C.E. Effect of cyclosporin A in murine leishmaniasis. Trop. Med. Parasitol. 1989, 40, 32–38. [Google Scholar] [PubMed]
- Hatzigeorgiou, D.E.; Geng, J.; Zhu, B.; Zhang, Y.; Liu, K.; Rom, W.N.; Fenton, M.J.; Turco, S.J.; Ho, J.L. Lipophosphoglycan from Leishmania suppresses agonist-induced interleukin 1 beta gene expression in human monocytes via a unique promoter se-quence. Proc. Natl. Acad. Sci. USA 1996, 93, 14708–14713. [Google Scholar] [CrossRef] [PubMed]
- Cillari, E.; Dieli, M.; Maltese, E.; Milano, S.; Salerno, A.; Liew, F.Y. Enhancement of macrophage IL-1 production by Leishmania major infection in vitro and its inhibition by IFN-g. J. Immunol. 1989, 143, 2001–2005. [Google Scholar] [PubMed]
- Matte, C.; Olivier, M. Leishmania-Induced Cellular Recruitment during the Early Inflammatory Response: Modulation of Proinflammatory Mediators. J. Infect. Dis. 2002, 185, 673–681. [Google Scholar] [CrossRef]
- Ghalib, H.W.; Piuvezam, M.R.; Skeiky, Y.A.; Siddig, M.; Hashim, F.A.; El-Hassan, A.M.; Russo, D.M.; Reed, S.G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Investig. 1993, 92, 324–329. [Google Scholar] [CrossRef]
- Nylen, S.R.; Maurya, L.; Eidsmo, K.D.; Manandhar, S.S.; Sacks, D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 2007, 204, 805–817. [Google Scholar] [CrossRef]
- Panaro, M.; Acquafredda, A.; Lisi, S.; Lofrumento, D.; Mitolo, V.; Sisto, M.; Fasanella, A.; Trotta, T.; Bertani, F.; Consenti, B.; et al. Nitric oxide production by macrophages of dogs vaccinated with killed Leishmania infantum promastigotes. Comp. Immunol. Microbiol. Infect. Dis. 2001, 24, 187–195. [Google Scholar] [CrossRef]
- Pinelli, E.; Gebhard, D.; Mommaas, A.; van Hoeij, M.; Langermans, J.A.; Ruitenberg, E.; Rutten, V.P. Infection of a canine macrophage cell line with Leishmania infantum: Determination of nitric oxide production and anti-leishmanial activity. Vet. Parasitol. 2000, 92, 181–189. [Google Scholar] [CrossRef]
- Ghalib, H.W.; Whittle, J.A.; Kubin, M.; Hashim, F.A.; El-Hassan, A.M.; Grabstein, K.H.; Trinchieri, G.; Reed, S.G. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J. Immunol. 1995, 154, 4623–4629. [Google Scholar]
- Sacks, D.L.; Lal, S.L.; Shrivastava, S.N.; Blackwell, J.; Neva, F.A. An analysis of T cell responsiveness in Indian kala-azar. J. Immunol. 1987, 138, 908–913. [Google Scholar]
- Mosser, D.M.; Edelson, P.J. Activation of the alternative complement pathway by Leishmania promastigotes: Parasite lysisand attachment to macrophages. J. Immunol. 1984, 132, 1501–1505. [Google Scholar] [PubMed]
- Mosser, D.M.; Burke, S.K.; Coutavas, E.E.; Wedgwood, J.F.; Edelson, P.J. Leishmania species: Mechanisms of complement activation by five strains of promastigotes. Exp. Parasitol. 1986, 62, 394–404. [Google Scholar] [CrossRef]
- Laurenti, M.D.; Corbett, C.; Sotto, M.; Sinhorini, I.; Goto, H. The role of complement in the acute inflammatory process in the skin and in host–parasite interaction in hamsters inoculated with Leishmania (Leishmania) chagasi. Int. J. Exp. Pathol. 1996, 77, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Brittingham, A.; Mosser, D. Exploitation of the complement system by Leishmania promastigotes. Parasitol. Today 1996, 12, 444–447. [Google Scholar] [CrossRef]
- Rosenthal, L.A.; Sutterwala, F.S.; Kehrli, M.E.; Mosser, D.M. Leishmania major-human macrophage interactions: Cooperation between Mac-1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infect. Immun. 1996, 64, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.M. Role of macrophage complement and lectin-like receptors in binding Leishmania parasites to host macrophages. Immunol. Lett. 1985, 11, 227–232. [Google Scholar] [CrossRef]
- Ueno, N.; Wilson, M.E. Receptor-mediated phagocytosis of Leishmania: Implications for intracellular survival. Trends Parasitol. 2012, 28, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.A.P.; Nascimento, A.A.d.S.; Saab, N.A.A.; Fugiwara, R.T.; Pessoa, G.C.D.; Koerich, L.B.; Pereira, M.H.; Araújo, R.N.; Sant’Anna, M.R.V.; Gontijo, N.F. Evasion of the complement system by Leishmania through the uptake of factor H, a complement regulatory protein. Acta Trop. 2021, 224, 106152. [Google Scholar] [CrossRef]
- Ambrosio, A.R.; Bavia, L.; Hiraiwa, P.M.; Tirado, T.C.; Figueiredo, F.B.; de Messias-Reason, I.J. The lectin pathway of complement and the initial recognition of Leishmania infantum promastigotes. Life Sci. 2021, 282, 119793. [Google Scholar] [CrossRef]
- Vargas-Inchaustegui, D.A.; Tai, W.; Xin, L.; Hogg, A.E.; Corry, D.B.; Soong, L. Distinct roles for MyD88 and toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 2009, 77, 2948–2956. [Google Scholar] [CrossRef]
- Argueta-Donohué, J.; Wilkins-Rodríguez, A.A.; Aguirre-García, M.; Gutiérrez-Kobeh, L. Differential phagocytosis of Leishmania mexicana promastigotes and amastigotes by monocyte-derived dendritic cells. Microbiol. Immunol. 2016, 60, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, L.; Trevelin, S.C.; Nascimento, M.S.; Lima-Jùnior, D.S.; Costa, D.L.; Almeida, R.P.; Cunha, F.Q.; Silva, J.S.; Carregaro, V. Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection. Infect. Immun. 2015, 83, 4604–4616. [Google Scholar] [CrossRef] [PubMed]
- Liese, J.; Schleicher, U.; Bogdan, C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 2007, 37, 3424–3434. [Google Scholar] [CrossRef] [PubMed]
- Revest, M.; Donaghy, L.; Cabillic, F.; Guiguen, C.; Gangneux, J.-P. Comparison of the immunomodulatory effects of L. donovani and L. major excreted–secreted antigens, particulate and soluble extracts and viable parasites on human dendritic cells. Vaccine 2008, 26, 6119–6123. [Google Scholar] [CrossRef]
- Ato, M.; Maroof, A.; Zubairi, S.; Nakano, H.; Kakiuchi, T.; Kaye, P.M. Loss of Dendritic Cell Migration and Impaired Resistance to Leishmania donovani Infection in Mice Deficient in CCL19 and CCL21. J. Immunol. 2006, 176, 5486–5493. [Google Scholar] [CrossRef]
- Sato, N.; Ahuja, S.K.; Quinones, M.; Kostecki, V.; Reddick, R.L.; Melby, P.C.; Kuziel, W.A.; Ahuja, S.S. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokin, B Cell Outgrowth, and Sustained Neutrophilic Inflammation. J. Exp. Med. 2000, 192, 205–218. [Google Scholar]
- von Stebut, E.; Belkaid, Y.; Jakob, T.; Sacks, D.L.; Udey, M.C. Uptake of Leishmania major amastigotes results in activation and in-terleukin 12 release from murine skin–derived dendritic cells: Implications for the initiation of Anti-Leishmania immunity. J. Exp. Med. 1998, 188, 1547–1552. [Google Scholar] [CrossRef]
- Favali, C.; Tavares, N.; Clarencio, J.; Barral, A.; Barral-Netto, M.; Brodskyn, C. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J. Leukoc. Biol. 2007, 82, 1401–1406. [Google Scholar] [CrossRef][Green Version]
- Resende, M.; Moreira, D.; Augusto, J.; Cunha, J.; Neves, B.; Cruz, M.T.; Estaquier, J.; Cordeiro-da-Silva, A.; Silvestre, R. Leishmania-infected MHC Class II high dendritic cells polarize CD4+ T cells toward a nonprotective T-bet+ IFN- + IL-10+ phenotype. J. Immunol. 2013, 191, 262–273. [Google Scholar] [CrossRef]
- Amaral, V.F.; Ransatto, V.A.; Conceicão-Silva, F.; Molinaro, E.; Ferreira, V.; Coutinho, S.G.; McMahon-Pratt, D.; Grimaldi, G. Leishmania amazonensis: The Asian rhesus macaques (Macaca mulatta) as an experimental model for study of cutaneous leishmaniasis. Exp. Parasitol. 1996, 82, 34–44. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, S.D.C.; Pessoa-E-Silva, R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; da C. Oliveira, C.N.; de Lorena, V.M.B.; de Paiva-Cavalcanti, M. The Equivocal Role of Th17 Cells and Neutrophils on Immunopathogenesis of Leishmaniasis. Front. Immunol. 2017, 8, 1437. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Brombacher, F. T helper 1/T helper 2 cells and resistance/susceptibility to Leishmania infection: Is this paradigm still relevant? Front. Immunol. 2012, 2012. 3, 80. [Google Scholar]
- Castes, M.; Cabrera, M.; Trujillo, D.; Convit, J. T-cell subpopulations, expression of interleukin-2 receptor, and production of inter-leukin-2 and gamma interferon in human American cutaneous leishmaniasis. J. Clin. Microbiol. 1988, 26, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Frankenburg, S.; Kofsky, Y.; Gross, A. In vitro secretion of cytokines by human mononuclear cells of individuals during and after cutaneous leishmaniasis infection. Parasite Immunol. 1993, 15, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Holaday, B.J.; Pompeu, M.M.D.L.; Evans, T.; Braga, D.N.D.M.; Texeira, M.J.; Sousa, A.D.Q.; Sadick, M.D.; Vasconcelos, A.W.; Abrams, J.S.; Pearson, R.D.; et al. Correlates of Leishmania-Specific Immunity in the Clinical Spectrum of Infection with Leishmania chagasi. J. Infect. Dis. 1993, 167, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Amit, A.; Dikhit, M.R.; Singh, A.; Kumar, V.; Suman, S.S.; Singh, A.; Kumar, A.; Thakur, A.K.; Das, V.R.; Das, P.; et al. Immunization with Leishmania donovani protein disulfide isomerase DNA construct induces Th1 and Th17 dependent immune response and protection against experimental visceral leishmaniasis in Balb/c mice. Mol. Immunol. 2017, 82, 104–113. [Google Scholar] [CrossRef] [PubMed]
- McMahon-Pratt, D.; Alexander, J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 2004, 201, 206–224. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Khare, P.; Joshi, S.; Kushawaha, P.K.; Sundar, S.; Dube, A. Th1 stimulatory proteins of Leishmania donovani: Comparative cellular and protective responses of rTriose phosphate isomerase, rProtein disulfide isomerase and rElongation factor-2 in combination with rHSP70 against visceral leishmaniasis. PLoS ONE 2014, 9, e108556. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Barreto, A.S.; Bomfim, L.G.S.; Gomes, M.C.; Ferreira, N.L.C.; da Cruz, G.S.; Magalhães, L.S.; de Jesus, A.R.; Palatnik-De-Sousa, C.B.; Corrêa, C.B.; et al. Multifunctional, TNF-α and IFN-γ-Secreting CD4 and CD8 T Cells and CD8High T Cells Are Associated with the Cure of Human Visceral Leishmaniasis. Front. Immunol. 2021, 12, 773983. [Google Scholar] [CrossRef]
- Hejazi, S.; Hoseini, S.; Javanmard, S.; Zarkesh, S.; Khamesipour, A. Interleukin-10 and Transforming Growth Factor-β in Early and Late Lesions of Patients with Leishmania major induced Cutaneous Leishmaniasis. Iran. J. Parasitol. 2012, 7, 53–60. [Google Scholar] [PubMed]
- Bhowmick, S.; Ravindran, R.; Ali, N. IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine. BMC Microbiol. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Aoki, J.I.; Fernandes, J.C.R.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Acuña, S.M.; Müller, K.E.; Vanderlinde, R.H.; Floeter-Winter, L.M. Arginine and polyamines fate in Leishmania infection. Front. Microbiol. 2018, 15, 2682. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Wang, W.; Fu, S.P.; Wang, J.F.; Liu, H.M.; Xie, S.S.; Liu, B.R.; Li, Y.; Lv, Q.K.; Li, Z.Q.; et al. IL-21 modulates release of proinflammatory cytokines in LPS-stimulated macrophages through distinct signaling path-ways. Mediat. Inflamm. 2013, 2013, 548073. [Google Scholar] [CrossRef]
- Poudel, B.; Yorek, M.S.; Mazgaeen, L.; Brown, S.A.; Kanneganti, T.-D.; Gurung, P. Acute IL-4 Governs Pathogenic T Cell Responses during Leishmania major Infection. ImmunoHorizons 2020, 4, 546–560. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hoffmann, K.F.; Mendez, S.; Kamhawi, S.; Udey, M.C.; Wynn, T.; Sacks, D.L. The Role of Interleukin (IL)-10 in the Persistence of Leishmania major in the Skin after Healing and the Therapeutic Potential of Anti–IL-10 Receptor Antibody for Sterile Cure. J. Exp. Med. 2001, 194, 1497–1506. [Google Scholar] [CrossRef]
- Belkaid, Y.; Piccirillo, C.A.; Mendez, S. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002, 420, 633–637. [Google Scholar] [CrossRef]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4+CD25-Foxp3- Th1 cells are source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef]
- Kane, M.M.; Mosser, D.M. The Role of IL-10 in Promoting Disease Progression in Leishmaniasis. J. Immunol. 2001, 166, 1141–1147. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Maurya, R.; Nylén, S.; Ansari, N.; Rai, M.; Sundar, S.; Sacks, D. IL-10 Neutralization Promotes Parasite Clearance in Splenic Aspirate Cells from Patients with Visceral Leishmaniasis. J. Infect. Dis. 2011, 204, 1134–1137. [Google Scholar] [CrossRef]
- Schwarz, T.; Remer, K.A.; Nahrendorf, W.; Masic, A.; Siewe, L.; Muller, W.; Roers, A.; Moll, H. T Cell-Derived IL-10 Determines Leishmaniasis Disease Outcome and Is Suppressed by a Dendritic Cell Based Vaccine. PLoS Pathog. 2013, 9, e1003476. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Majumdar, S.; Adhikari, A.; Bhattacharya, P.; Mukherjee, A.K.; Majumdar, S.B.; Majumdar, S. Treatment with IP-10 induces host-protective immune response by regulating the T regulatory cell functioning in Leishmania donovani-infected mice. Med. Microbiol. Immunol. 2011, 200, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Mendez, S.; Reckling, S.K.; Piccirillo, C.A.; Sacks, D.L.; Belkaid, Y. Role for CD4+ CD25+ Regulatory T Cells in Reactivation of Persistent Leishmaniasis and Control of Concomitant Immunity. J. Exp. Med. 2004, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Masterson, J.; Sun, J.; Soong, L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonen-sis infection. J. Immunol. 2005, 174, 7147–7153. [Google Scholar] [CrossRef]
- Rai, A.K.; Thakur, C.P.; Singh, A.; Seth, T.; Srivastava, S.K.; Singh, P.; Mitra, D.K. Regulatory T Cells Suppress T Cell Activation at the Pathologic Site of Human Visceral Leishmaniasis. PLoS ONE 2012, 7, e31551. [Google Scholar] [CrossRef]
- Rodriguez-Pinto, D.; Navas, A.; Blanco, V.M.; Ramírez, L.; Garcerant, D.; Cruz, A.; Craft, N.; Saravia, N.G. Regulatory T Cells in the Pathogenesis and Healing of Chronic Human Dermal Leishmaniasis Caused by Leishmania (Viannia) Species. PLoS Negl. Trop. Dis. 2012, 6, e1627. [Google Scholar] [CrossRef]
- Rodriguez-Pinto, D.; Saravia, N.G.; McMahon-Pratt, D. CD4 T cell activation by B cells in human Leishmania (Viannia)infection. BMC Infect. Dis. 2014, 14, 108. [Google Scholar] [CrossRef]
- Ehrlich, A.; Castilho, T.M.; Goldsmith-Pestana, K.; Chae, W.J.; Bothwell, A.L.; Sparwasser, T.; McMahon-Pratt, D. The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection. J. Immunol. 2014, 193, 2961–2970. [Google Scholar] [CrossRef]
- Anderson, C.F.; Stumhofer, J.S.; Hunter, C.A.; Sacks, D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in non-healing Leishmania major infection. J. Immunol. 2009, 183, 4619–4627. [Google Scholar] [CrossRef]
- Castilho, T.M.; Goldsmith-pestana, K.; Lozano, C.; Valderrama, L.; Saravia, N.G.; Mcmahon-pratt, D. Murine model of chronic L. (Viannia) panamensis infection: Role of IL-13 in disease. Eur. J. Immunol. 2010, 40, 2816–2829. [Google Scholar] [CrossRef]
- Katara, G.K.; Raj, A.; Kumar, R.; Avishek, K.; Kaushal, H.; Ansari, N.A.; Bumb, R.A.; Salotra, P. Analysis of localized immune responses reveals presence of Th17 and Treg cells in cutaneous leishmaniasis due to Leishmania tropica. BMC Immunol. 2013, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Espir, T.T.; Figueira, L.D.P.; Naiff, M.D.F.; Costa, A.; Ramalho-Ortigao, M.; Malheiro, A.; Franco, A.M.R. The Role of Inflammatory, Anti-Inflammatory, and Regulatory Cytokines in Patients Infected with Cutaneous Leishmaniasis in Amazonas State, Brazil. J. Immunol. Res. 2014, 2014, 481750. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Sabur, A.; Kamran, M.; Shadab, M.; Das, S.; Ali, N. Effector functions of Th17 cells are regulated by IL-35 and TGF-β in visceral leishmaniasis. FASEB J. 2021, 35, e21755. [Google Scholar] [CrossRef] [PubMed]
- Khatonier, R.; Ahmed, G.; Sarmah, P.; Narain, K.; Khan, A.M. Immunomodulatory role of Th17 pathway in experimental visceral leishmaniasis. Immunobiology 2021, 226, 152148. [Google Scholar] [CrossRef]
- Nateghi, R.M.; Keshavarz, V.H.; Eskandari, S.E.; Miramin, M.A.; Shahrestani, S.T.; Sarraf-Nejad, A.; Khamesipour, A. Differential in vitro CD4+/CD8+ T-cell response to live vs. killed Leishmania major. Parasite Immunol. 2010, 32, 101–110. [Google Scholar] [CrossRef]
- Santos, C.D.; Boaventura, V.; Ribeiro Cardoso, C.; Tavares, N.; Lordelo, M.J.; Noronha, A.; Costa, J.; Borges, V.M.; de Oliveira, C.I.; Van Weyenbergh, J.; et al. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. J. Investig. Dermatol. 2013, 133, 1533–1540. [Google Scholar] [CrossRef]
- Joshi, T.; Rodriguez, S.; Perovic, V.; Cockburn, I.; Stäger, S. B7-H1 Blockade Increases Survival of Dysfunctional CD8+ T Cells and Confers Protection against Leishmania donovani Infections. PLoS Pathog. 2009, 5, e1000431. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Singh, N.; Singh, A.K.; Rai, M.; Sacks, D.; Sundar, S.; Nylén, S. CD8 T cell exhaustion in human visceral leishmaniasis. J. Infect. Dis. 2014, 209, 290–299. [Google Scholar] [CrossRef]
- Clarencio, J.; de Oliveira, C.I.; Favali, C.; Medina, O.; Caldas, A.; Costa, C.H.; Costa, D.L.; Brodskyn, C.; Barral, A.; Barral-Netto, M. Could the lower frequency of CD8+CD18+CD45RO+ lymphocytes be biomarkers of human VL? Int. Immunol. 2009, 21, 137–144. [Google Scholar] [CrossRef][Green Version]
- Bourreau, E.; Ronet, C.; Couppié, P.; Sainte-Marie, D.; Tacchini-Cottier, F.; Launois, P. IL-10 producing CD8+ T cells in human infection with Leishmania guyanensis. Microbes Infect. 2007, 9, 1034–1041. [Google Scholar] [CrossRef]
- Saha, S.; Mondal, S.; Ravindran, R.; Bhowmick, S.; Modak, D.; Mallick, S.; Rahman, M.; Kar, S.; Goswami, R.; Guha, S.K.; et al. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: The significance of amphotericin B in the control of Leishmania donovani infection in India. J. Immunol. 2007, 179, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Katara, G.K.; Ansari, N.A.; Verma, S.; Ramesh, V.; Salotra, P. Foxp3 and IL-10 Expression Correlates with Parasite Burden in Lesional Tissues of Post Kala Azar Dermal Leishmaniasis (PKDL) Patients. PLoS Negl. Trop. Dis. 2011, 5, e1171. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.L.; Cotterell, S.E.; Gorak, P.M.; Engwerda, C.R.; Kaye, P.M. Blockade of CTLA-4 enhances host resistance to the intra-cellular pathogen, Leishmania donovani. J. Immunol. 1998, 161, 4153–4160. [Google Scholar] [PubMed]
- Zubairi, S.; Sanos, S.L.; Hill, S.; Kaye, P.M. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing ofLeishmania donovani. Eur. J. Immunol. 2004, 34, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Jouanne, C.; Lão, S.D.S.; Szabo, A.; Guillet, J.G.; Antoine, J.C. Antigen presentation capacity of murine macrophages infected with Leishmania amazonensis amastigotes. J. Immunol. 1993, 151, 2050–2061. [Google Scholar]
- Lang, T.; de Chastellier, C.; Frehel, C.; Hellio, R.; Metezeau, P.; Leao, S.; Antoine, J.C. Distribution of MHC class I and of MHC class II molecules in macrophages infected with Leishmania amazonensis. J. Cell. Sci. 1994, 107, 69–82. [Google Scholar] [CrossRef]
- Fruth, U.; Solioz, N.; Louis, J.A. Leishmania major interferes with antigen presentation by infected macrophages. J. Immunol. 1993, 150, 1857–1864. [Google Scholar]
- De Almeida, M.; Cardoso, S.; Barral-Netto, M. Leishmania (Leishmania) chagasi infection alters the expression of cell adhesion and costimulatory molecules on human monocyte and macrophage. Int. J. Parasitol. 2003, 33, 153–162. [Google Scholar] [CrossRef]
- Kaye, M.; Rogers, N.J.; Curry, A.J.; Scott, J.C. Deficient expression of co-stimulatory molecules on Leishmania infected macrophages. Eur. J. Immunol. 1994, 24, 2850–2854. [Google Scholar] [CrossRef]
- Saha, B.; Das, G.; Vohra, H.; Ganguly, N.K.; Mishra, G.C. Macrophage-T cell interaction in experimental visceral leishmaniasis: Failure to express costimulatory molecules on Leishmania infected macrophages and its implication in the suppression of cell-mediated immunity. Eur. J. Immunol. 1995, 25, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Soong, L.; Xu, J.-C.; Grewal, I.; Kima, P.; Sun, J.; Longley, B.; Ruddle, N.H.; McMahon-Pratt, D.; Flavell, R.A. Disruption of CD40–CD40 Ligand Interactions Results in an Enhanced Susceptibility to Leishmania amazonensis Infection. Immunity 1996, 4, 263–273. [Google Scholar] [CrossRef]
- Kamanaka, M.; Yu, P.; Yasui, T.; Oshida, K.; Kawabe, T.; Horii, T.; Kishimoto, T.; Kikutani, H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell mediated immunity. Immunity 1996, 4, 275–281. [Google Scholar] [CrossRef]
- Heinzel, F.P.; Rerko, R.M.; Hujer, A.M. Underproduction of Interleukin-12 in Susceptible Mice during Progressive Leishmaniasis Is Due to Decreased CD40 Activity. Cell. Immunol. 1998, 184, 129–142. [Google Scholar] [CrossRef]
- Awasthi, A.; Mathur, R.; Khan, A.; Joshi, B.N.; Jain, N.; Sawant, S.; Boppana, R.; Mitra, D.; Saha, B. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J. Exp. Med. 2003, 197, 1037–1043. [Google Scholar] [CrossRef]
- Nyambura, L.W.; Jarmalavicius, S.; Walden, P. Impact of Leishmania donovani infection on the HLA I self peptide repertoire of human macrophages. PLoS ONE 2018, 13, e0200297. [Google Scholar] [CrossRef]
- Rezvan, H.; Khodadadi, A.; Ali, S.A. CTL responses to DCs stimulated with Leishmania antigens detected by DCs expressing Leishmania gp63. Iran. J. Immunol. 2014, 11, 65–73. [Google Scholar]
- Imbert, S.; Palous, M.; Meyer, I.; Dannaoui, E.; Mazier, D.; Datry, A.; Fekkar, A. In VitroCombination of Voriconazole and Miltefosine against Clinically Relevant Molds. Antimicrob. Agents Chemother. 2014, 58, 6996–6998. [Google Scholar] [CrossRef]
- Ashwin, H.; Sadlova, J.; Vojtkova, B.; Becvar, T.; Lypaczewski, P.; Schwartz, E.; Greensted, E.; Van Bocxlaer, K.; Pasin, M.; Lipinski, K.S.; et al. Characterization of a new Leishmania major strain for use in a controlled human infection model. Nat. Commun. 2021, 12, 215. [Google Scholar] [CrossRef]
- Gavron, D.; Saul, A. Pioneer of Tropical Medicine; Balaban Publishers: Rehovot, Israel, 1997; p. 92. ISBN 0-86689-045-9. [Google Scholar]
- Nadim, A.; Javadian, E.; Mohebali, M. The experience of leishmanization in the Islamic Republic of Iran. EMHJ East. Mediter-Ranean Health J. 1997, 3, 284–289. [Google Scholar] [CrossRef]
- Koufman, Z.; Egoz, N.; Greenblatt, C.L.; Handman, E.; Montilio, B.; Even-Paz, Z. Observations on immunization against cutaneous leish-maniasis in Israel. Isr. J. Med. Sci. 1978, 14, 218–222. [Google Scholar] [PubMed]
- Nadim, A.; Javadian, E.; Tahvildar-Bidruni, G.; Ghorbani, M. Effectiveness of leishmanization in the control of cutaneous leishman-iasis. Bull. Soc. Pathol. Exot. Filiales. 1983, 76, 4377–4383. [Google Scholar]
- Seyed, N.; Peters, N.C.; Rafati, S. Translating Observations from Leishmanization into Non-Living Vaccines: The Potential of Dendritic Cell-Based Vaccination Strategies Against Leishmania. Front. Immunol. 2018, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.L. Vaccines against tropical parasitic diseases: A persisting answer to a persisting problem. Nat. Immunol. 2014, 15, 403–405. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Todd, D.; Daneshvar, H.; Burchmore, R. The potential of live attenuated vaccines against Cutaneous Leishmaniasis. Exp. Parasitol. 2020, 210, 107849. [Google Scholar] [CrossRef]
- Noazin, S.; Modabber, F.; Khamesipour, A.; Smith, P.G.; Moulton, L.H.; Nasseri, K.; Sharifi, I.; Khalil, E.A.; Bernal, I.D.V.; Antunes, C.M.; et al. First generation leishmaniasis vaccines: A review of field efficacy trials. Vaccine 2008, 26, 6759–6767. [Google Scholar] [CrossRef]
- Daneshvar, H.; Coombs, G.H.; Hagan, P.; Phillips, R.S. Leishmania mexicana and Leishmania major: Attenuation of Wild? Type Parasites and Vaccination with the Attenuated Lines. J. Infect. Dis. 2003, 187, 1662–1668. [Google Scholar] [CrossRef]
- Nagill, R.; Kaur, S. Vaccine candidates for leishmaniasis: A review. Int. Immunopharmacol. 2011, 11, 1464–1488. [Google Scholar] [CrossRef]
- De Oliveira, B.C.; Duthie, M.S.; Alves, P.V.R. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum. Vaccin. Immunother. 2020, 16, 919–930. [Google Scholar] [CrossRef]
- Pandey, S.C.; Kumar, A.; Samant, M. Genetically modified live attenuated vaccine: A potential strategy to combat visceral leishmaniasis. Parasite Immunol. 2020, 42, e12732. [Google Scholar] [CrossRef]
- Volpedo, G.; Huston, R.H.; Holcomb, E.A.; Pacheco-Fernandez, T.; Gannavaram, S.; Bhattacharya, P.; Nakhasi, H.L.; Satoskar, A.R. From infection to vaccination: Reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev. Vaccines 2021, 20, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Titus, R.G.; Gueiros-Filho, F.J.; de Freitas, L.A.; Beverley, S.M. Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. USA 1995, 92, 10267–11071. [Google Scholar] [CrossRef] [PubMed]
- Veras, P.; Brodskyn, C.; Balestieri, F.; De Freitas, L.; Ramos, A.; Queiroz, A.; Barral, A.; Beverley, S.; Barral-Netto, M. A dhfr-ts- Leishmania major Knockout Mutant Cross-protects against Leishmania amazonensis. Mem. Inst. Oswaldo Cruz. 1999, 94, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.F.; Teva, A.; Oliveira-Neto, M.P.; Silva, A.J.; Pereira, M.S.; Cupolillo, E.; Porrozzi, R.; Coutinho, S.G.; Pirmez, C.; Beverley, S.M.; et al. Study of the safety, immunogenicity and efficacy of attenuated and killed Leishmania major vaccines in a rhesus monkey (Macaca mulatta) model of the human disease. Mem. Inst. Oswaldo Cruz. 2002, 97, 1041–1048. [Google Scholar] [CrossRef]
- Alexander, J.; Coombs, G.H.; Mottram, J. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1998, 161, 6794–6801. [Google Scholar]
- Saravia, N.G.; Escorcia, B.; Osorio, Y.; Valderrama, L.; Brooks, D.; Arteaga, L.; Coombs, G.; Mottram, J.; Travi, B.L. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in non-murine models. Vaccine 2006, 24, 4247–4259. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Debrabant, A.; Duncan, R.; Muller, J.; Salotra, P.; Sreenivas, G.; Salisbury, J.; Nakhasi, H.L. Centrin Gene Disruption Impairs Stage-specific Basal Body Duplication and Cell Cycle Progression in Leishmania. J. Biol. Chem. 2004, 279, 25703–25710. [Google Scholar] [CrossRef]
- Fiuza, J.A.; Santiago, H.; Selvapandiyan, A.; Gannavaram, S.; Ricci, D.; Bueno, L.L.; Bartholomeu, D.C.; Correa-Oliveira, R.; Nakhasi, H.L.; Fujiwara, R.T. Induction of immunogenicity by live attenuated L. donovani centrin parasite in dogs. Vaccine 2013, 31, 1785–1792. [Google Scholar] [CrossRef][Green Version]
- Fiuza, J.A.; Gannavaram, S.; Santiago, H.D.C.; Selvapandiyan, A.; Souza, D.M.; Passos, L.S.A.; de Mendonça, L.Z.; Lemos-Giunchetti, D.D.S.; Ricci, N.D.; Bartholomeu, D.C.; et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 2015, 33, 280–288. [Google Scholar] [CrossRef]
- Viana, K.F.; Fiuza, J.A.; Gannavaam, S.; Dey, R.; Selvapandiyan, A.; Bartholomeu, D.C.; da Silveira-Lemos, D.; Bueno, L.L.; Dutra, W.O.; Fujiwara, R.T.; et al. Application of rapid in vitro co-culture system of macrophages and T-cell subsets to assess the immunogenicity of dogs vaccinated with live attenuated Leishmania donovani centrin deleted parasites (LdCen-/-). Parasites Vectors 2016, 9, 250. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Joshi, A.B.; Ismail, N.; Gannavaram, S.; Debrabant, A.; Akue, A.D.; Kukuruga, M.A.; Selvapandiyan, A.; et al. Live Attenuated Leishmania donovani Centrin Knock Out Parasites Generate Non-inferior Protective Immune Response in Aged Mice against Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2016, 10, e0004963. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Kaul, A.; Bhattacharya, P.; Gannavaram, S.; Nakhasi, H.L. Immunization with Live Attenuated Leishmania donovani Centrin-/- Parasites Is Efficacious in Asymptomatic Infection. Front. Immunol. 2017, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, J.A.; Dey, R.; Davenport, D.; Abdeladhim, M.; Meneses, C.; Oliveira, F.; Kamhawi, S.; Valenzuela, J.G.; Gannavaram, S.; Nakhasi, H.L. Intradermal Immunization of Leishmania donovani Centrin Knock-Out Parasites in Combination with Salivary Protein LJM19 from Sand Fly Vector Induces a Durable Protective Immune Response in Hamsters. PLoS Negl. Trop. Dis. 2016, 10, e0004322. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Dagur, P.K.; Karmakar, S.; Ismail, N.; Joshi, A.B.; Akue, A.D.; KuKuruga, M.; McCoy, J.P., Jr.; Dey, R.; et al. Live Attenuated Leishmania donovani Centrin Gene–Deleted Parasites Induce IL-23–Dependent IL-17–Protective Immune Response against Visceral Leishmaniasis in a Murine Model. J. Immunol. 2018, 200, 163–176. [Google Scholar] [CrossRef]
- Afshar, M.J.A.; Elikaee, S.; Saberi, R.; Mohtasebi, S.; Mohebali, M. Expression analysis of centrin gene in promastigote and amastigote forms of Leishmania infantum iranian isolates: A promising target for live attenuated vaccine development against canine leishmaniasis. BMC Vet. Res. 2021, 17, 162. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef]
- Elikaee, S.; Mohebali, M.; Rezaei, S.; Eslami, H.; Khamesipour, A.; Keshavarz, H.; Eshraghian, M.R. Development of a new live attenuated Leishmania major p27 gene knockout: Safety and immunogenicity evaluation in BALB/c mice. Cell. Immunol. 2018, 332, 24–31. [Google Scholar] [CrossRef]
- Elikaee, S.; Mohebali, M.; Rezaei, S.; Eslami, H.; Khamesipour, A.; Keshavarz, H.; Eshraghian, M.R. Leishmania major p27 gene knockout as a novel live attenuated vaccine candidate: Protective immunity and efficacy evaluation against cutaneous and visceral leishmaniasis in BALB/c mice. Vaccine 2019, 37, 3221–3228. [Google Scholar] [CrossRef]
- Solana, J.C.; Ramírez, L.; Cook, E.C.; Hernández-García, E.; Sacristán, S.; Martín, M.E.; Manuel González, V.; Reguera, R.M.; Balaña-Fouce, R.; Fresno, M.; et al. Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice. Vaccines 2020, 8, 141. [Google Scholar] [CrossRef]
- Soto, M.; Ramírez, L.; Solana, J.C.; Cook, E.; Hernández-García, E.; Requena, J.M.; Iborra, S. Inoculation of the Leishmania infantum HSP70-II Null Mutant Induces Long-Term Protection against L. amazonensis Infection in BALB/c Mice. Microorganisms 2021, 9, 363. [Google Scholar] [CrossRef]
- Didwania, N.; Shadab, M.; Sabur, A.; Ali, N. Alternative to Chemotherapy—The Unmet Demand against Leishmaniasis. Front. Immunol. 2017, 8, 1779. [Google Scholar] [CrossRef] [PubMed]
- Noazin, S.; Khamesipour, A.; Moulton, L.H.; Tanner, M.; Nasseri, K.; Modabber, F.; Sharifi, I.; Khalil, E.; Bernal, I.D.V.; Antunes, C.M.; et al. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis—A meta-analysis. Vaccine 2009, 27, 4747–4753. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, K.W.; Birnbaum, R.; Haskell, J.; Vanchinathan, V.; Greger, S.; Narayan, R.; Chang, P.-L.; Tran, T.A.; Hickerson, S.M.; Beverley, S.M.; et al. Killed but Metabolically Active Leishmania infantum as a Novel Whole-Cell Vaccine for Visceral Leishmaniasis. Clin. Vaccine Immunol. 2012, 19, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, M.; Bahrami, F.; Niknam, H.M. Vaccination with whole-cell killed or recombinant leishmanial protein and toll-like receptor agonists against Leishmania tropica in BALB/c mice. PLoS ONE 2018, 13, e0204491. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Abdlwahab, A.A. Investigating the Existence of Ribosomal Protein L5 Gene in Syrian Strain of Leishmania tropica Genome: Sequencing It and Evaluating Its Immune Response as DNA Vaccine. J. Parasitol. Res. 2021, 2021, 6617270. [Google Scholar] [CrossRef]
- Mutiso, J.M.; Macharia, J.C.; Kiio, M.N.; Ichagichu, J.M.; Rikoi, H.; Gicheru, M.M. Development of Leishmania vaccines: Predicting the future from past and present experience. J. Biomed. Res. 2013, 27, 85–102. [Google Scholar] [CrossRef]
- Sinha, S.; Sundaram, S.; Singh, A.P.; Tripathi, A. A gp63 based vaccine candidate against Visceral Leishmaniasis. Bioinformation 2011, 5, 320–325. [Google Scholar] [CrossRef]
- Bhowmick, S.; Ravindran, R.; Ali, N. gp63 in Stable Cationic Liposomes Confers Sustained Vaccine Immunity to Susceptible BALB/c Mice Infected with Leishmania donovani. Infect. Immun. 2008, 76, 1003–1015. [Google Scholar] [CrossRef]
- Mazumder, S.; Maji, M.; Ali, N. Potentiating Effects of MPL on DSPC Bearing Cationic Liposomes Promote Recombinant GP63 Vaccine Efficacy: High Immunogenicity and Protection. PLoS Negl. Trop. Dis. 2011, 5, e1429. [Google Scholar] [CrossRef]
- Rezvan, H.; Rees, R.; Ali, S. Immunogenicity of MHC Class I Peptides Derived from Leishmania mexicana Gp63 in HLA-A2.1 Transgenic (HHDII) and BALB/C Mouse Models. Iran. J. Parasitol. 2012, 7, 27–40. [Google Scholar]
- Rezvan, H. Immunogenicity of HLA-DR1 Restricted Peptides Derived from Leishmania major gp63 Using FVB/N-DR1 Transgenic Mouse Model. Iran. J. Parasitol. 2013, 8, 273–279. [Google Scholar] [PubMed]
- Silva, L.P.; Paciello, M.O.; Teixeira, W.P.A.; Rivas, A.V.; Agular, R.W.S.; Cangussu, A.S.R.; Barbosa, L.C.B.; Marchetto, R.; Giunchetti, R.C.; Viana, K.F. Immunogenicity of HLA-DR1 and HLA-A2 peptides derived from Leishmania major Gp63 in golden hamsters. Parasite Immunol. 2020, 42, e12780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, J.; Li, J.; Zhou, Q.; Chen, H.; Zheng, Z.; Chen, Q.; Chen, D.; Chen, J. The immunogenicity and protective immunity of multi-epitopes DNA prime-protein boost vaccines encoding Amastin-Kmp-11, Kmp11-Gp63 and Amastin-Gp63 against visceral leishmaniasis. PLoS ONE 2020, 15, e0230381. [Google Scholar] [CrossRef] [PubMed]
- Motamedpour, L.; Dalimi, A.; Pirestani, M.; Ghafarifar, F. In silico analysis and expression of a new chimeric antigen as a vaccine candidate against cutaneous leishmaniasis. Iran. J. Basic Med. Sci. 2020, 23, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Mougneau, E.; Altare, F.; Wakil, A.E.; Zheng, S.; Coppola, T.; Wang, Z.-E.; Waldmann, R.; Locksley, R.M.; Glaichenhaus, N. Expression Cloning of a Protective Leishmania Antigen. Science 1995, 268, 563–566. [Google Scholar] [CrossRef]
- Hugentobler, F.; Yam, K.K.; Gillard, J.; Mahbuba, R.; Olivier, M.; Cousineau, B. Immunization against Leishmania major Infection Using LACK- and IL-12-Expressing Lactococcus lactis Induces Delay in Footpad Swelling. PLoS ONE 2012, 7, e30945. [Google Scholar] [CrossRef]
- Hugentobler, F.; Di Roberto, R.B.; Gillard, J.; Cousineau, B. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine 2012, 30, 5726–5732. [Google Scholar] [CrossRef]
- Salari, S.; Sharifi, I.; Keyhani, A.R.; Almani, P.G.N. Evaluation of a new live recombinant vaccine against cutaneous leishmaniasis in BALB/c mice. Parasites Vectors 2020, 13, 415. [Google Scholar] [CrossRef]
- Lakhal-Naouar, I.; Koles, N.; Rao, M.; Morrison, E.B.; Childs, J.M.; Alving, C.R.; Aronson, N.E. Transcutaneous immunization using SLA or rLACK skews the immune response towards a Th1 profile but fails to protect BALB/c mice against a Leishmania major challenge. Vaccine 2019, 37, 516–523. [Google Scholar] [CrossRef]
- Naseri, H.; Eskandari, F.; Jaafari, M.R.; Khamesipour, A.; Abbasi, A.; Badiee, A. PEGylation of cationic liposomes encapsulating soluble Leishmania antigens reduces the adjuvant efficacy of liposomes in murine model. Parasite Immunol. 2017, 39, e12492. [Google Scholar] [CrossRef]
- Hezarjaribi, H.Z.; Soosaraei, M.; Fakhar, M.; Akhtari, J.; Rafiei, A.; Jorjani, O.N. Preparation and characterization of a nanoliposomal vaccine of pcLACK candidate against cutaneous leishmaniasis. Infect. Disord. Drug Targets 2020, 21, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Bhaumik, S.; Haldar, A.K.; Naskar, K.; De, T.; Dana, S.K.; Walden, P.; Roy, S. Hybrid Cell Vaccination Resolves Leishmania donovani Infection by Eliciting a Strong CD8 + Cytotoxic T-Lymphocyte Response with Concomitant Suppression of Interleukin-10 (IL-10) but Not IL-4 or IL-13. Infect. Immun. 2007, 75, 5956–5966. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Karagouni, E. Cellular vaccination with bone marrow-derived dendritic cells pulsed with a peptide of Leishmania infantum KMP-11 and CpG oligonucleotides induces protection in a murine model of visceral leishmaniasis. Vaccine 2011, 29, 5053–5064. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Amit, A.; Kumar, A.; Dikhit, M.; Pandey, K.; Das, P.; Bimal, S. A New Vaccine Strategy of Dendritic Cell Presented Kinetoplastid Membrane (KMP-11) as Immunogen for Control against Experimental Visceral Leishmaniasis. Modern Res. Inflamm. 2017, 6, 15–28. [Google Scholar] [CrossRef][Green Version]
- Santos, D.M.; Carneiro, M.W.; de Moura, T.R.; Soto, M.; Luz, N.F.; Prates, D.B.; Irache, J.M.; Brodskyn, C.; Barral, A.; Netto, M.B.; et al. PLGA nanoparticles loaded with KMP-11 stimulate innate immunity and induce the killing of Leishmania. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 985–995. [Google Scholar] [CrossRef]
- Salari, S.; Sharifi, I.; Bamorovat, M.; Almani, P.G.N. The immunity of the recombinant prokaryotic and eukaryotic subunit vaccines against cutaneous leishmaniasis. Microb. Pathog. 2021, 153, 104807. [Google Scholar] [CrossRef]
- Saraiva, E.M.; Barbosa, A.d.F.; Santos, F.N.; Borja-Cabrera, G.P.; Nico, D.; Souza, L.O.P.; Mendes-Aguiar, C.d.O.; de Souza, E.P.; Fampa, P.; Parra, L.E.; et al. The FML-vaccine (Leishmune®) against canine visceral leishmaniasis: A transmission blocking vaccine. Vaccine 2006, 24, 2423–2431. [Google Scholar] [CrossRef]
- Foroughi-Parvar, F.; Hatam, G.-R.; Sarkari, B.; Kamali-Sarvestani, E. Leishmania infantum FML pulsed-dendritic cells induce a protective immune response in murine visceral leishmaniasis. Immunotherapy 2015, 7, 3–12. [Google Scholar] [CrossRef]
- Ahmadabad, H.N.; Shafiei, R.; Hatam, G.R.; Emameh, R.Z.; Aspatwar, A. Cytokine profile and nitric oxide levels in peritoneal macrophages of BALB/c mice exposed to the fucose-mannose ligand of Leishmania infantum combined with glycyrrhizin. Parasites Vectors 2020, 13, 363. [Google Scholar] [CrossRef]
- Brito, R.; Ruiz, J.; Cardoso, J.; Ostolin, T.; Reis, L.; Mathias, F.; Aguiar-Soares, R.; Roatt, B.; Corrêa-Oliveira, R.; Resende, D.; et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines 2020, 8, 252. [Google Scholar] [CrossRef]
- Ostolin, T.L.V.D.P.; Gusmão, M.R.; Mathias, F.A.S.; Cardoso, J.M.D.O.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Ruiz, J.C.; Resende, D.D.M.; de Brito, R.C.F.; Reis, A.B. A chimeric vaccine combined with adjuvant system induces immunogenicity and protection against visceral leishmaniasis in BALB/c mice. Vaccine 2021, 39, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Costa, L.E.; Carvalho, A.M.R.S.; Ludolf, F.; Santos, T.T.D.O.; Vale, D.L.; Roatt, B.; Menezes-Souza, D.; et al. A vaccine composed of a hypothetical protein and the eukaryotic initiation factor 5a from Leishmania braziliensis cross-protection against Leishmania amazonensis infection. Immunobiology 2017, 222, 251–260. [Google Scholar] [CrossRef]
- Martins, V.T.; Lage, D.P.; Duarte, M.C.; Costa, L.E.; Garde, E.; Rodrigues, M.R.; Chávez-Fumagalli, M.A.; Menezes-Souza, D.; Roatt, B.M.; Tavares, C.A.P.; et al. A new Leishmania-specific hypothetical protein, LiHyT, used as a vaccine antigen against visceral leishmaniasis. Acta Trop. 2016, 154, 73–81. [Google Scholar] [CrossRef]
- Santos, T.T.; Martins, V.T.; Lage, D.P.; Costa, L.E.; Salles, B.C.; Carvalho, A.M.; Dias, D.S.; Ribeiro, P.A.; Chávez-Fumagalli, M.A.; de Ávila, R.A.M.; et al. Probing the efficacy of a heterologous Leishmania/L. viannia braziliensis recombinant enolase as a candidate vaccine to restrict the development of L. infantum in BALB/c mice. Acta Trop. 2017, 171, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Prakash, J.; Shukla, H.; Das, K.C.; Tripathi, T.; Dubey, V.K. Design of a multi-epitope subunit vaccine for immune-protection against Leishmania parasite. Pathog. Glob. Heal. 2020, 114, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Atapour, A.; Ghalamfarsa, F.; Naderi, S.; Hatam, G. Designing of a Novel Fusion Protein Vaccine Candidate Against Human Visceral Leishmaniasis (VL) Using Immunoinformatics and Structural Approaches. Int. J. Pept. Res. Ther. 2021, 27, 1885–1898. [Google Scholar] [CrossRef]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Parra, L.; Borja-Cabrera, G.P.; Santos, F.; Souza, L.; Palatnik-De-Sousa, C.; Menz, I. Safety trial using the Leishmune® vaccine against canine visceral leishmaniasis in Brazil. Vaccine 2007, 25, 2180–2186. [Google Scholar] [CrossRef]
- Aguiar-Soares, R.; Roatt, B.; Mathias, F.; Reis, L.; Cardoso, J.; Brito, R.; Ker, H.; Corrêa-Oliveira, R.; Giunchetti, R.; Reis, A. Phase I and II Clinical Trial Comparing the LBSap, Leishmune®, and Leish-Tec® Vaccines against Canine Visceral Leishmaniasis. Vaccines 2020, 8, 690. [Google Scholar] [CrossRef]
- Ministerio da Agricultura, Pecuaria e Abastecimento (MAPA). Suspensao da Licence de Fabricacao e Comercialixacao do Produto Leishmune®. Nota Tecnia n 038/2014/DFIP/SDA. Brasila. 2014. Available online: http://www.agricultura.gov.br/assuntos/politica-agricola/arquivos/nota-tecnica-dfip-38-14-leishmune.pdf/view (accessed on 11 November 2021).
- Bongiorno, G.; Paparcone, R.; Manzillo, V.F.; Oliva, G.; Cuisinier, A.-M.; Gradoni, L. Vaccination with LiESP/QA-21 (CaniLeish®) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs—A preliminary xenodiagnosis study. Veter-Parasitol. 2013, 197, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Costa, M.M.S.; Coelho, E.A.F.; Michalick, M.S.M.; de Freitas, E.; Melo, M.N.; Tafuri, W.L.; Resende, D.; Hermont, V.; Abrantes, C.D.F.; et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 2008, 26, 5888–5895. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Veter. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ikeogu, N.M.; Akaluka, G.N.; Edechi, C.A.; Salako, E.S.; Onyilagha, C.; Barazandeh, A.F.; Uzonna, J.E. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms 2020, 8, 1201. [Google Scholar] [CrossRef]
- Grimaldi, G., Jr.; Teva, A.; dos-Santos, C.B.; Santos, F.N.; Pinto, I.-S.; Fux, B.; Leite, G.R.; Falqueto, A. Field trial of efficacy of the Leish-tec® vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS ONE 2017, 12, e0185438. [Google Scholar] [CrossRef]
- Moreno, J.; Vouldoukis, I.; Martin, V.; McGahie, D.; Cuisinier, A.-M.; Gueguen, S. Use of a LiESP/QA-21 Vaccine (CaniLeish) Stimulates an Appropriate Th1-Dominated Cell-Mediated Immune Response in Dogs. PLoS Negl. Trop. Dis. 2012, 6, e1683. [Google Scholar] [CrossRef]
- European Medicines Agency. CaniLeish: Summary of Product Characteristics. 2011. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_the_public/veterinary/002232/WC500104955.pdf (accessed on 20 January 2022).
- Velez, R.; Domenech, E.; Rodríguez-Cortés, A.; Barrios, D.; Tebar, S.; Fernández-Arévalo, A.; Aguilar, R.; Dobaño, C.; Alberola, J.; Cairó, J.; et al. Evaluation of canine leishmaniosis vaccine CaniLeish® under field conditions in native dog populations from an endemic Mediterranean area–A randomized controlled trial. Acta Trop. 2020, 205, 105387. [Google Scholar] [CrossRef]
- European Medicines Agency. Lentifend: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/letifend-epar-product-information_en.pdf (accessed on 20 January 2022).
- Cotrina, J.F.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmanio-sis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Cacheiro-Llaguno, C.; Parody, N.; Renshaw-Calderón, A.; Osuna, C.; Alonso, C.; Carnés, J. Vaccination with LetiFend® reduces circulating immune complexes in dogs experimentally infected with L. infantum. Vaccine 2020, 38, 890–896. [Google Scholar] [CrossRef]
- Giunchetti, R.C.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Coura-Vital, W.; de Abreu, R.T.; Malaquias, L.C.C.; Gontijo, N.F.; et al. A killed Leishmania vaccine with sand fly saliva extract and saponin adjuvant displays immunogenicity in dogs. Vaccine 2008, 26, 623–638. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares, R.D.; Vitoriano-Souza, J.; Coura-Vital, W.; Braga, S.L.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; de Lana, M.; Figueiredo Gontijo, N.; et al. Performance of LBSap Vaccine after Intradermal Challenge with L. infantum and Saliva of Lu. longipalpis: Immunogenicity and Parasi-tological Evaluation. PLoS ONE 2012, 7, e49780. [Google Scholar] [CrossRef]
- Lemesre, J.L.; Holzmuller, P.; Cavaleyra, M.; Gonçalves, R.B.; Hottin, G.; Papierok, G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine 2005, 23, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Lemesre, J.L.; Holzmuller, P.; Gonçalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: Double-blind randomised efficacy field trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Holzmuller, P.; Cavaleyra, M.; Moreaux, J.; Kovacic, R.; Vincendeau, P.; Papierok, G.; Lemesre, J.-L. Lymphocytes of dogs immunised with purified excreted-secreted antigens of Leishmania infantum co-incubated with Leishmania infected macrophages produce IFN gamma resulting in nitric oxide-mediated amastigote apoptosis. Vet. Immunol. Immunopathol. 2005, 106, 247–257. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Reis, L.E.S.; Cardoso, J.M.D.O.; Mathias, F.A.S.; de Brito, R.C.F.; da Silva, S.M.; Gontijo, N.D.F.; Ferreira, S.D.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- Shermeh, A.S.; Zahedifard, F.; Habibzadeh, S.; Taheri, T.; Rafati, S.; Seyed, N. Evaluation of protection induced by in vitro maturated BMDCs presenting CD8+ T cell stimulating peptides after a heterologous vaccination regimen in BALB/c model against Leishmania major. Exp. Parasitol. 2021, 223, 108082. [Google Scholar] [CrossRef]
- Das, A.; Ali, N. Vaccine Development Against Leishmania donovani. Front. Immunol. 2012, 3, 99. [Google Scholar] [CrossRef]
- Liu, M.A.; Wahren, B.; Hedestam, G.B.K. DNA vaccines: Recent developments and future possibilities. Hum. Gene Ther. 2006, 17, 1051–1061. [Google Scholar] [CrossRef]
- Alarcon, J.B.; Waine, G.W.; McManus, D.P. DNA Vaccines: Technology and Application as Anti-parasite and Anti-microbial Agents. Adv. Parasitol. 1999, 42, 343–410. [Google Scholar] [CrossRef]
- Dunning, N. Leishmania vaccines: From leishmanization to the era of DNA technology. Biosci. Horiz. 2009, 2, 73–82. [Google Scholar] [CrossRef]
- Tewary, P.; Jain, M.; Sahani, M.H.; Saxena, S.; Madhubala, R. A Heterologous Prime-Boost Vaccination Regimen Using ORFF DNA and Recombinant ORFF Protein Confers Protective Immunity against Experimental Visceral Leishmaniasis. J. Infect. Dis. 2005, 191, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Fairweather, N.; Button, L.L.; McMaster, W.R.; Kahl, L.P.; Liew, F.Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J. Immunol. 1990, 145, 2281–2285. [Google Scholar]
- Dumonteil, E.; Jesus, R.S.M.; Javier, E.O.; Del Rosario, G.M.M. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 2003, 21, 2161–2168. [Google Scholar] [CrossRef]
- Rosado-Vallado, M.; Mut-Martin, M.; García-Miss Mdel, R.; Dumonteil, E. Aluminium phosphate potentiates the efficacy of DNA vaccines against Leishmania mexicana. Vaccine 2005, 23, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Tsagozis, P.; Karagouni, E.; Dotsika, E. Dendritic Cells Pulsed with Peptides of GP63 Induce Differential Protection against Experimental Cutaneous Leishmaniasis. Int. J. Immunopathol. Pharmacol. 2004, 17, 343–352. [Google Scholar] [CrossRef]
- Forster, K.M.; Hartwig, D.D.; Oliveira, T.L.; Bacelo, K.L.; Schuch, R.; Amaral, M.G.; Dellagostin, O.A. DNA prime-protein boost based vaccination with a conserved region of leptospiral immunoglobulin-like A and B proteins enhances protection against leptospirosis. Mem. Inst. Oswaldo Cruz 2015, 110, 989–995. [Google Scholar] [CrossRef]
- Aguilar-Be, I.; Zardo, R.D.S.; de Souza, E.P.; Borja-Cabrera, G.P.; Vallado, M.E.R.; Mut-Martin, M.; García-Miss, M.D.R.; Palatnik-De-Sousa, C.B.; Dumonteil, E. Cross-Protective Efficacy of a Prophylactic Leishmania donovani DNA Vaccine against Visceral and Cutaneous Murine Leishmaniasis. Infect. Immun. 2005, 73, 812–819. [Google Scholar] [CrossRef]
- Campos-Neto, A.; Webb, J.R.; Greeson, K.; Coler, R.N.; Skeiky, Y.A.W.; Reed, S.G. Vaccination with Plasmid DNA Encoding TSA/LmSTI1 Leishmanial Fusion Proteins Confers Protection against Leishmania major Infection in Susceptible BALB/c Mice. Infect. Immun. 2002, 70, 2828–2836. [Google Scholar] [CrossRef]
- Méndez, S.; Gurunathan, S.; Kamhawi, S.; Belkaid, Y.; Moga, M.A.; Skeiky, Y.A.W.; Campos-Neto, A.; Reed, S.; Seder, R.A.; Sacks, D. The Potency and Durability of DNA- and Protein-Based Vaccines Against Leishmania major Evaluated Using Low-Dose, Intradermal Challenge. J. Immunol. 2001, 166, 5122–5128. [Google Scholar] [CrossRef]
- Ahmed, S.B.H.; Touihri, L.; Chtourou, Y.; Dellagi, K.; Bahloul, C. DNA based vaccination with a cocktail of plasmids encoding immunodominant Leishmania (Leishmania) major antigens confers full protection in BALB/c mice. Vaccine 2009, 27, 99–106. [Google Scholar] [CrossRef]
- Saldarriaga, O.A.; Travi, B.L.; Park, W.; Perez, L.E.; Melby, P.C. Immunogenicity of a multicomponent DNA vaccine against visceral leishmaniasis in dogs. Vaccine 2006, 24, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Soto, M.; Carrión, J.; Alonso, C.; Requena, J.M. Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine 2004, 22, 3865–3876. [Google Scholar] [CrossRef] [PubMed]
- Carrión, J. Mechanisms of immunity to Leishmania major infection in mice: The contribution of DNA vaccines coding for two novel sets of histones (H2A–H2B or H3–H4). Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Schwartz, J.; Calvo, A.; Blanco, L.; Larrea, E.; Irache, J.M.; Sanmartín, C.; Coulman, S.A.; Soto, M.; Birchall, J.C.; et al. Skin vaccination using microneedles coated with a plasmid DNA cocktail encoding nucleosomal histones of Leishmania spp. Int. J. Pharm. 2017, 533, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Baharia, R.K.; Tandon, R.; Sahasrabuddhe, A.A.; Sundar, S.; Dube, A. Nucleosomal Histone Proteins of L. donovani: A Combination of Recombinant H2A, H2B, H3 and H4 Proteins Were Highly Immunogenic and Offered Optimum Prophylactic Efficacy against Leishmania Challenge in Hamsters. PLoS ONE 2014, 9, e97911. [Google Scholar] [CrossRef]
- Carneiro, M.W.; Santos, D.M.; Fukutani, K.F.; Clarencio, J.; Miranda, J.C.; Brodskyn, C.; Barral, A.; Barral-Netto, M.; Soto, M.; de Oliveira, C.I. Vaccination with L. infantum chagasi Nucleosomal Histones Confers Protection against New World Cutaneous Leishmaniasis Caused by Leishmania braziliensis. PLoS ONE 2012, 7, e52296. [Google Scholar] [CrossRef]
- Mortazavidehkordi, N.; Farjadfar, A.; Khanahmad, H.; Ghayour Najafabadi, Z.; Hashemi, N.; Fallah, A.; Najafi, A.; Kia, V.; Hejazi, S.H. Evaluation of a novel lentiviral vaccine expressing KMP11-HASPB fusion protein against Leishmania infantum in BALB/c mice. Parasite Immunol. 2016, 38, 670–677. [Google Scholar] [CrossRef]
- Mortazavidehkordi, N.; Fallah, A.; Abdollahi, A.; Kia, V.; Khanahmad, H.; Najafabadi, Z.G.; Hashemi, N.; Estiri, B.; Roudbari, Z.; Najafi, A.; et al. A lentiviral vaccine expressing KMP11-HASPB fusion protein increases immune response to Leishmania major in BALB/C. Parasitol. Res. 2018, 117, 2265–2273. [Google Scholar] [CrossRef]
- Dalimi, A.; Nasiri, V.D. Construction and Immunogenicity Assessment of pEGFP-N1-KMP11-GP96 (Fusion) as a DNA Vaccine Candidate against Leishmania major Infection in BALB/c Mice. Iran. J. Parasitol. 2020, 15, 11–21. [Google Scholar] [CrossRef]
- Guha, R.; Gupta, D.; Rastogi, R.; Vikram, R.; Krishnamurthy, G.; Bimal, S.; Roy, S.; Mukhopadhyay, A. Vaccination with Leishmania Hemoglobin Receptor–Encoding DNA Protects Against Visceral Leishmaniasis. Sci. Transl. Med. 2013, 5, 202ra121. [Google Scholar] [CrossRef]
- Das, S.; Freier, A.; Boussoffara, T.; Das, S.; Oswald, D.; Losch, F.O.; Selka, M.; Sacerdoti-Sierra, N.; Schönian, G.; Wiesmüller, K.-H.; et al. Modular Multiantigen T Cell Epitope–Enriched DNA Vaccine Against Human Leishmaniasis. Sci. Transl. Med. 2014, 6, 234ra56. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.; Van Hoeven, N.; Macmillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.-C.; Stinchcomb, D.T.; Reed, S.G. Heterologous Immunization with Defined RNA and Subunit Vaccines Enhances T Cell Responses That Protect Against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Reyes, M.A.; Baylón-Pacheco, L.; Espíritu-Gordillo, P.; Galindo-Gómez, S.; Tsutsumi, V.; Rosales-Encina, J.L. Effect of Prophylactic Vaccination with the Membrane-Bound Acid Phosphatase Gene of Leishmania mexicana in the Murine Model of Localized Cutaneous Leishmaniasis. J. Immunol. Res. 2021, 2021, 6624246. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Sacks, D.L.; Brown, D.R.; Reiner, S.L.; Charest, H.; Glaichenhaus, N.; Seder, R.A. Vaccination with DNA Encoding the Immunodominant LACK Parasite Antigen Confers Protective Immunity to Mice Infected with Leishmania major. J. Exp. Med. 1997, 186, 1137–1147. [Google Scholar] [CrossRef]
- Gonzalo, R.M.; Rodriguez, J.R.; Rodriguez, D.; Gonzalez-Aseguinolaza, G.; Larraga, V.; Esteban, M. Protective immune response against cutaneous leishmaniasis by prime/booster immunization regimens with vaccinia virus recombinants expressing Leishmania infantum p36/LACK and IL-12 in combination with purified p36. Microbes Infect. 2001, 3, 701–711. [Google Scholar] [CrossRef]
- Ramos, I.; Alonso, A.; Marcen, J.; Peris, A.; Castillo, J.; Colmenares, M.; Larraga, V. Heterologous prime-boost vaccination with a non-replicative vaccinia recombinant vector expressing LACK confers protection against canine visceral leishmaniasis with a predominant Th1-specific immune response. Vaccine 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Ramiro, M.J.; Zárate, J.J.; Hanke, T.; Rodriguez, D.; Rodriguez, J.R.; Esteban, M.; Lucientes, J.; Castillo, J.A.; Larraga, V. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine 2003, 21, 2474–2484. [Google Scholar] [CrossRef]
- Ramos, I.; Alonso, A.; Peris, A.; Marcen, J.; Abengozar, M.; Alcolea, P.; Castillo, J.; Larraga, V. Antibiotic resistance free plasmid DNA expressing LACK protein leads towards a protective Th1 response against Leishmania infantum infection. Vaccine 2009, 27, 6695–6703. [Google Scholar] [CrossRef]
- Jorjani, O.; Ghaffarifar, F.; Sharifi, Z.; Dalimi, A.; Ziaei-Hezarjaribi, H.; Talebi, B. LACK Gene’s Immune Response Induced by Cocktail DNA Vaccine with IL-12 Gene Against Cutaneous Leishmaniasis in BALB/c Mice. Avicenna J. Med. Biotechnol. 2018, 10, 134–140. [Google Scholar]
- Ghaffarifar, F.; Jorjani, O.; Sharifi, Z.; Dalimi, A.; Hassan, Z.M.; Tabatabaie, F.; Khoshzaban, F.; Hezarjaribi, H.Z. Enhancement of immune response induced by DNA vaccine cocktail expressing complete LACK and TSA genes against Leishmania major. APMIS 2013, 121, 290–298. [Google Scholar] [CrossRef]
- Maspi, N.; Ghaffarifar, F.; Sharifi, Z.; Dalimi, A.; Dayer, M.S. Comparative Assessment of Induced Immune Responses Following Intramuscular Immunization with Fusion and Cocktail of LeIF, LACK and TSA Genes Against Cutaneous Leishmaniasis in BALB/c Mice. Arch. Immunol. Ther. Exp. 2018, 66, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Sangani, G.; Mohebali, M.; Jajarmi, V.; Khamesipour, A.; Bandehpour, M.; Mahmoudi, M.; Zahedi-Zavaram, H. Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens. Iran. J. Basic Med. Sci. 2019, 22, 1493–1501. [Google Scholar] [PubMed]
- Alcolea, P.J.; Alonso, A.; Esteban, A.; Peris, P.; Cortés, A.; Castillo, J.A.; Larraga, V. IL12 p35 and p40 subunit genes administered as pPAL plasmid constructs do not improve protection of pPAL-LACK vaccine against canine leishmaniasis. PLoS ONE 2019, 14, e0212136. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Younis, B.M.; Osman, M.; Khalil, E.A.; Santoro, F.; Furini, S.; Wiggins, R.; Keding, A.; Carraro, M.; Musa, A.E.; Abdarahaman, M.A.; et al. Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan. Mol. Ther. 2021, 29, 2366–2377. [Google Scholar] [CrossRef]
- Oliveira-Da-Silva, J.A.; Lage, D.P.; Ramos, F.F.; Machado, A.S.; Tavares, G.S.; Mendonça, D.V.; Pereira, I.A.; Martins, V.T.; Carvalho, L.M.; Ludolf, F.; et al. Leishmania infantum pyridoxal kinase evaluated in a recombinant protein and DNA vaccine to protects against visceral leishmaniasis. Mol. Immunol. 2020, 124, 161–171. [Google Scholar] [CrossRef]
- Martínez-Flórez, A.; Martori, C.; Monteagudo, P.L.; Rodriguez, F.; Alberola, J.; Rodríguez-Cortés, A.; Muntsant, C.M. Sirolimus enhances the protection achieved by a DNA vaccine against Leishmania infantum. Parasites Vectors 2020, 13, 294. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).