Abstract
The past decades of cancer immunotherapy research have provided profound evidence that the immune system is capable of inducing durable tumor regression. Although many commercialized anti-cancer immunotherapies are available to patients, these treatment options only scrape the surface of the potential immune-related treatment possibilities for cancer. Additionally, many individuals are ineligible for established immunotherapies due to their cancer type. The adoptive cell transfer of autologous tumor-infiltrating lymphocytes has been used in humans for over 30 years to treat metastatic melanoma, and continued modifications are making it increasingly more effective against other types of cancer. This comprehensive review outlines this therapy from its infancy through to the present day, bringing to light modifications and optimizations to the traditional workflow, as well as highlighting the influence of new methods and technologies.
1. Introduction
In 2011, the United States Food and Drug Administration (FDA) approved the checkpoint inhibitor (CPI), ipilimumab, as the first immunotherapeutic for the treatment of metastatic melanoma [1]. Following this approval, CPI therapy has been an increasingly inclusive treatment option, and continues to be the most widely used class of immunotherapeutic treatment for cancer [2]. CPI effectiveness against cancer is achieved by indiscriminately untethering the immune system, including pre-existing antitumor immunity [3,4,5]. Adoptive cell therapy (ACT) makes up another class of immunotherapy in which an individual’s own immune cells are removed, manipulated, and administered back to them with the goal of providing a tumor-specific, cell mediated response against cancer [6]. This broad method includes chimeric antigen receptor T cells (CAR-T), engineered T cell receptor (TCR-T), and tumor infiltrating lymphocytes (TILs). CAR-T and TCR-T therapies involve extensive genetic modifications to arm the cells with unique, cancer specific receptors [6,7,8]. TIL-based ACT is a distinctive cell therapy, in which tumor-derived immune cells are expanded in a multi-step process and infused back into the individual (see Section 2.4). An abundance of intratumoral lymphocytes is typically a positive prognostic indicator [9,10,11], suggesting that tumor-infiltrating immune cells play an important role in tumor eradication. Therefore, the expansion and re-infusion of these cells is a promising approach. Significant responses can be achieved with the therapeutic use of these cells with no genetic manipulation, predominantly in patients with metastatic melanoma. With this extensive process comes numerous obstacles, including obtaining and expanding tumor-reactive TILs, managing adverse events, and increasing the effectiveness against other types of cancer. While genetic modification is not a defining component of the therapy, its integration may be crucial in the advancement of TIL-based ACT. Here, we aim to examine and discuss the history, obstacles, and modifications of this therapy.
2. History
2.1. Early Experiments
Adoptive cell therapy using TILs as it is known today is the product of decades of landmark findings and experiments. The earliest documented appreciation of lymphocyte infiltration to tumor tissue dates back to 1921. Investigating the variable post-operative survival rates experienced by his patients, Dr. William C. MacCarty evaluated the histology of resected tumors. He found that patients who lived longer after surgery had lymphocytes that were, “intimately associated with cancer cells” [12]. The importance of infiltration of tumor tissue by lymphocytes became increasingly evident [13,14], but the therapeutic use did not come until much later.
The Surgery Branch at the National Cancer Institute (NCI) was the pioneering force behind adoptive cell therapies and conducted extensive research using lymphokine-activated killer (LAK) cells which set the stage for TIL in both the laboratory and clinic. Preparation of LAK cells involved isolating lymphocytes from peripheral whole blood of individuals with cancer, which could be then activated and expanded in culture for the adoptive cell treatment of the individual [15]. The discovery of interleukin-2 (IL-2) as an imperative agent in the function and response of immune cells allowed for the long-term culture and maintenance of T cells [16,17,18]. The development of recombinant IL-2 at the NCI in 1983 allowed for large-scale production of the growth factor which was instrumental in propelling the clinical use of the therapies [19]. Successful in vitro and animal models [18,20,21] gave way to a clinical trial reported in 1985 [22]. Twenty-five patients across six types of metastatic cancer were treated with the autologous adoptive cell transfer of LAK demonstrating the safety and feasibility of the therapy, with one patient experiencing complete regression of tumor [22].
The NCI subsequently demonstrated that TIL had obvious advantages over LAK. In addition to exemplifying reactive potential against LAK-resistant tumors, TIL produced better fold expansions, more successful synergy with exogenous IL-2 and cyclophosphamide, and produced more durable and powerful anti-tumor responses [23]. The NCI developed a method to obtain TILs involving the fragmentation and enzymatic digestion of resected tumor followed by culture in IL-2-containing medium for roughly 60 days [24]. After successfully using the protocol in murine experiments [23,25], a landmark study was reported in 1987 where TILs from melanoma patients were not only expanded to a clinical scale, but also showed functional reactivity against autologous tumor [26]. Pilot studies at the NCI showed that autologous adoptive transfer of these cells was safe, feasible, and improved with dose escalation of cyclophosphamide, IL-2, and TIL quantity [27,28]. In 1988, the group published a special report of preliminary clinical results in the New England Journal of Medicine showing that combination of cyclophosphamide, IL-2, and autologous TIL adoptive transfer had 40–60% objective response rate against metastatic melanoma [29]. This study defined TIL-based ACT as a promising treatment option and opened the door for institutions around the world.
2.2. Optimizing Methods: The Rapid Expansion Protocol
The clinical reports of the late 1980s set the standard for TIL development and administration protocol. The 1988 publication also brought insight into cell types that comprised TILs. Seventy-four to one hundred percent of the cells in the infusion product were CD3+ T cells, elucidating why IL-2 (formerly known as T cell growth factor) was so crucial for the feasibility of adoptive cell therapies. While essential for production, solitary use of IL-2 for cell expansion resulted in TILs lacking longevity due to dependency on the growth factor [30]. This posed a clinical barrier given the intolerability of IL-2 therapy [31].
Discoveries in T cell biology and advanced culture methods allowed for rational alteration of TIL production methods and resulted in the development of the rapid expansion protocol (REP), now a paramount aspect of TIL-based ACT. After isolation and initial expansion of TILs from tumors, cells would undergo this process, involving the 14-day culturing of cells in medium containing IL-2, anti-CD3 monoclonal antibodies (OKT3), and allogenic feeder cells [32,33].
By the 1990s, the main components of the CD3 subcomplex (γ, δ, ε) were mapped and understood to play a vital role in antigen-dependent TCR signaling [34]. The addition of OKT3 to the REP was found to improve the expansion and long-term culture of T cells with antigen-independence by stimulating the TCR/CD3 complex [35,36,37]. The OKT3 approach also dampened the IL-2-dependency in vivo, and when applied to a murine adoptive T cell transfer model demonstrated that the autologous cells retained tumor immunogenicity and were capable of eliminating cancer in mice [38]. This 1991 study also demonstrated that in vitro expansion with anti-CD3 monoclonal antibodies resulted in improved fold expansion and produced cells with increased longevity after transfer, likely due to the upregulation of IL-2 receptors resulting from CD3-mediated activation, a mechanism not observed with IL-2 stimulation alone.
The last key component of the REP is the allogenic feeder cells. It was shown in the 1980s that accessory cells in the T cell culture environment could profoundly accentuate lymphocyte growth [39,40]. While early experiments used feeder cells derived from various anatomical sites of animals, Riddle et al. described a method to use peripheral blood-derived mononuclear cells as feeder cells to produce virus-specific T cells for murine adoptive cell transfer experiments [41]. The irradiation of these cells allowed retention of their native costimulatory abilities but rendered them incompetent for replication [42]. While the use of feeder cells transcends T cell expansion, the mechanisms by which these cells work in the context of TILs is still largely unknown. By the 2000s, the NCI reported the adoption of REP into a number of clinical trials.
2.3. Discovering Correlates of Response and Optimizations to Therapy
Since the early NCI clinical trials in the 1980s, clear correlates of response continued to be identified both in the ex vivo workflow and in the patient. An early correlate identified was the profound effect that ex vivo culture time had on the efficacy of treatment. In 1991, Aebersold et al. identified a 45-day threshold. TIL functionality was detrimentally impeded when culture times prior to infusion exceeded this threshold, and TILs cultured less than 45 days demonstrated greatly improved in vitro tumor lysis and patient outcomes [43]. Three years later, the team reported a more in-depth analysis of TIL age, supporting the need to avoid lengthy culture times before infusion [44]. Given the need for shortening ex vivo time, another method was developed.
From the compelling findings of the significant effects of culture time came the development of minimally cultured TILs, also referred to as young TILs. Until the late 2000s, only individual TIL cultures that demonstrated reactive potential would proceed through the REP process and be infused to the patient, known as the standard method. In contrast, the young TIL method involved pooling all TIL cultures from one patient into the REP [45,46,47], which almost halved the total ex vivo time [45,46]. An important association with shorter culture time and the young TIL method was found to be telomere length [45,48]. Telomers are repetitions of nucleotide sequences flanking the ends of chromosomes and associate with specific trans-acting factors [49]. Every cycle of cell division results in the shortening of these regions, having downstream influences on cellular function [49,50]. The resulting consequences are detrimental in the context of TIL therapy, and include cellular senescence, loss of function, and reduced expression of CD27/CD28 costimulatory proteins important for T cell activation [48,50]. Young TIL trials have repeatedly shown that this method results in longer persistence of TIL in the patient and better clinical outcomes than the aforementioned standard method, and these benefits have been attributed to curtailing telomer shortening [45,46,47,51,52,53]. The overwhelming evidence of the inverse relation of culture time and TIL efficacy has become influential in the clinical use of the therapy.
The phenotypic variation observed in the infusion product was also linked to patient responses, although much less black and white than the effect of culture time. As previously stated, it has been known since early TIL trials that infusion products are ostensibly T lymphocytes [29]. While phenotypes of CD4-positive (CD4+, helper-cell functions) and CD8-positive (CD8+, killer-cell functions) cells were quantitated in the special report, no trend in patient outcome could be attributed to phenotype. The appreciation of increased CD8+ T cell populations in responding patients in a 1994 NCI publication brought to light a possible correlation between phenotype and clinical outcome [44], which could be expected due to the direct cytolytic abilities of CD8+ T cells. These TILs were much more functional in vitro against autologous tumor when compared to that of the non-responders, but the benefit was much less exaggerated in the clinical response, although apparent. As TIL-based ACT became more feasible, other notable groups reported the importance of CD8-dense TIL products [54,55,56]. Importantly, effective ACT requires phenotypic diversity. This was highlighted by clinical trials involving TIL- and PBMC-derived antigen-specific clonal CD8+ T cells. Unfortunately these studies showed to be largely ineffective with no subjects experiencing an overall response [57,58,59]. While much of the inefficacy was likely due to the narrow antigen specificity of the TILs, it is now well understood that CD4+ T cells are crucial for accentuating the response of CD8+ cytotoxic TILs [60,61]. Investigations to better understand phenotypic contributions of TILs are continually underway. In 2020 the NCI reported that metastatic melanoma responders in a TIL-based ACT trial had a subpopulation of CD8+ stem-like T cells that exhibited high in vivo replicative potential, longevity, and antitumor immunity after administration and correlated with positive outcome [62]. These cells were restricted to a CD39− and CD69− phenotype. Infusion products that contained only the double positive, terminally differentiated counterpart significantly hindered a successful outcome for the patient. This study further exemplifies the need for more research on ideal phenotypes and ways to identify and promote the production to increase the effectiveness of TIL-based ACT.
Lymphodepletion is a crucial component of ACT for the function and persistence of the infused cells [63,64]. While many trials investigated the ideal doses and frequencies of the non-myeloablative drugs, cyclophosphamide and fludarabine, the benefit of the more drastic depletion method of total-body irradiation (TBI) was also investigated as well as the combination of the two [63,64,65,66]. While TBI was arguably a slightly better preconditioning method, it did not yield results that outweighed the toxicities experienced by the patients [67]. Today, non-myeloablative lymphodepletion prior to ACT of low culture time TIL infusion product is the international norm. Although the general workflow of TIL-based ACT has changed very little over the decades, the multistep protocol leaves much room for optimization and alteration to improve patient outcome.
2.4. Current Standard of Care and Therapeutic Process
The cyclic workflow of TIL adoptive transfer, as it pertains to our institution, is illustrated in Figure 1 below. Following surgical removal, the tumor is manually fragmented with a scalpel. The initial expansion phase begins when the fragments are left to culture individually in a 24-well plate with IL-2 supplemented medium to produce young TILs. The lymphocytes residing in the tumor will egress the tissue and overflow into the medium, as well as exhibit cytotoxicity against the autologous tumor, typically eliminating it entirely from the culture [45]. After sufficient growth, the remaining young TILs are advanced to the REP containing irradiated allogenic feeder cells, OKT3, and IL-2. While these components are tantamount to the REP, some aspects vary between institutions, such as the use of bioreactors or specialized gas-permeable cultureware [56,68,69]. To maximize TIL proliferation, the clearance of waste and access to oxygen must keep up with the expanding cell culture. Bioreactors, such as the commonly used WAVE, oxygenate the culture through rocking and agitation while continuously renewing media [68,70]. Alternatively, gas-permeable vessels, such as G-Rex, remain static as oxygen and CO2 are exchanged through a silicon membrane base [71]. Both methods are capable of producing high yield REPs and fulfill good manufacturing practice requirements for clinical use [68,69]. The infusion of TILs is preceded by nonmyeloablative lymphodepletion, typically cyclophosphamide and fludarabine, then followed with IL-2 administration. Aliquots of TILs and tumor can be saved throughout the process for ex vivo experiments, and even subsequent expansion and treatment.
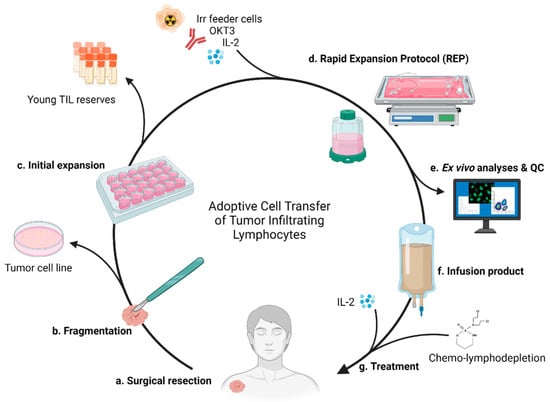
Figure 1.
TIL production and administration. (a) Full or partial surgical resection of tumor. (b) The tumor is minced via scalpel. Tumor material can be saved to establish cell lines for future assays. (c) Fragments are placed into individual wells of a 24-well plate with culture medium. Harvested young TILs can be saved for future autologous therapy or experiments, while others (d) proceed to REP with irradiated allogenic feeder cells, OKT3, and IL-2 for massive expansion. Use of bioreactor or gas-permeable flasks. (e) Quality of the cells are analyzed prior creation of (f) the infusion product. (g) Treatment of the patient includes the preparative lymphodepletion, administration of the autologous TIL product, and subsequent IL-2 administration.
3. Treatment Complications and Resolutions
3.1. Surgery
Currently, there is no clinically approved TIL therapy for the treatment of cancer. As the treatment is available only through clinical trial, there are strict eligibility requirements for admittance. One initial limiting factor for eligibility is surgical candidacy, for which a patient can be excluded for a number of reasons. Two of the most common reasons are surgical inaccessibility to the tumor and clinical deterioration of the patient. As described above, TIL therapy begins with full or partial surgical resection of the tumor which can be performed either through open surgery, or minimally invasive endoscopic surgery. Accessibility is not frequently a hurdle in case of metastatic melanoma as these tumors often metastasize to lymph nodes, cutaneous, and subcutaneous sites. Tumors from other cancer types, however, can be difficult to access surgically, or arise in sites that pose difficulty to sterilely obtain. Gastrointestinal cancers are specifically difficult to resect without contamination. Though it has had little exploration in the context of TIL therapy, ultrasound-guided biopsy may address not only these issues, but also further reduce the invasiveness [72]. As surgical and imaging technologies improve, TIL adoptive therapy will become increasingly more available to patients with these types of cancer.
3.2. Expansion Complications
The ex vivo expansion phases required to produce large quantities of phenotypically effective TILs presents the next barrier to treatment. As previously discussed, a high CD8:CD4 ratio is a good indicator for effective TIL therapy alongside short ex vivo time (see Section 2.3). The outcomes of these ex vivo expansion steps can be greatly impacted by small changes in the culture environment. The development of the rapid expansion protocol demonstrated the importance of integrating discoveries in T cell biology, and the same approach should be taken to further improve the production process. Recent attention to costimulatory elements and their manipulation currently demonstrates potential for improving the speed and quality of TIL expansion for ACT.
3.2.1. Expansion Resolutions Targeting 4-1BB
In recent years, the potential of 4-1BB (CD137) as a costimulatory target for TIL-based ACT has been investigated. The theory for propagating this pathway to the benefit of TIL target is multifocal. As a “second signal” costimulatory molecule, 4-1BB ligation triggers downstream NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation, driving T cell survival [73], and is highly expressed on activated CD8+ cells [74]. Addition of this agonistic antibody toward the costimulatory protein could drive the prolific stimulation of desired cytotoxic T cell populations within the tumor fragments. Additionally, anti-4-1BB could benefit TIL expansion by acting on intratumoral dendritic cells in tumor fragments that also express the target protein. This interaction has been shown to drive dendritic cell maturation and upregulation of MHC-II and costimulatory proteins that could augment TIL proliferation [75].
Researchers at MD Andersen Cancer Center (MDACC) experimented with the propagation of this pathway by introducing anti-4-1BB agonistic antibodies to initial expansion cultures of triple negative breast cancer with IL-2 [76]. They found that this addition not only increased CD8+ T cell populations, but also aided in boosting the functionality of these cells in a flow cytometry-based redirected killing assay. In a subsequent publication, the group further explored the potential of 4-1BB activation in both cutaneous and uveal melanoma [77]. Contrary to the previously described TIL-based ACT workflow above (see Section 2.4), the authors did not begin the pre-REP phase in 24-well plates but began the culture by adding tumor fragments to a specialized flask with medium containing “3-signal medium” (OKT3, anti-4-1BB, and IL-2). After 2 weeks of minor culture maintenance, cell quantities not only mirrored, but exceeded those of typical pre-REP quantities. Consistent with their previous finding, CD8+ phenotype was favored, and functionality was retained. The authors reason that the REP phase could altogether be bypassed if more tumor fragments were used in the initial expansion phase.
Our institution explored 4-1BB agonism in a sarcoma model [78]. The expansion and functionality of CD8+ TILs in this report were consistent with the previously discussed MDACC findings. In depth analysis of the TILs, however, showed that 4-1BB and CD3 combined stimulation resulted in not only favorable Th1-associated phenotype such as interferon gamma (IFNγ), but also disadvantageous Th2-associated cytokines (IL-4, IL-5, IL-13), which is supported by previous findings [79]. Additionally, while CD8+ T cells preferentially benefit from this costimulation, regulatory T cell populations can also counterintuitively be expanded [80]. The extent to which this could have TIL-suppressive or antitumor effects is not clear but should be taken into consideration during future investigation.
3.2.2. Expansion Resolutions Targeting CTLA-4
While agonism of the costimulatory protein 4-1BB has a profound effect on the TILs, inhibiting negative-regulators of activation may also be a promising approach to making the therapeutic TIL product more feasible. The immunological checkpoint, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), mediates immunosuppression when agonized by its ligand, CD80/CD86, expressed by target cells [81,82]. Our group demonstrated that the addition of CTLA-4-blocking antibodies to tumor fragments from ovarian cancer metastases during the pre-REP initial expansion phase improved the outcome of the expansion in a number of ways [83]. Not only did anti-CTLA-4 addition result in the expansion of TIL from more patients, but also resulted in more TILs per fragment, and better CD8:CD4 ratio. To our knowledge, this currently is the only report investigating the introduction of a checkpoint inhibitor directly to the TIL culture to assist with in vitro expansion.
Modification of the cellular environment has proven to help overcome the barriers brought forth in the ex vivo phases. 4-1BB and CTLA-4 manipulation have shown potential in broadening the availability of clinical grade TILs. While both of these approaches have been tested in diverse cancer types, it is important to recognize that expansion complications still remain a barrier to care for patients with metastatic melanoma. Other costimulatory targets and their combination have yet to be explored, but these changes could not only make treatment effective in other types of cancer, but also further improve outcome in patients with melanoma.
3.3. Severe Adverse Events
Altering the immunological landscape to boost anticancer immunity inherently has the potential to elicit immune-mediated toxicities in the patient. TIL-based ACT is a unique immunotherapy, as the risk for autoimmune side effects is less than others, such as CPI [3,4,5] and CAR-T [7]. This is likely because the cells administered are mostly anti-tumor with little to no off-target reactivity and not exacerbated by cutting the breaks of immunoregulatory pathways, as seen in CPI [3,4,5]. Some autoimmune reactions such as vitiligo, uveitis, and vasculitis are sometimes experienced [63,84,85]. Toxicities during treatment are typically due to preparatory lymphodepletion from cyclophosphamide and fludarabine, and systemic IL-2 administration [85]. Fever, chills, hypotension, hypoxia, and dyspnea are among the most common side effects experienced; vomiting, diarrhea, renal failure, and atrial fibrillation are less common [63,84,85] and are typically short-lived.
3.4. The Effect of CPI-Resistance on TIL Therapy
Cancer immunotherapies should not be thought of as one single brand. The diversity of the disease renders many cancer patients unresponsive to current commercial therapies such as ipilimumab and nivolumab. However, this should not imply that cellular immunotherapies will be unproductive in such cases, as concluded by Besser et al. in a 2013 clinical trial report showing that Ipilimumab resistance had virtually no effect on TIL therapy in melanoma [86]. In 2018, our group showed that CPI-resistant melanoma can still produce functional, tumor-reactive TILs that can mediate regression [87]. In a recent data analysis of clinical trials at our own institution, we have further concluded that disease progression after CPI therapy does not negate the ability for response to adoptive TIL therapy [88]. Even more recently the TIL-based ACT product, Lifileucel produced by Iovance Biotherapeutics, was shown to produce a durable response against metastatic melanoma in large cohorts of anti-PD-1 and PL-L1 refractory patients [89]. While CPI-resistance does not definitively nullify the success of TIL-based ACT, methods for optimizing treatment of this patient population should continue to be explored.
4. TIL-Based ACT in Non-Melanoma Solid Tumors
TIL-based ACT is arguably the best treatment option for individuals with refractory metastatic melanoma; however, few individuals with non-melanoma cancers have reaped the rewards of this therapy. Metastases from melanoma are susceptible to TIL-based ACT in all anatomic locations, including the brain [65,90] hinting at the potential of this therapy in other cancer types. While non-epithelial cancers such as non-small cell lung cancer, gliomas, hepatocellular carcinoma, and pancreatic tumors are much more resistant to the therapy, infusion-scale TIL have been produced, demonstrating the feasibility for treatment of these cancers with TIL [91,92,93,94,95]. Brain tumors have unique hurdles for cellular therapies, specifically the blood–brain barrier, an immunosuppressive environment [96], and invasive surgery in the case of TIL. The dearth of research in this area is likely not limited to these factors; however, Liu et al. demonstrated that glioma-derived TILs could be efficiently expanded in 16 out of 16 cases using IL-2, -15, and -21 [95]. The improved phenotype and quantity of TILs obtained with this method could ameliorate the responses observed in a 1999 report in which one out of six individuals with malignant gliomas had a complete response after TIL-based ACT [97]. A phase I clinical trial in patients with hepatocellular carcinoma showed that 80% of the cohort had no evidence of disease after 14 months [93], but a phase II trial has yet to follow. Beyond these reports, Iovance Biotherapeutics is filling many gaps in clinical TIL-based ACT studies by including multiple solid tumor cancer types into clinical trials, some of which combine other therapies (NCT03610490, NCT03449108, NCT03215810). The success of CPIs across multiple cancer types has drawn recent attention toward other immunotherapies, namely TIL-based ACT for broader cancer types. Attempts at treating these patients with TILs has helped elucidate the factors contributing to less effective responses. Recent studies have repurposed modern therapies and technologies to make adoptive transfer of TIL work better for other groups of patients, notably combination with other immunotherapies and specific targeting.
4.1. Checkpoint Inhibition Combination
Our institution has shown that ovarian cancer is among the cancers for which ACT is safe and feasible, but it does not result in responses comparable to melanoma [56,98]. It is becoming increasingly clear that there are multiple CPI-based targets that can help augment the therapy both at the in vivo expansion phases (see Section 3.2) and as a combination therapy. While both ovarian cancer and metastatic melanoma show high T cell infiltration of the tumor, REP fold expansion, and post-REP T cell effector memory phenotype, the infusion products derived from ovarian tumors appear to have debilitating phenotypic differences such as high expression of exhaustion markers and unfavorable CD8:CD4 ratios [98,99]. This ratio is not only post-REP, but also reflected in the tumor itself, illustrating the need for a priming therapy prior to tumor resection for TIL.
To combat the evidence of exhaustion and unfavorable CD8:CD4 ratios observed in the previous studies, our group strategically combined marketed CPI therapies with TIL therapy for the treatment of six individuals with ovarian cancer [100]. The CTLA-4 inhibitor, Ipilimumab, was given in the priming phase before tumor resection, while the PD-1 inhibitor, Nivolumab, was administered after TIL infusion during the effector phase. The goal was to exacerbate the anti-tumor response, and although it is hard to compare with earlier ovarian cancer studies, CPI combination seemingly led to more lucrative expansions, more favorable CD8:CD4 ratio, and better tumor reactivity in vitro. Clinical results showed one partial response, one long-term stable disease for 12 months. The study demonstrated that CPI combination can augment the response and help skew to the desired CD8+ phenotype.
While we have shown that such combinations could play a role to overcome the specific barriers in ovarian cancer, there is currently very little literature on the topic. However, the plethora of ongoing clinical trials as seen in Table 1, suggest that the potential of CPI combination with TIL-based ACT will soon be better understood.

Table 1.
Current TIL-based ACT clinical trials involving CPI combination.
4.2. Specific Targeting
All anti-cancer immunotherapies are dependent on the ability of the immune system to discern healthy tissue from cancer. Metastatic melanoma is the most immunogenic cancer [101], which likely facilitates the neoantigenicity that plays a key role in its susceptibility to the administered TILs [64]. Our institution with others have investigated neoantigen-specificity in TILs with the use of barcoded neopeptide:MHC multimers [102]. Our study, currently in submission, revealed that neoantigen specificity abundance in TIL infusion product correlated with survival, while lacking such specificity resulted in progressive disease. In responders, these neoantigen-reactive TILs were engrafted into the host, detectible in peripheral blood, and active in the tumor microenvironment, acting as the driving force behind tumor regression. These findings also underline the importance of tumor neoatigenicity for successful outcome. While there are presumably many other factors, this could also help explain the inefficacy of TIL therapy in moderate to low mutational cancers such as ovarian and breast cancer [76,100,101].
In addition to combination of other therapies, TIL-based ACT could be improved by ensuring that cells infused to the patient react specifically and strongly to their own tumor cells. Broad approaches involving the adoptive transfer of clonal TILs have fallen short of traditional TIL-based ACT (see Section 2.3) and some non-melanoma cancer mutations have little overlap and require in extensive, broad screening for a highly personalized process [103,104]. Such an approach would involve the identification of cancer-restricted target(s), evidence of reactive TIL to the target(s), and isolation and expansion of these TILs.
Individual accounts of this process have been reported. In 2018, the NCI reported a case of complete durable regression of metastatic breast cancer after treatment with ACT of TIL directed towards four unique, cancer-specific, somatic mutations [105]. These mutations were identified via whole-exome sequencing and RNA sequencing. TILs expanded from the tumor recognizing peptide-MHC complexes presenting epitopes of four of these unique mutations were isolated, expanded, and shown to be functional in the ex vivo killing of autologous tumor as well as mediating tumor regression in the patient. While this approach could be replicated, the methods used are far from practical for the treatment of many patients.
Methods of target identification and T cell reactivity are typically expensive and laborious. Whole genome, whole exome, and next generation sequencing are beyond the in-house capabilities of many institutions and cancer treatment facilities. Similarly, conventional tests to identify T cell reactivity to peptides requires special technical skill and usually industrial collaboration. Recently developed technologies are helping to mainstream these personalized approaches [106]. Some new methods depend on epitope libraries and wet lab analysis such as signaling and antigen-presenting bifunctional receptors (SABRs) [107] and peptide-MHC-TCR hybrid molecules (MCRs) [108], while others rely more heavily on epitope predictive informatic analyses such as the recently reported, ProTECT-prediction pipeline [109]. The use of DNA-barcoded multimers as described above could also be applied to other cancer types to identify and expand neoantigen-reactive TILs. Although these advances offer clear potential for broadening the treatment abilities of TIL-based ACT for individuals with non-melanoma cancers, further investigation is needed for clinical translation.
5. Genetic Modification of TILs
Genetic modification has revolutionized cell-based therapies in cancer. Though research in this area has grown in recent years, genetic modification is not new to TIL therapy. Retroviral transduction has been used at the NCI since the early 1990s in the earliest attempts to better equip these cells to eradicate cancer [110,111]. While this technique is still widely used and revised in TIL studies [112], different technologies are continuously adopted to TIL therapy as they are developed.
5.1. IL-2
While countless genetic alterations are hypothesized and tested in animal models, few have reached the clinic. In 2008, researchers at the NCI transduced TILs with a gene coding for recombinant IL-2 [113]. As stated above, IL-2 is essential for T cell survival. This factor is included in in vitro TIL cultures and expansions as well as administered to the patient during the course of TIL therapy, frequently leading to painful toxicities. The goal of this study was to compensate for the low IL-2 production capacity of the TILs by equipping them with means of autocrine IL-2 production. Success could result in increased in vivo longevity of the TILs and in turn improved patient outcome. Additionally, systemic IL-2 treatment during therapy could be decreased or discontinued. The in vitro results showed promise, as six out of the eight transduced patient samples produced IL-2 upon autologous tumor stimulation and persisted longer than non-transduced TILs. Unfortunately, the clinical trial was inconsistent with the in vitro findings. The patient outcome across the study was reduced compared to previous trials with non-genetically modified TILs. Only one patient infused with transduced product had sustained TILs after 1 month of treatment without exogenous IL-2 and experienced a partial response. The authors suggest that the extended culture time of the TILs due to lengthy transduction process was a contributing factor for the poor in vivo responses. As genetic manipulation tools improve, IL-2 may be a further investigated target of manipulation in the future to improve TIL therapy.
5.2. IL-12
IL-12 is an important cytokine in perpetuating anti-tumor Th1 responses [114,115]. However, like IL-2 and TNFα, systemic use results in painful clinical toxicity [116]. In 2015, NCI researchers transduced human TILs with an inducible gene coding for IL-12 [117]. To add a layer of regulation, an NFAT (nuclear factor of activated T cells) promotor preceded the IL-12 coding region, resulting in an NFAT.IL-12 protein. The goal of this study was to equip TILs with the ability to generate IL-12 upon activation in the tumor microenvironment after being reinfused into the patient.
The study was designed as a cell dose escalation, with a low dose cohort receiving 0.001 to 0.1 × 109 transduced TILs, and a high-dose cohort receiving 0.3 to 3 × 109 cells. Only one out of 17 patients in the low-dose cohort showed an objective response, while 10 out of 16 patients benefited in the high dose cohort. While higher cell dose correlated with tumor regression, these patients still experienced severe toxicities. Additionally, responses tended to be short lived, and after one month post infusion, only 8 of the 33 total patients showed in vivo sustenance of the transduced TILs. While the NFAT addition to the single-chain IL-12 gene was intended to confine the response to the tumor, high serum IL-12 and IFNγ levels indicated that the TILs were being activated outside of the tumor microenvironment, which was likely a contributing factor to the toxicities. This indicates that either the promotor has a basal level activity that results in constitutional expression of the transduced gene, or that non-tumor elements are causing the activation of the TILs and in turn, the expression of NFAT.IL-12. A way to overcome the latter could be to ensure that the infused TILs are tumor specific. The authors consider that a different promotor may result in a lower basal level expression of the gene.
5.3. CXCR2
While enhancing the in vivo longevity and cytotoxic capabilities of TILs could result in improved patient outcome, it is also important to ensure that infused cells localize to tumor sites. In melanoma models, CXCL1 and CXCL8 have been shown to be produced by cells in the TME [118,119,120], leading to the exploration of their natural receptor, CXCR2, for potential improvement of adoptive cell therapies. To overcome the lack of receptor expression in T cells, two groups, led by Patrick Hwu, have demonstrated the ability for CXCR2 retrovirally transduced T cells to chemotactically home to tumor sites both in vitro [120] and in vivo mouse model [121] with CXCR2-dependency. In these pre-clinical studies, the T cells elicit functional activity and increase the survival of animals. In 2016 our group reported a CXCR2 TIL transfection method via mRNA electroporation [122], but this method has not proceeded further. However, the adoptive cell transfer of CXCR2-retrovirally transduced TILs [123] is currently undergoing clinical trial at MDACC (NCT01740557).
5.4. Gene Knockouts
Rather than arming cells with the ability to produce immune-perpetuating and inflammatory signals, many groups have attempted to genetically eliminate immunosuppressive and exhaustive elements from them. Given the success of CPI therapy, PD-1 is seen as an attractive target. While combination studies using PD-1-targeting drugs and TIL-based ACT are being conducted (see Section 4.1), it may also be advantageous to delete PD-1 from infused TILs altogether. Anti-PD-1 monoclonal antibodies act systemically and are associated with multiple toxicities, as previously discussed. To avoid cutting the brakes from the entire immune system, deletion of PD-1 from TIL infusion products could possibly result in a more cancer-directed, exhaustion-averse immune response with less toxicity. Effective PD-1 deletion in TILs at a clinical-scale has been reported using techniques such as ZFN [124] and TALEN [125], and more recently our institute has utilized CRISPR-mediated methods to improve this efficiency even further [126]. While these studies demonstrate feasibility in clinical-scale TIL products and suggest improved performance of the product by way of increased IFNγ secretion, this combination is yet to enter clinical trials. Contrarily, two recent, unrelated clinical studies have reported successful CRISPR-mediated knockout of the PD1 gene for adoptive transfer of autologous T cells for cancer therapy [127,128], however, neither of these studies involved TILs and reported low knockout efficiency.
An internal checkpoint, cytokine-induced SH2 protein (CISH), has been found to impede anti-tumor CD8+ T cell responses, primarily through suppressing TCR avidity [129]. A recently reported study from the NCI and University of Minnesota used CRISPR to eliminate the CISH gene from TILs [130]. Favorable functional outcomes, such as increased tumor lysis and neoantigen reactivity, resulted from the inactivation of this internal checkpoint. Phenotypic changes include increased TCR avidity and PD-1 expression. While the latter effect is counterintuitive to treatment, anti-PD-1 combination promoted tumor regression and survival in a murine model. A phase I/II clinical trial exploring the use of CISH knockout TIL-based ACT (NCT04426669) is currently underway. As chaperone technologies are developed to detect off target hits and knockout efficacy [131,132,133], the CRISPR/Cas9 method continues to become increasingly accessible and may soon play an integral role in TIL-based ACT. Figure 2 illustrates these interventions in the standard workflow as well as those previously discussed for the improvement of this therapy.
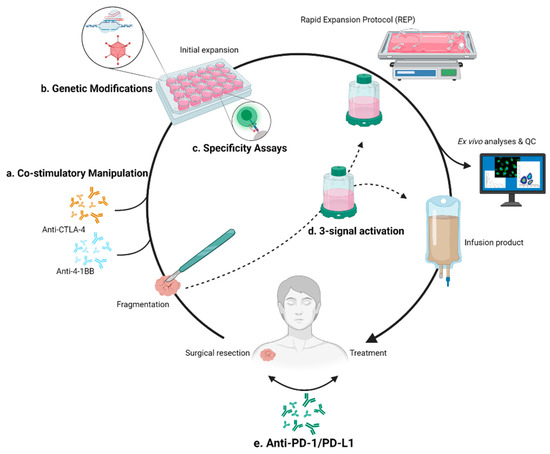
Figure 2.
Modifications of TIL-based ACT workflow. (a) Addition of monoclonal antibodies targeting CTLA-4 and 4-1BB to improve the initial expansion phase of the workflow. (b) Genetic modification of TIL via CRISPR/Cas9 or viral transduction to strategically knockout or knock-in desired elements. (c) Identification of tumor-specific TILs to improve cancer-directed response. (d) A 3-signal activation method described by MDACC. Replaces initial expansion phase, and possibly could be used to bypass REP. (e) Priming and/or combination checkpoint inhibition therapies.
6. Conclusions
The recent revived attention to adoptive TIL therapy suggests that the future of the therapy will likely be similar to its past. The innovative application of the advances and discoveries in other fields continues to shape and improve this evolving therapy. Figure 2 illustrates the interventions discussed in this work that are currently being investigated. While methods capitalizing on CPI, CRISPR/Cas9 and other biotechnologies are in the process of clinical translation, few patients have felt the benefits of these laboratory accomplishments. Although further pre-clinical and clinical studies are needed for many modifications to the therapy, the future of TIL-based ACT will likely be highly personalized, interdisciplinary, and an effective treatment option in a wide variety of cancers.
Author Contributions
Writing—original draft preparation and visualization, T.M.H.; writing—review and editing, C.A.C.; project administration, T.M.H. and Ö.M., review and editing, I.M.S. and Ö.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Images created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexander, W. The Checkpoint Immunotherapy Revolution: What Started as a Trickle Has Become a Flood, despite Some Daunting Adverse Effects, New Drugs, Indications, and Combinations Continue to Emerge. Pharm. Ther. 2016, 41, 185–191. [Google Scholar]
- Haslam, A.; Prasad, V. Estimation of the Percentage of Us Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, upon Programmed Cell Death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. Available online: http://www.Jstor.Org/Stable/2890840 (accessed on 16 March 2021). [CrossRef] [PubMed] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Met, Ö.; Jensen, K.M.; Chamberlain, C.A.; Donia, M.; Svane, I.M. Principles of Adoptive T Cell Therapy in Cancer. Semin. Immunopathol. 2019, 41, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.C.; Maus, M.V. Recent Advances and Discoveries in the Mechanisms and Functions of CAR T Cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Zhou, W.L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.D.; et al. Genetically Engineered T Cells for Cancer Immunotherapy. Signal Transduct. Target. Ther. 2019, 4, 35. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Melanoma: A Systematic Review and Meta-Analysis. Oncoimmunology 2019, 8, e1593806. [Google Scholar] [CrossRef] [Green Version]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- MacCarty, W.C. Factors Which Influence Longevity in Cancer. A Study of 293 Cases. Ann. Surg. 1922, 76, 9–12. [Google Scholar] [PubMed]
- Elston, C.W.; Bagshawe, K.D. Cellular Reaction in Trophoblastic Tumours. Br. J. Cancer 1973, 28, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paola, M.; Angelini, L.; Bertolotti, A.; Colizza, S. Host Resistance in Relation to Survival in Breast Cancer. Br. Med. J. 1974, 4, 268–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mule, J.J.; Shu, S.; Schwarz, S.L.; Rosenberg, S.A. Adoptive Immunotherapy of Established Pulmonary Metastases with LAK Cells and Recombinant Interleukin-2. Science 1984, 225, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Mochizuki, D.; Gillis, S. T-Cell Growth Factors: Interleukin 2. Immunol. Today 1980, 1, 113–117. [Google Scholar] [CrossRef]
- Ruscetti, F.W.; Gallo, R.C. Human T-Lymphocyte Growth Factor: Regulation of Growth and Function of T-Lymphocytes. Blood 1981, 57, 379–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, B.E.A.; Viazumder, A.; Zhang, H.U.A.Z.; Rosenberg, S.A. Lymphokine-Activated Killer Cell Phenomenon, Lysis of Natural Killer-Resistant Fresh Solid Tumor Cells by Interleukin 2-Activated Autologous Human Peripheral Blood Lymphocytes. J. Exp. Med. 1982, 155, 1823–1841. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Grimm, E.A.; Mcgrogan, M.; Doyle, M.; Kawasaki, E.; Koths, K.; Mark, D.F. Biological Activity of Recombinant Human Interleukin-2 Produced in Escherichia Coli. Science 1984, 223, 1412–1415. [Google Scholar] [CrossRef]
- Lotze, M.T.; Grimm, E.A.; Mazumder, A.; Strausser, J.L.; Rosenberg, S.A. Lysis of Fresh and Cultured Autologous Tumor by Human Lymphocytes Cultured in T-Cell Growth Factor. Cancer Res. 1981, 41, 4420–4425. [Google Scholar]
- Lafreniere, R.; Rosenstein, M.S.; Rosenberg, S.A. Optimal Methods for Generating Expanded Lymphokine Activated Killer Cells Capable of Reducing Established Murine Tumors in vivo. J. Immunol. Methods 1986, 94, 37–49. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the Systemic Administration of Autologous Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 to Patients with Metastatic Cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Muul, L.M.; Solomon, D.; Rosenberg, S.A. Expansion of Human Tumor Infiltrating Lymphocytes for Use in Immunotherapy Trials. J. Immunol. Methods 1987, 102, 127–141. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Spiess, P.J.; Schwarz, S. In Vitro Growth of Murine T Cells. V. The Isolation and Growth of Lymphoid Cells Infiltrating Syngeneic Solid Tumors. J. Immunol. 1979, 51, 1852–1854. [Google Scholar]
- Muul, L.M.; Spiess, P.J.; Director, E.P.; Rosenberg, S.A. Identification of Specific Cytolytic Immune Responses against Autologous Tumor in Humans Bearing Malignant Melanoma. J. Immunol. 1987, 138, 989–995. [Google Scholar]
- Kradin, R.L.; Boyle, L.A.; Preffer, F.I.; Callahan, R.J.; Barlai-Kovach, M.; Strauss, H.W.; Dubinett, S.; Kurnick, J.T. Tumor-Derived Interleukin-2-Dependent Lymphocytes in Adoptive Immunotherapy of Lung Cancer. Cancer Immunol. Immunother. 1987, 24, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Solomon, D.; Avis, F.P.; Chang, A.E.; Freerksen, D.L.; Linehan, W.M.; Lotze, M.T.; Robertson, C.N.; Seipp, C.A.; Simon, P.; et al. Immunotherapy of Patients with Advanced Cancer Using Tumor-Infiltrating Lymphocytes and Recombinant Interleukin-2: A Pilot Study. J. Clin. Oncol. 1988, 6, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of Tumor Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. NEJM 1988, 299, 230–234. [Google Scholar] [CrossRef]
- Cheever, M.A.; Thompson, J.A.; Kern, D.E.; Greenberg, P.D. Interleukin-2 Administered In Vivo Induces the Growth and Augments the Function of Cultured T Cells in vivo. J. Biol. Response Mod. 1984, 3, 462–467. [Google Scholar]
- Marabondo, S.; Kaufman, H.L. High-Dose Interleukin-2 (IL-2) for the Treatment of Melanoma: Safety Considerations and Future Directions. Expert Opin. Drug Saf. 2017, 16, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.R.; Greenberg, P.D. High Efficient Transduction of T Lymphocytes Using Rapid Expansion Methods (“REM”). U.S. Patent 6,040,177, 21 March 2000. [Google Scholar]
- Flyer, D.C.; Clary, K.W. Modified Rapid Expansion Methods (“Modified-REM”) for In Vitro Propagation of T Lymphocytes. U.S. Patent 6,316,257 B1, 13 November 2001. [Google Scholar]
- Clevers, H.; Alarcon, B.; Wileman, T.; Terhorst, C. The T Cell Receptor/CD3 Complex: A Dynamic Protein Ensemble. Annu. Rev. Immunol. 1988, 6, 629–662. [Google Scholar] [CrossRef] [PubMed]
- Londei, M.; Grubeck-Loebenstein, B.; De Berardinis, P.; Greenall, C.; Feldmann, M. Efficient Propagation and Cloning of Human T Cells in the Absence of Antigen by Means of OKT3, Interleukin 2, and Antigen-Presenting Cells. Scand. J. Immunol. 1988, 27, 35–46. [Google Scholar] [CrossRef]
- Riddell, S.R.; Greenberg, P.D. The Use of Anti-CD3 and Anti-CD28 Monoclonal Antibodies to Clone and Expand Human Antigen-Specific T Cells. J. Immunol. Methods 1990, 128, 189–201. [Google Scholar] [CrossRef]
- Minguet, S.; Swamy, M.; Alarcón, B.; Luescher, I.F.; Schamel, W.W.A. Full Activation of the T Cell Receptor Requires Both Clustering and Conformational Changes at CD3. Immunity 2007, 26, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Crossland, K.D.; Lee, V.K.; Chen, W.; Riddell, S.R.; Greenberg, P.D.; Cheever, M.A. T Cells from Tumor-Immune Mice Nonspecifically Expanded in Vitro with Anti-CD3 plus IL-2 Retain Specific Function in Vitro and Can Eradicate Disseminated Leukemia in Vivo. J. Immunol. 1991, 146, 4414–4420. [Google Scholar] [PubMed]
- Gerber, M.; Guichard, M.; Pioch, Y.; Dubois, J.B. The Influence of Interleukin-2, Feeder Cells, and Timing of Irradiation on the Radiosensitivity of Human T Lymphocytes Assessed by the Colony-Forming Assay. Radiat. Res. 1989, 120, 164–176. [Google Scholar] [CrossRef]
- Cerottini, J.; Maria, A.N.I.; Mingari, C. Direct Demonstration of the Clonogenic Potential of Every Human Peripheral Blood T Cell. J. Exp. Med. 1983, 157, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Riddell, S.R.; Watanabe, K.S.; Goodrich, J.M.; Li, C.R.; Agha, M.E.; Greenberg, P.D. Restoration of Viral Immunity in Immunodeficient Humans by the Adoptive Transfer of T Cell Clones. Science 1992, 257, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.; García-Pérez, E.; Meana, Á.; Larcher, F.; Del Río, M. Feeder Layer Cell Actions and Applications. Tissue Eng. Part B Rev. 2015, 21, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Aebersold, P.; Hyatt, C.; Johnson, S.; Hines, K.; Korcak, L.; Sanders, M.; Lotz, M.; Topalian, S.; Yang, J.; Rosenberg, S.A. Lysis of Autologous Melanoma Cells by Tumor Infiltrating Lymphocytes: Association with Clinical Response. J. Natl. Cancer Inst. 1991, 83, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, D.J.; Hom, S.S.; Dadmarz, R.; White, D.E.; Yannelli, J.R.; Steinberg, S.M.; Rosenberg, S.A.; Topalian, S.L. In Vitro Predictors of Therapeutic Response in Melanoma Patients Receiving Tumor-Infiltrating Lymphocytes and Interleukin-2. J. Clin. Oncol. 1994. [Google Scholar] [CrossRef]
- Tran, K.Q.; Zhou, J.; Durflinger, K.H.; Langhan, M.M.; Shelton, T.E.; Wunderlich, J.R.; Robbins, P.F.; Rosenberg, S.A.; Dudley, M.E. Minimally Cultured Tumor-Infiltrating Lymphocytes Display Optimal Characteristics for Adoptive Cell Therapy. J. Immunother. 2008, 31, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Besser, M.J.; Shapira-Frommer, R.; Treves, A.J.; Zippel, D.; Itzhaki, O.; Schallmach, E.; Kubi, A.; Shalmon, B.; Hardan, I.; Catane, R.; et al. Minimally Cultured or Selected Autologous Tumor-Infiltrating Lymphocytes after a Lympho-Depleting Chemotherapy Regimen in Metastatic Melanoma Patients. J. Immunother. 2009, 32, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, O.; Hovav, E.; Ziporen, Y.; Levy, D.; Kubi, A.; Zikich, D.; Hershkovitz, L.; Treves, A.J.; Shalmon, B.; Zippel, D.; et al. Establishment and Large-Scale Expansion of Minimally Cultured Young Tumor Infiltrating Lymphocytes for Adoptive Transfer Therapy. J. Immunother. 2011, 34, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shen, X.; Huang, J.; Hodes, R.J.; Rosenberg, S.A.; Robbins, P.F. Telomere Length of Transferred Lymphocytes Correlates with In Vivo Persistence and Tumor Regression in Melanoma Patients Receiving Cell Transfer Therapy. J. Immunol. 2005, 175, 7046–7052. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, E.H. Structure and Function of Telomeres. Nature 1991, 354, 737–740. [Google Scholar] [CrossRef]
- Hodes, R.J.; Hathcock, K.S.; Weng, N.P. Telomeres in T and B Cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef]
- Dudley, M.E.; Gross, C.A.; Somerville, R.P.T.; Hong, Y.; Schaub, N.P.; Rosati, S.F.; White, D.E.; Nathan, D.; Restifo, N.P.; Steinberg, S.M.; et al. Randomized Selection Design Trial Evaluating CD8+-Enriched versus Unselected Tumor-Infiltrating Lymphocytes for Adoptive Cell Therapy for Patients with Melanoma. J. Clin. Oncol. 2013, 31, 2152–2159. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.E.; Gross, C.A.; Langhan, M.M.; Garcia, M.R.; Sherry, R.M.; Yang, J.C.; Phan, G.Q.; Kammula, U.S.; Hughes, M.S.; Citrin, D.E.; et al. CD8+ Enriched “Young” Tumor Infiltrating Lymphocytes Can Mediate Regression of Metastatic Melanoma. Clin. Cancer Res. 2010, 16, 6122–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donia, M.; Junker, N.; Ellebaek, E.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Characterization and Comparison of “standard” and “Young” Tumour-Infiltrating Lymphocytes for Adoptive Cell Therapy at a Danish Translational Research Institution. Scand. J. Immunol. 2012, 75, 157–167. [Google Scholar] [CrossRef]
- Besser, M.J.; Shapira-Frommer, R.; Treves, A.J.; Zippel, D.; Itzhaki, O.; Hershkovitz, L.; Levy, D.; Kubi, A.; Hovav, E.; Chermoshniuk, N.; et al. Clinical Responses in a Phase II Study Using Adoptive Transfer of Short-Term Cultured Tumor Infiltration Lymphocytes in Metastatic Melanoma Patients. Clin. Cancer Res. 2010, 16, 2646–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radvanyi, L.G.; Bernatchez, C.; Zhang, M.; Fox, P.S.; Miller, P.; Chacon, J.; Wu, R.; Lizee, G.; Mahoney, S.; Alvarado, G.; et al. Specific Lymphocyte Subsets Predict Response to Adoptive Cell Therapy Using Expanded Autologous Tumor-Infiltrating Lymphocytes in Metastatic Melanoma Patients. Clin. Cancer Res. 2012, 18, 6758–6770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, M.; Westergaard, M.C.W.; Milne, K.; Nielsen, M.; Borch, T.H.; Poulsen, L.G.; Hendel, H.W.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes in Patients with Metastatic Ovarian Cancer: A Pilot Study. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.M.; Marincola, F.M.; Leitman, S.F.; Seipp, C.A.; et al. A Phase I Study of Nonmyeloablative Chemotherapy and Adoptive Transfer of Autologous Tumor Antigen-Specific T Lymphocytes in Patients with Metastatic Melanoma. J. Immunother. 2002, 25, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, M.E.; Wunderlich, J.; Nishimura, M.I.; Yu, D.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Sherry, R.; et al. Adoptive Transfer of Cloned Melanoma-Reactive T Lymphocytes for the Treatment of Patients with Metastatic Melanoma. J. Immunother. 2001, 24, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.; Thompson, J.A.; Byrd, D.; Riddell, S.R.; Roche, P.; Celis, E.; Greenberg, P.D. Adoptive T Cell Therapy Using Antigen-Specific CD8+ T Cell Clones for the Treatment of Patients with Metastatic Melanoma: In Vivo Persistence, Migration, and Antitumor Effect of Transferred T Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16168–16173. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.E.; Wunderlich, J.R.; Shelton, T.E.; Even, J.; Rosenberg, S.A. Generation of Tumor-Infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. J. Immunother. 2003, 26, 332–342. [Google Scholar] [CrossRef]
- Antony, P.A.; Piccirillo, C.A.; Akpinarli, A.; Finkelstein, S.E.; Speiss, P.J.; Surman, D.R.; Palmer, D.C.; Chan, C.-C.; Klebanoff, C.A.; Overwijk, W.W.; et al. CD8+ T Cell Immunity Against a Tumor/Self-Antigen Is Augmented by CD4+ T Helper Cells and Hindered by Naturally Occurring T Regulatory Cells. J. Immunol. 2005, 174, 2591–2601. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.; Lowery, F.J.; Copeland, A.R.; Bahadiroglu, E.; Mukherjee, R.; Jia, L.; Anibal, J.T.; Sachs, A.; Adebola, S.O.; Gurusamy, D.; et al. Stem-like CD8 T Cells Mediate Response of Adoptive Cell Immunotherapy Against Human Cancer. Science 2020, 370, 1328–1334. [Google Scholar] [CrossRef]
- Dudley, M.E.; Yang, J.C.; Sherry, R.; Hughes, M.S.; Royal, R.; Kammula, U.; Robbins, P.F.; Huang, J.P.; Citrin, D.E.; Leitman, S.F.; et al. Adoptive Cell Therapy for Patients with Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens. J. Clin. Oncol. 2008, 26, 5233–5239. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive Cell Transfer: A Clinical Path to Effective Cancer Immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive Cell Transfer Therapy Following Non-Myeloablative but Lymphodepleting Chemotherapy for the Treatment of Patients with Refractory Metastatic Melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer Regression and Autoimmunity in Patients after Clonal Repopulation with Antitumor Lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, S.L.; Dudley, M.E.; Citrin, D.E.; Somerville, R.P.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients with Metastatic Melanoma. J. Clin. Oncol. 2016, 34, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Larsen, S.M.; Met, Ö.; Svane, I.M. Simplified Protocol for Clinical-Grade Tumor-Infiltrating Lymphocyte Manufacturing with Use of the Wave Bioreactor. Cytotherapy 2014, 16, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.F.; Brenner, L.J.; Gerdemann, U.; Ngo, M.C.; Sili, U.; Liu, H.; Wilson, J.; Dotti, G.; Heslop, H.E.; Leen, A.M.; et al. Accelerated Production of Antigen-Specific T Cells for Preclinical and Clinical Applications Using Gas-Permeable Rapid Expansion Cultureware (G-Rex). J. Immunother. 2010, 33, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Hollyman, D.; Stefanski, J.; Przybylowski, M.; Bartido, S.; Borquez-Ojeda, O.; Taylor, C.; Yeh, R.; Capacio, V.; Olszewska, M.; Hosey, J.; et al. Manufacturing Validation of Biologically Functional T Cells Targeted to CD19 Antigen for Autologous Adoptive Cell Therapy. J. Immunother. 2009, 32, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Forget, M.A.; Malu, S.; Liu, H.; Toth, C.; Maiti, S.; Kale, C.; Haymaker, C.; Bernatchez, C.; Huls, H.; Wang, E.; et al. Activation and Propagation of Tumor-Infiltrating Lymphocytes on Clinical-Grade Designer Artificial Antigen-Presenting Cells for Adoptive Immunotherapy of Melanoma. J. Immunother. 2014, 37, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Ullenhag, G.J.; Sadeghi, A.M.; Carlsson, B.; Ahlström, H.; Mosavi, F.; Wagenius, G.; Tötterman, T.H. Adoptive T-Cell Therapy for Malignant Melanoma Patients with TILs Obtained by Ultrasound-Guided Needle Biopsy. Cancer Immunol. Immunother. 2012, 61, 725–732. [Google Scholar] [CrossRef]
- Arch, R.H.; Thompson, C.B. 4-1BB and Ox40 Are Members of a Tumor Necrosis Factor (TNF)-Nerve Growth Factor Receptor Subfamily That Bind TNF Receptor-Associated Factors and Activate Nuclear Factor ΚB. Mol. Cell. Biol. 1998, 18, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Song, D.G.; Poussin, M.; Yamamoto, T.; Best, A.; Li, C.; Coukos, G.; Powell, D.J. CD137 Accurately Identifies and Enriches for Naturally Occurring Tumor-Reactive T Cells in Tumor. Clin. Cancer Res. 2014, 20, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacon, J.A.; Sarnaik, A.A.; Chen, J.Q.; Creasy, C.; Kale, C.; Robinson, J.; Weber, J.; Hwu, P.; Pilon-Thomas, S.; Radvanyi, L. Manipulating the Tumor Microenvironment Ex Vivo for Enhanced Expansion of Tumor-Infiltrating Lymphocytes for Adoptive Cell Therapy. Clin. Cancer Res. 2015, 21, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harao, M.; Forget, M.A.; Roszik, J.; Gao, H.; Babiera, G.V.; Krishnamurthy, S.; Chacon, J.A.; Li, S.; Mittendorf, E.A.; Desnyder, S.M.; et al. 4-1BB-Enhanced Expansion of CD8 TIL from Triple-Negative Breast Cancer Unveils Mutation-Specific CD8 T Cells. Cancer Immunol. Res. 2017, 5, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavera, R.J.; Forget, M.A.; Kim, Y.U.; Sakellariou-Thompson, D.; Creasy, C.A.; Bhatta, A.; Fulbright, O.J.; Ramachandran, R.; Thorsen, S.T.; Flores, E.; et al. Utilizing T-Cell Activation Signals 1, 2, and 3 for Tumor-Infiltrating Lymphocytes (TIL) Expansion: The Advantage over the Sole Use of Interleukin-2 in Cutaneous and Uveal Melanoma. J. Immunother. 2018, 41, 399–405. [Google Scholar] [CrossRef]
- Nielsen, M.; Krarup-Hansen, A.; Hovgaard, D.; Petersen, M.M.; Loya, A.C.; Westergaard, M.C.W.; Svane, I.M.; Junker, N. In Vitro 4-1BB Stimulation Promotes Expansion of CD8+ Tumor-Infiltrating Lymphocytes from Various Sarcoma Subtypes. Cancer Immunol. Immunother. 2020, 69, 2179–2191. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, Y.H.; Choi, B.K.; Kwon, P.M.; Lee, H.-W.; Kwon, B.S. 4-1BB Triggers IL-13 Production from T Cells to Limit the Polarized, Th1-Mediated Inflammation. J. Leukoc. Biol. 2007, 81, 1455–1465. [Google Scholar] [CrossRef]
- Elpek, K.G.; Yolcu, E.S.; Franke, D.D.H.; Lacelle, C.; Schabowsky, R.-H.; Shirwan, H. Ex Vivo Expansion of CD4 + CD25 + FoxP3 + T Regulatory Cells Based on Synergy between IL-2 and 4-1BB Signaling. J. Immunol. 2007, 179, 7295–7304. [Google Scholar] [CrossRef] [Green Version]
- Melero, I.; Hervas-Stubbs, S.; Glennie, M.; Pardoll, D.M.; Chen, L. Immunostimulatory Monoclonal Antibodies for Cancer Therapy. Nat. Rev. Cancer 2007, 7, 95–106. [Google Scholar] [CrossRef]
- Teft, W.A.; Kirchhof, M.G.; Madrenas, J. A Molecular Perspective of CTLA-4 Function. Annu. Rev. Immunol. 2006, 24, 65–97. [Google Scholar] [CrossRef] [Green Version]
- Friese, C.; Harbst, K.; Borch, T.H.; Westergaard, M.C.W.; Pedersen, M.; Kverneland, A.; Jönsson, G.; Donia, M.; Svane, I.M.; Met, Ö. CTLA-4 Blockade Boosts the Expansion of Tumor-Reactive CD8+ Tumor-Infiltrating Lymphocytes in Ovarian Cancer. Sci. Rep. 2020, 10, 3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, R.; Donia, M.; Ellebaek, E.; Borch, T.H.; Kongsted, P.; Iversen, T.Z.; Hölmich, L.R.; Hendel, H.W.; Met, Ö.; Andersen, M.H.; et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated Il2 Regimen. Clin. Cancer Res. 2016, 22, 3734–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.C. Toxicities Associated with Adoptive T-Cell Transfer for Cancer. Cancer J. 2015, 21, 506–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besser, M.J.; Shapira-Frommer, R.; Itzhaki, O.; Treves, A.J.; Zippel, D.B.; Levy, D.; Kubi, A.; Shoshani, N.; Zikich, D.; Ohayon, Y.; et al. Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma: Intent-to-Treat Analysis and Efficacy after Failure to Prior Immunotherapies. Clin. Cancer Res. 2013, 19, 4792–4800. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.; Borch, T.H.; Draghi, A.; Gokuldass, A.; Rana, A.H.M.; Pedersen, M.; Nielsen, M.; Kongsted, P.; Kjeldsen, J.W.; Westergaard, C.W.M.; et al. T Cells Isolated from Patients with Checkpoint Inhibitor-Resistant Melanoma Are Functional and Can Mediate Tumor Regression. Ann. Oncol. 2018, 29, 1575–1581. [Google Scholar] [CrossRef]
- Borch, T.H.; Andersen, R.; Ellebaek, E.; Met, Ö.; Donia, M.; Marie Svane, I. Future Role for Adoptive T-Cell Therapy in Checkpoint Inhibitor-Resistant Metastatic Melanoma. J. Immunother. Cancer 2020, 8, e000668. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Hong, J.J.; Rosenberg, S.A.; Dudley, M.E.; Yang, J.C.; White, D.E.; Butman, J.A.; Sherry, R.M. Successful Treatment of Melanoma Brain Metastases with Adoptive Cell Therapy. Clin. Cancer Res. 2010, 16, 4892–4898. [Google Scholar] [CrossRef] [Green Version]
- Ben-Avi, R.; Farhi, R.; Ben-Nun, A.; Gorodner, M.; Greenberg, E.; Markel, G.; Schachter, J.; Itzhaki, O.; Besser, M.J. Establishment of Adoptive Cell Therapy with Tumor Infiltrating Lymphocytes for Non-small Cell Lung Cancer Patients. Cancer Immunol. Immunother. 2018, 67, 1221–1230. [Google Scholar] [CrossRef]
- Ma, Y.; Ou, J.; Lin, T.; Chen, L.; Wang, J.; Qiao, D.; Lai, S.; Duan, C.; Chen, Y.; Chang, R.; et al. Phenotypic Analysis of Tumor-infiltrating Lymphocytes from non-small Cell Lung Cancer and Their Potential Application for Adoptive Cell Therapy. Immunopharmacol. Immunotoxicol. 2020, 42, 319–329. [Google Scholar] [CrossRef]
- Jiang, S.S.; Tang, Y.; Zhang, Y.; Weng, D.; Zhou, Z.; Pan, K.; Pan, Q.; Wang, Q.; Liu, Q.; He, J.; et al. A Phase I Clinical Trial Utilizing Autologous Tumor-infiltrating Lymphocytes in Patients with Primary Hepatocellular Carcinoma. Oncotarget 2015, 6, 41339–41349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.; Liu, H.; Malafa, M.; Centeno, B.; Hodul, P.; Pimiento, J.; Pilon-Thomas, S.; Sarnaik, A.A. Expansion of Tumor-infiltrating Lymphocytes (TIL) from Human Pancreatic Tumors. J. Immunother. Cancer. 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Meng, Q.; Bartek, J., Jr.; Poiret, T.; Persson, O.; Rane, L.; Rangelova, E.; Illies, C.; Peredo, I.H.; Luo, X.; et al. Tumor-infiltrating Lymphocytes (TILs) from Patients with Glioma. Oncoimmunology 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodmer, S.; Strommer, K.; Frei, K.; Siepl, C.; Tribolet, N.; Heid, I.; Fontana, A. Immunosupression and Transforming Growth Factor-beta in Glioblastoma. Preferential Production of Transforming Growth Factor-beta 2. J. Immunol. 1989, 143, 3222–3229. [Google Scholar] [PubMed]
- Quattrocchi, K.B.; Miller, C.H.; Cush, S.; Bernard, S.A.; Dull, S.T.; Smith, M.; Gudeman, S.; Varia, M.A. Pilot Study of Local Autologous Tumor Infiltrating Lymphocytes for the Treatment of Recurrent Malignant Gliomas. J. Neurooncol. 1999, 45, 141–157. [Google Scholar] [CrossRef]
- Westergaard, M.C.W.; Andersen, R.; Chong, C.; Kjeldsen, J.W.; Pedersen, M.; Friese, C.; Hasselager, T.; Lajer, H.; Coukos, G.; Bassani-Sternberg, M.; et al. Tumour-Reactive T Cell Subsets in the Microenvironment of Ovarian Cancer. Br. J. Cancer 2019, 120, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Kverneland, A.H.; Pedersen, M.; Wulff Westergaard, M.C.; Nielsen, M.; Borch, T.H.; Olsen, L.R.; Aasbjerg, G.; Santegoets, S.J.; van der Burg, S.H.; Milne, K.; et al. Adoptive Cell Therapy in Combination with Checkpoint Inhibitors in Ovarian Cancer. Oncotarget 2020, 11, 2092–2105. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Heeke, C.; Kristensen, N.P.; Tvingsholm, S.A.; Borch, A.; Draghi, A.; Crowther, M.D.; Carri, I.; Munk, K.K.; Holm, J.S.; Bjerregaard, A.; et al. Engraftment of Neoantigen-Reactive CD8+ T Cells Affects the Clinical Outcome in Patients Receiving Adoptive Transfer of Tumor-Infiltrating Lymphocytes. J. Clin. Invest. 2021. In submission. [Google Scholar]
- Tran, E.; Ahmadzadeh, M.; Lu, Y.; Gros, A.; Turcotte, S.; Paul, F.; Gartner, J.J.; Zheng, Z.; Li, Y.F.; Ray, S.; et al. Immunogenicity of Somatic Mutations in Human Gastrointestinal Cancers. Science 2015, 350, 1387–1390. [Google Scholar] [CrossRef]
- Hansen, U.K.; Ramskov, S.; Bjerregaard, A.M.; Borch, A.; Andersen, R.; Draghi, A.; Donia, M.; Bentzen, A.K.; Marquard, A.M.; Szallasi, Z.; et al. Tumor-Infiltrating T Cells From Clear Cell Renal Cell Carcinoma Patients Recognize Neoepitopes Derived From Point and Frameshift Mutations. Front. Immunol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune Recognition of Somatic Mutations Leading to Complete Durable Regression in Metastatic Breast Cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Kast, F.; Klein, C.; Umaña, P.; Gros, A.; Gasser, S. Advances in Identification and Selection of Personalized Neoantigen/T-Cell Pairs for Autologous Adoptive T Cell Therapies. Oncoimmunology 2021, 10, 1869389. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, A.V.; Leonard, M.T.; Jeppson, J.D.; Swift, M.; Li, G.; Wong, S.; Peng, S.; Zaretsky, J.M.; Heath, J.R.; Ribas, A.; et al. T Cell Antigen Discovery via Signaling and Antigen-Presenting Bifunctional Receptors. Nat. Methods 2019, 16, 191–198. [Google Scholar] [CrossRef]
- Kisielow, J.; Obermair, F.J.; Kopf, M. Deciphering CD4+ T Cell Specificity Using Novel MHC–TCR Chimeric Receptors. Nat. Immunol. 2019, 20, 652–662. [Google Scholar] [CrossRef]
- Rao, A.A.; Madejska, A.A.; Pfeil, J.; Paten, B.; Salama, S.R.; Haussler, D. ProTECT—Prediction of T-Cell Epitopes for Cancer Therapy. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Hwu, P.; Yannelli, J.; Kriegler, M.; Anderson, W.F.; Perez, C.; Chiang, Y.; Schwarz, S.; Cowherd, R.; Delgado, C.; Mule, J.; et al. Functional and Molecular Characterization of Tumor-Infiltrating Lymphocytes Transduced with Tumor Necrosis Factor-α CDNA for the Gene Therapy of Cancer in Humans. J. Immunol. 1993, 150, 4104–4115. [Google Scholar] [PubMed]
- Hwu, P.; Rosenberg, S.A. The Use of Gene-Modified Tumor-Infiltrating Lymphocytes for Cancer Therapy. Ann. N. Y. Acad. Sci. 1994, 716, 188–203. [Google Scholar] [CrossRef]
- Weinstein-Marom, H.; Gross, G.; Levi, M.; Brayer, H.; Schachter, J.; Itzhaki, O.; Besser, M.J. Genetic Modification of Tumor-Infiltrating Lymphocytes via Retroviral Transduction. Front. Immunol. 2020, 11, 584148. [Google Scholar] [CrossRef]
- Heemskerk, B.; Liu, K.E.; Dudley, M.E.; Johnson, L.A.; Downey, S.; Zheng, Z.; Shelton, T.E.; Robbins, P.F.; Morgan, R.A.; Rosenberg, S.A. Adoptive Cell Therapy for Patients with Melanoma, Using Tumor- Infiltrating Lymphocytes Genetically Engineered to Secrete Interleukin-2. Hum. Gene Ther. 2008, 19, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 Family Cytokines: Immunological Playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunda, B.M.J.; Luistro, L.; Warrier, R.R.; Wright, R.B.; Hubbard, B.R.; Murphy, M.; Wolf, S.F.; Gately, M.K. Antitumor and Antimetastatic Activity of Interleukin 12 against Murine Tumors. J. Exp. Med. 1993, 178, 1223–1230. [Google Scholar] [CrossRef]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12 Associated Toxicity and Interferon-γ Production. Blood 1997, 90, 2541–2548. [Google Scholar] [CrossRef]
- Zhang, L.; Morgan, R.A.; Beane, J.D.; Zheng, Z.; Dudley, M.E.; Kassim, S.H.; Nahvi, A.V.; Ngo, L.T.; Sherry, R.M.; Phan, G.Q.; et al. Tumor-Infiltrating Lymphocytes Genetically Engineered with an Inducible Gene Encoding Interleukin-12 for the Immunotherapy of Metastatic Melanoma. Clin. Cancer Res. 2015, 21, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Haghnegahdar, H.; Du, J.; Wang, D.Z.; Strieter, R.M.; Burdick, M.D.; Nanney, L.B.; Cardwell, N.; Luan, J.; Shattuck-Brandt, R.; Richmond, A. The Tumorigenic and Angiogenic Effects of MGSA/GRO Proteins in Melanoma. J. Leukoc. Biol. 2000, 67, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kruizinga, R.C.; Bestebroer, J.; Berghuis, P.; de Haas, C.J.C.; Links, T.P.; de Vries, E.G.E.; Walenkamp, A.M.E. Role of Chemokines and Their Receptors in Cancer. Curr. Pharm. Des. 2009, 15, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Wang, G.; Westwood, J.A.; Pachynski, R.K.; Tiffany, H.L.; Marincola, F.M.; Wang, E.; Young, H.A.; Murphy, P.M.; Hwu, P. Redirecting Migration of T Cells to Chemokine Secreted from Tumors by Genetic Modification with CXCR2. Hum. Gene Ther. 2002, 13, 1971–1980. [Google Scholar] [CrossRef]
- Peng, W.; Ye, Y.; Rabinovich, B.A.; Liu, C.; Lou, Y.; Zhang, M.; Whittington, M.; Yang, Y.; Overwijk, W.W.; Lizée, G.; et al. Transduction of Tumor-Specific T Cells with CXCR2 Chemokine Receptor Improves Migration to Tumor and Antitumor Immune Responses. Clin. Cancer Res. 2010, 16, 5458–5468. [Google Scholar] [CrossRef] [Green Version]
- Idorn, M.; Straten, P.T.; Svane, I.M.; Met, Ö. Transfection of Tumor-Infiltrating T Cells with MRNA Encoding CXCR2. Methods Mol. Biol. 2016, 1428, 261–276. [Google Scholar] [CrossRef]
- Forget, M.A.; Tavera, R.J.; Haymaker, C.; Ramachandran, R.; Malu, S.; Zhang, M.; Wardell, S.; Fulbright, O.J.; Toth, C.L.; Gonzalez, A.M.; et al. A Novel Method to Generate and Expand Clinical-Grade, Genetically Modified, Tumor-Infiltrating Lymphocytes. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Beane, J.D.; Lee, G.; Zheng, Z.; Mendel, M.; Abate-Daga, D.; Bharathan, M.; Black, M.; Gandhi, N.; Yu, Z.; Chandran, S.; et al. Clinical Scale Zinc Finger Nuclease-Mediated Gene Editing of PD-1 in Tumor Infiltrating Lymphocytes for the Treatment of Metastatic Melanoma. Mol. Ther. 2015, 23, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Ritthipichai, K.; Machin, M.; Juillerat, A.; Poirot, L.; Fardis, M.; Chartier, C. Genetic Modification of Iovance’s TIL through TALEN-Mediated Knockout of PD-1 as a Strategy to Empower TIL Therapy for Cancer. Ann. Oncol. 2020, 31, S720. [Google Scholar] [CrossRef]
- Chamberlain, C.A.; Bennett, E.P.; Kverneland, A.; Jensen, K.M.; Svane, I.M.; Donia, M.; Met, Ö. Highly Efficient CRISPR/Cas9-mediated Knockout of PD-1 for Tumor-infiltrating Lymphocyte-based Adoptive T cell Therapy. 2021; Manuscript in preparation. [Google Scholar]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-Engineered T Cells in Patients with Refractory Cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and Feasibility of CRISPR-Edited T Cells in Patients with Refractory Non-Small-Cell Lung Cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Palmer, D.C.; Guittard, G.C.; Franco, Z.; Crompton, J.G.; Eil, R.L.; Patel, S.J.; Ji, Y.; Van Panhuys, N.; Klebanoff, C.A.; Sukumar, M.; et al. Cish Actively Silences TCR Signaling in CD8+ T Cells to Maintain Tumor Tolerance. J. Exp. Med. 2015, 212, 2095–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, D.C.; Webber, B.R.; Patel, Y.; Johnson, M.J.; Kariya, C.M.; Lahr, W.S.; Parkhurst, M.R.; Gartner, J.J.; Prickett, T.D.; Lowery, F.J.; et al. Internal Checkpoint Regulates T Cell Neoantigen Reactivity and Susceptibility to PD1 Blockade. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J.S. Cas-OFFinder: A Fast and Versatile Algorithm That Searches for Potential off-Target Sites of Cas9 RNA-Guided Endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for SgRNA Design, off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Zheng, Z.; Nguyen, N.T.; Liebers, M.; Topkar, V.V.; Thapar, V.; Wyvekens, N.; Khayter, C.; Iafrate, A.J.; Le, L.P.; et al. GUIDE-Seq Enables Genome-Wide Profiling of off-Target Cleavage by CRISPR-Cas Nucleases. Nat. Biotechnol. 2015, 33, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).