The Effects of Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic Acid, on Neuroinflammation and Oxidative Stress in Astrocytes Stimulated with Lipopolysaccharide and Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Method
2.1. Cell Culture
2.2. Pharmacological Treatment
2.3. Real-Time PCR
2.4. Cell Viability
2.5. Statistical Analysis
3. Results
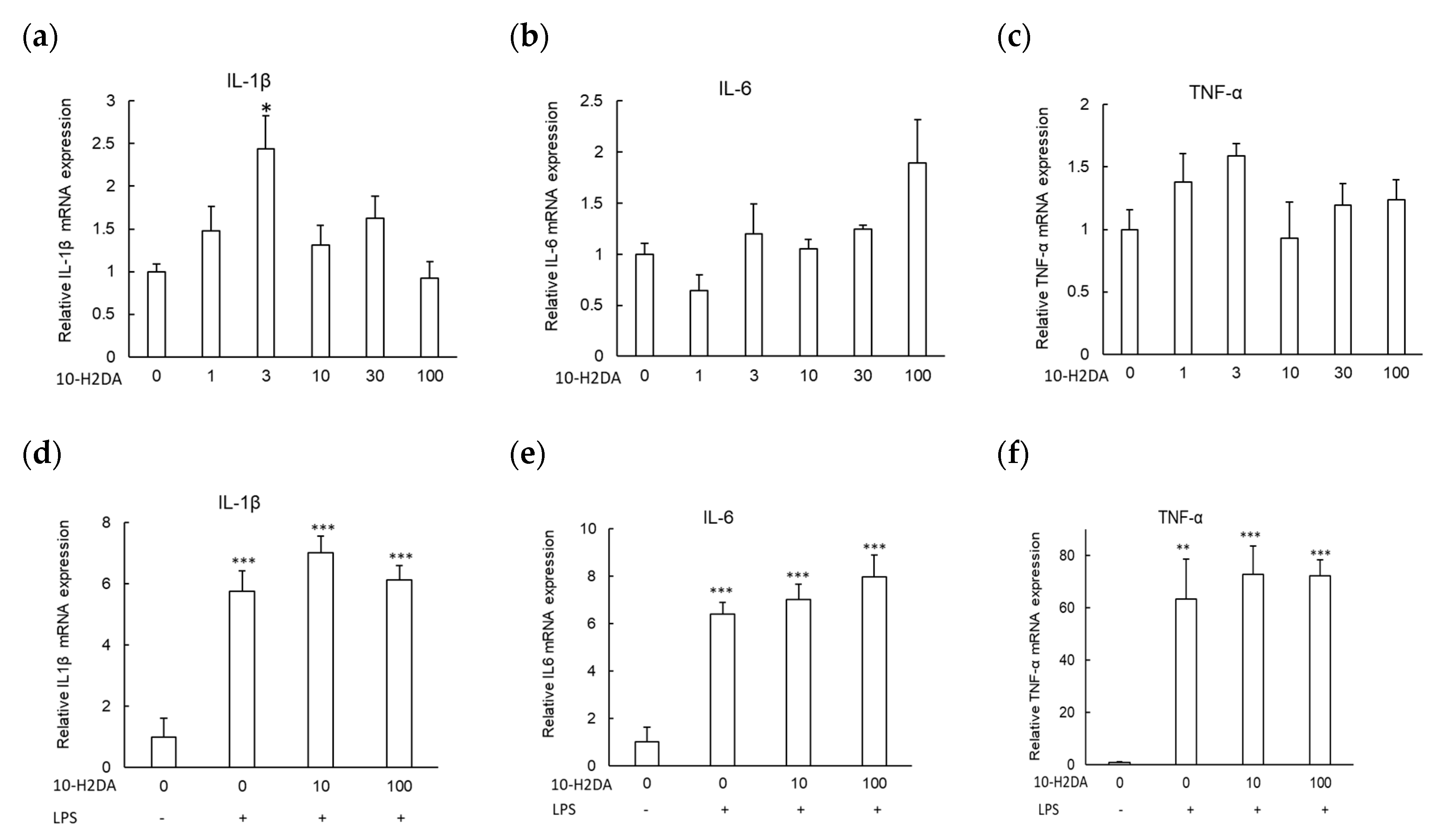
3.1. The Effect of 10-H2DA on the Expression of Proinflammatory Cytokine Genes
3.2. The Effect of 10-H2DA on the Expression of Neurotrophic Factors
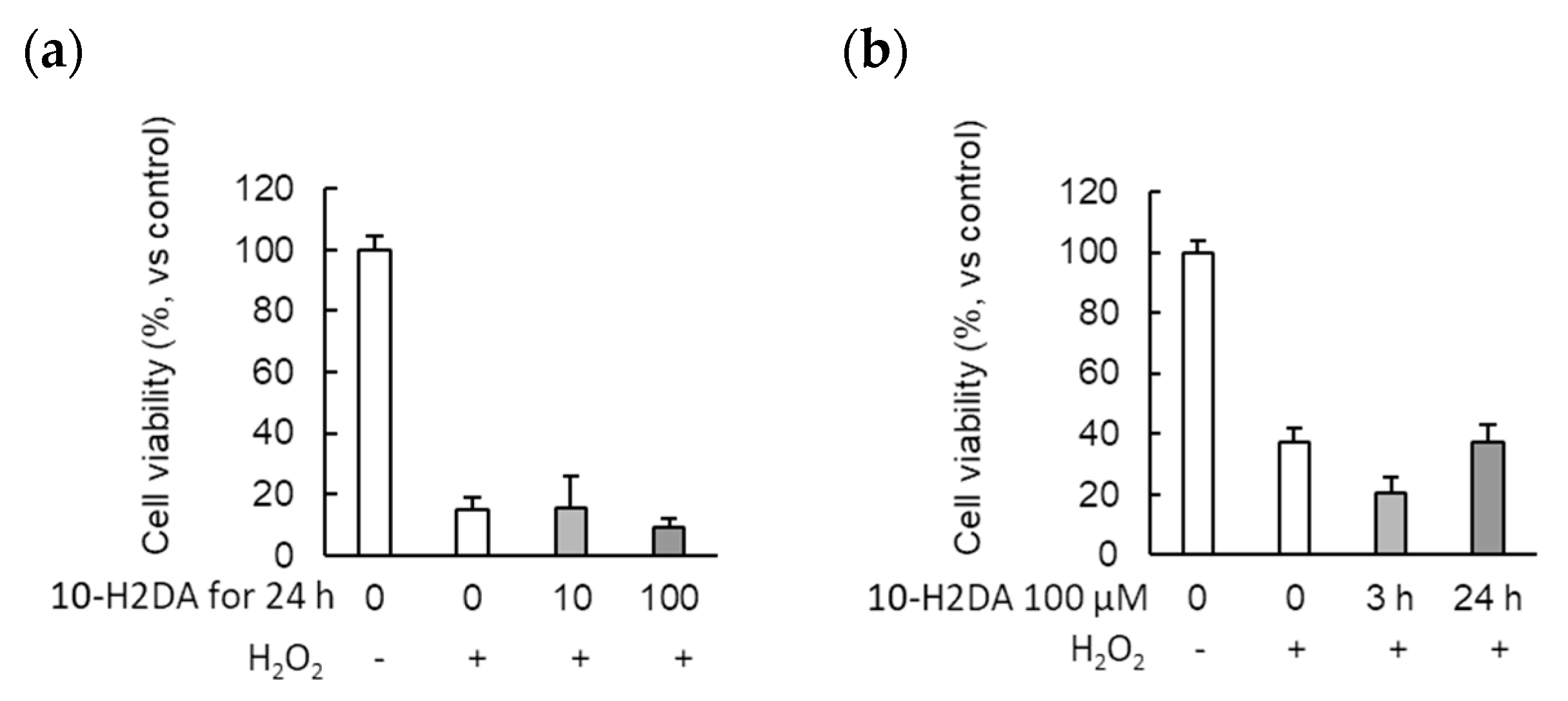
3.3. The Effect of 10-H2DA on the Cell Viability of Astrocytes Undergoing Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 10-H2DA | 10-Hydroxy-trans-2-decenoic acid |
| BDNF | Brain-derived neurotrophic factor |
| Covid-19 | Coronavirus disease 2019 |
| CNS | Central nervous system |
| DMEM | Dulbecco’s modified Eagle medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| FBS | Fetal bovine serum |
| FOXO | Forkhead box O transcription factor |
| GDNF | Glial cell line-derived neurotrophic factor |
| IFN | Interferon |
| IGF-1 | Insulin growth factor-1 |
| IL | Interleukin |
| H2O2 | Hydrogen peroxide |
| LPS | Lipopolysaccharide |
| NOS | Nitric oxide species |
| RJ | Royal jell |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-α |
References
- Iodice, F.; Cassano, V.; Rossini, P.M. Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer’s disease populations. Neurol. Sci. 2021, 42, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—A focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Nakamura, Z.M.; Nash, R.P.; Laughon, S.L.; Rosenstein, D.L. Neuropsychiatric Complications of COVID-19. Curr. Psychiatry Rep. 2021, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Bai, F.; Castoldi, R.; Barbanotti, D.; Falcinella, C.; Mulè, G.; Mondatore, D.; Tavelli, A.; Vegni, E.; Marchetti, G.; et al. Anxiety and depression symptoms after virological clearance of COVID-19: A cross-sectional study in Milan, Italy. J. Med. Virol. 2021, 93, 1175–1179. [Google Scholar] [CrossRef]
- Jaywant, A.; Vanderlind, W.M.; Alexopoulos, G.S.; Fridman, C.B.; Perlis, R.H.; Gunning, F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology 2021, 1–6. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal muscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (Covid-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hendawy, A.O. So, Antidepressant Drugs have Serious Adverse Effects, but what are the Alternatives? Nov. Appro. Drug Des. Dev. 2018, 4, 555636. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s disease: A focus on the effects of propolis and royal jelly. Oxid. Med. Cell Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379. [Google Scholar] [CrossRef]
- Ali, A.M.; Ahmed, A.H.; Smail, L. Psychological Climacteric Symptoms and Attitudes toward Menopause among Emirati Women. Int. J. Environ. Res. Public Health 2020, 17, 5028. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Corona Virus Disease 2019 (COVID-19): A pandemic that threatens physical and mental health by promoting physical inactivity. Sports Med. Health Sci. 2020, 2, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Canteiro, P.B.; Antero, D.C.; Tramontin, N.d.S.; Simon, K.U.; Mendes, C.; Anastácio Borges Correa, M.E.; Silveira, P.C.L.; Muller, A.P. Insulin treatment protects the brain against neuroinflammation by reducing cerebral cytokines and modulating mitochondrial function. Brain Res. Bull. 2019, 149, 120–128. [Google Scholar] [CrossRef]
- Perez-Dominguez, M.; Avila-Munoz, E.; Dominguez-Rivas, E.; Zepeda, A. The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regen. Res. 2019, 14, 817–825. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acaz-Fonseca, E.; Ortiz-Rodriguez, A.; Azcoitia, I.; Garcia-Segura, L.M.; Arevalo, M.-A. Notch signaling in astrocytes mediates their morphological response to an inflammatory challenge. Cell Death Discov. 2019, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, J.; Merkel, L.; Heckers, S.; Gudi, V.; Schwab, H.M.; Stangel, M. Investigation of Neuregulin-1 and Glial Cell-Derived Neurotrophic Factor in Rodent Astrocytes and Microglia. J. Mol. Neurosci. 2019, 67, 484–493. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Han, X.; Tang, F.; Gao, D. Mechanism of methylation and acetylation of high GDNF transcription in glioma cells: A review. Heliyon 2019, 5, e01951. [Google Scholar] [CrossRef] [Green Version]
- Amtul, Z.; Hill, D.J.; Arany, E.J.; Cechetto, D.F. Altered Insulin/Insulin-Like Growth Factor Signaling in a Comorbid Rat model of Ischemia and β-Amyloid Toxicity. Sci. Rep. 2018, 8, 5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, K.; Koarashi, K.; Kawabe, K.; Itakura, M.; Nakajima, H.; Moriyama, M.; Nakamura, Y. Insulin expression in cultured astrocytes and the decrease by amyloid β. Neurochem. Int. 2018, 119, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Hendawy, A.O. Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic acid, for Psychiatric and Neurological Disorders: How helpful could it be? Edelweiss. J. Food Sci. Technol. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Nakajima, S.; Numakawa, T.; Adachi, N.; Yoon, H.S.; Odaka, H.; Ooshima, Y.; Kunugi, H. The inactivation of extracellular signal-regulated kinase by glucagon-like peptide-1 contributes to neuroprotection against oxidative stress. Neurosci. Lett. 2016, 616, 105–110. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-Hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Casedas, G.; Bennett, A.C.; Gonzalez-Burgos, E.; Gomez-Serranillos, M.P.; Lopez, V.; Smith, C. Polyphenol-associated oxidative stress and inflammation in a model of LPS-induced inflammation in glial cells: Do we know enough for responsible compounding? Inflammopharmacology 2019, 27, 189–197. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, K.; Zhang, Y.-Z.; Zheng, Y.-F.; Hu, F.-L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic acid, as a Modulator of the Innate Immune Responses. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sugiyama, T.; Tokoro, S.; Neri, P.; Mori, H. Inhibition of interferon-gamma-induced nitric oxide production by 10-Hydroxy-trans-2-decenoic acid through inhibition of interferon regulatory factor-8 induction. Cell Immunol. 2012, 273, 73–78. [Google Scholar] [CrossRef]
- Sugiyama, T.; Takahashi, K.; Kuzumaki, A.; Tokoro, S.; Neri, P.; Mori, H. Inhibitory mechanism of 10-Hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-beta-induced nitric oxide production. Inflammation 2013, 36, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nitta, Y.; Fukumitsu, H.; Soumiya, H.; Ikeno, K.; Nakamura, T.; Furukawa, S. Antidepressant-Like Activity of 10-Hydroxy-trans-2-decenoic acid, a Unique Unsaturated Fatty Acid of Royal Jelly, in Stress-Inducible Depression-Like Mouse Model. Evid. Based Complementary Altern. Med. 2012, 2012, 139140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiser, M.J.; Grimshaw, V.; Wynalda, K.M.; Mohajeri, M.H.; Butt, C.M. Long-Term Administration of Queen Bee Acid (QBA) to Rodents Reduces Anxiety-Like Behavior, Promotes Neuronal Health and Improves Body Composition. Nutrients 2017, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- You, M.; Miao, Z.; Tian, J.; Hu, F. Trans-10-Hydroxy-2-decenoic acid protects against LPS-induced neuroinflammation through FOXO1-mediated activation of autophagy. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314. [Google Scholar] [CrossRef]
- Thevenet, J.; Marchi, U.D.; Domingo, J.S.; Christinat, N.; Bultot, L.; Lefebvre, G.; Sakamoto, K.; Descombes, P.; Masoodi, M.; Wiederkehr, A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte–neuron lactate and ketone body shuttle systems. FASEB J. 2016, 30, 1913–1926. [Google Scholar] [CrossRef] [Green Version]
- Scheld, M.; Fragoulis, A.; Nyamoya, S.; Zendedel, A.; Denecke, B.; Krauspe, B.; Teske, N.; Kipp, M.; Beyer, C.; Clarner, T. Mitochondrial Impairment in Oligodendroglial Cells Induces Cytokine Expression and Signaling. J. Mol. Neurosci. 2019, 67, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Pharaoh, G.A.; Marlin, M.C.; Masser, D.R.; Matsuzaki, S.; Wronowski, B.; Yeganeh, A.; Parks, E.E.; Premkumar, P.; Farley, J.A.; et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol. Metab. 2018, 9, 141–155. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Kunugi, H. The Effects of Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic Acid, on Neuroinflammation and Oxidative Stress in Astrocytes Stimulated with Lipopolysaccharide and Hydrogen Peroxide. Immuno 2021, 1, 212-222. https://doi.org/10.3390/immuno1030013
Ali AM, Kunugi H. The Effects of Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic Acid, on Neuroinflammation and Oxidative Stress in Astrocytes Stimulated with Lipopolysaccharide and Hydrogen Peroxide. Immuno. 2021; 1(3):212-222. https://doi.org/10.3390/immuno1030013
Chicago/Turabian StyleAli, Amira Mohammed, and Hiroshi Kunugi. 2021. "The Effects of Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic Acid, on Neuroinflammation and Oxidative Stress in Astrocytes Stimulated with Lipopolysaccharide and Hydrogen Peroxide" Immuno 1, no. 3: 212-222. https://doi.org/10.3390/immuno1030013
APA StyleAli, A. M., & Kunugi, H. (2021). The Effects of Royal Jelly Acid, 10-Hydroxy-trans-2-decenoic Acid, on Neuroinflammation and Oxidative Stress in Astrocytes Stimulated with Lipopolysaccharide and Hydrogen Peroxide. Immuno, 1(3), 212-222. https://doi.org/10.3390/immuno1030013






