A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation
Abstract
1. NK Cell Subpopulations
2. NK Cells in Pregnancy
3. NK Cells and Endometrial Disease
4. NK Cells in Autoimmune Diseases
5. NK Cells in Obesity
6. NK Cells in Infectious Diseases in Pregnancy
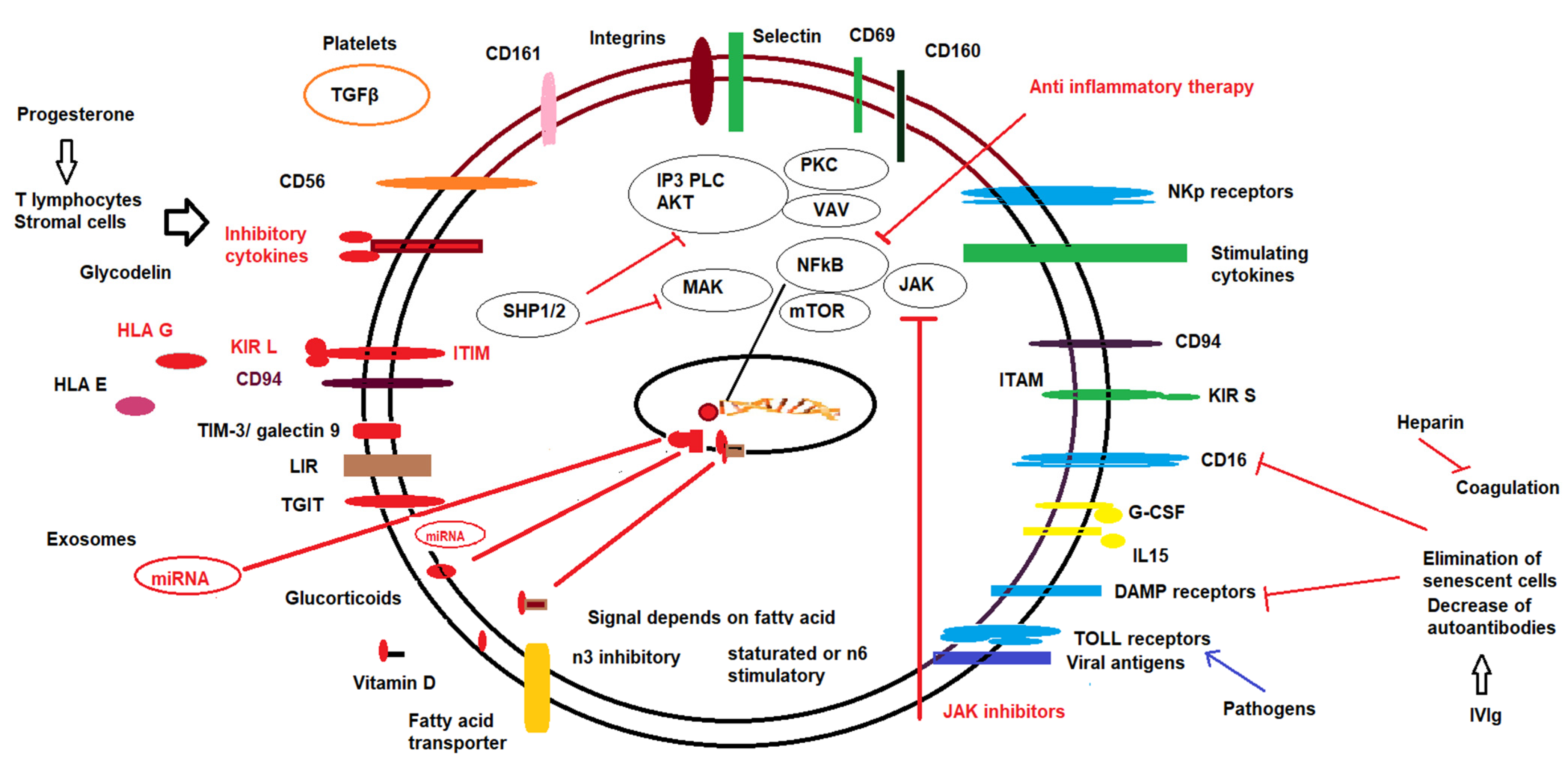
7. Pharmacological Modulation of NK Cells
7.1. Progesterone
7.2. Low Molecular Weight Heparin
7.3. Corticosteroids
7.4. Intravenous Immunoglobulins (IVIg)
7.5. Lipid Infusions
7.6. Vitamin D
7.7. Exosomes
7.8. Other Therapies
8. Conclusions
| Treatment Type | Effect on Peripheral NK Cells (Humans) | Local Cells | Animal Model | References |
|---|---|---|---|---|
| Progesterone | Inhibition on NK cytotoxicity by the progesterone-induced blocking factor | The indirect effect through T lymphocyte and stromal cell–cell cytokine secretion Progesterone-induced blocking factor | Increase tolerogenic response. There is an indirect effect of T cells and stromal cells. | [103,104,105,106,107,108,109,110,111,112,113] |
| Low molecular weight heparin | No direct effect | The indirect effect through T cell? | No direct reports | [114,115,116,117,118,119] |
| Corticosteroids | Decrease in cell number and decrease in cytotoxic response. | Downregulation of PD1/PD1L expression | Increase in tolerogenic responses | [120,121,122,123,124,125,126] |
| IVIg | Decrease in TH1 autoimmune cells. Modulation of NK cells | Reduction of deficient stromal cells. | Not reported | [127,128,129,130,131] |
| Lipid infusion | Decrease of the inflammatory burden and reduction in NK cytotoxic activity | Reduction of local transcription and secretion of IL15 and IL18 | Suppression cytotoxicity in vitro | [132,133,134,135,136,137,138] |
| Vitamin D | Decrease of in vitro cytotoxic response | Increase in tolerogenic responses | Modulation of iNKT cells which may affect NK cells | [139,140,141,142,143,144] |
| Exosomes | Increase in cytotoxic response against tumour cells | Decrease inflammatory burden increase tolerance | Increase tolerogenic NK cell responses | [145,146,147,148,149,150,151] |
| Intrauterine PBMC Platelets | Increase tolerogenic response via TGFβ | Increase in local tolerogenic response | [152,153,154,155] | |
| Medroxyprogesterone acetate | Increase in NK cell activity | Local decrease of IL-15 | Not described | [161,162] |
| Other small molecule therapy | Decrease peripheral NK cytotoxic response | Regulation of dNK | Not described | [164,165] |
| G-CSF | There is an indirect effect on NK cytotoxic response | Increase in tolerogenic response | Increase in tolerogenic response Tim-3 dependent | [166] |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef] [PubMed]
- Goodier, M.R.; Riley, E.M. Regulation of the human NK cell compartment by pathogens and vaccines. Clin. Transl. Immunol. 2021, 10, e1244. [Google Scholar] [CrossRef]
- Beaulieu, A.M. Memory responses by natural killer cells. J. Leukoc. Biol. 2018, 104, 1087–1096. [Google Scholar] [CrossRef]
- Beaulieu, A.M. Transcriptional and epigenetic regulation of memory NK cell responses. Immunol. Rev. 2021, 300, 125–133. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Sojka, D.K. Uterine Natural Killer Cell Heterogeneity: Lessons From Mouse Models. Front. Immunol. 2020, 11, 290. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, Z.; Peng, H. Tissue-resident NK cells and other innate lymphoid cells. Adv. Immunol. 2020, 145, 37–53. [Google Scholar]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Zhang, S.; Zhou, Y.; Wang, D.; Tao, S.; Ni, F. CD49a regulates the function of human decidual natural killer cells. Am. J. Reprod. Immunol. 2019, 81, e13101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Iizuka, K.; Kang, H.S.; Dokun, A.; French, A.R.; Greco, S.; Yokoyama, W.M. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 2002, 3, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Smyth, M.J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006, 176, 1517–1524. [Google Scholar] [CrossRef]
- Sojka, D.K.; Tian, Z.; Yokoyama, W.M. Tissue-resident natural killer cells and their potential diversity. Semin. Immunol. 2014, 26, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.; Berta, G.; Szekeres-Bartho, J. PIBF positive uterine NK cells in the mouse decidua. J. Reprod. Immunol. 2017, 119, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Csabai, T.; Pallinger, E.; Kovacs, A.F.; Miko, E.; Bognar, Z.; Szekeres-Bartho, J. Altered Immune Response and Implantation Failure in Progesterone-Induced Blocking Factor-Deficient Mice. Front. Immunol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Tong, L.; Xiang, L.; Shen, S.; Pan, C.; Liu, C.; Zhang, H. Correlations of the expression of γδ T cells and their co-stimulatory molecules TIGIT, PD-1, ICOS and BTLA with PR and PIBF in the peripheral blood and decidual tissues of women with unexplained recurrent spontaneous abortion. Clin. Exp. Immunol. 2021, 203, 55–65. [Google Scholar] [CrossRef]
- Lee, C.L.; Vijayan, M.; Wang, X.; Lam, K.K.W.; Koistinen, H.; Seppala, M.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N. Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype. Hum. Reprod. 2019, 34, 689–701. [Google Scholar] [CrossRef]
- Dixit, A.; Karande, A.A. Glycodelin regulates the numbers and function of peripheral natural killer cells. J. Reprod. Immunol. 2020, 137, 102625. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Feyaerts, D.; Kuret, T.; van Cranenbroek, B.; van der Zeeuw-Hingrez, S.; van der Heijden, O.W.H.; van der Meer, A.; Joosten, I.; van der Molen, R.G. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum. Reprod 2018, 33, 441–451. [Google Scholar] [CrossRef]
- Mendes, J.; Areia, A.L.; Rodrigues-Santos, P.; Santos-Rosa, M.; Mota-Pinto, A. Innate Lymphoid Cells in Human Pregnancy. Front. Immunol. 2020, 11, 551707. [Google Scholar] [CrossRef]
- De Mendonça Vieira, R.; Meagher, A.; Crespo, Â.C.; Kshirsagar, S.K.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Human Term Pregnancy Decidual NK Cells Generate Distinct Cytotoxic Responses. J. Immunol. 2020, 204, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Huhn, O.; Ivarsson, M.A.; Gardner, L.; Hollinshead, M.; Stinchcombe, J.C.; Chen, P.; Shreeve, N.; Chazara, O.; Farrell, L.E.; Theorell, J.; et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat. Commun. 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Cai, P.; Jin, L.; Sha, Q.; Yu, Q.; Zhang, W.; Jiang, C.; Liu, Q.; Zong, D.; Li, K.; et al. Single-cell profiling of the human decidual immune microenvironment in patients with recurrent pregnancy loss. Cell. Discov. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cui, L.; Xu, X.; Zhang, H.; Jiang, Y.; Ren, L.; Yang, C.; Liu, X.; Hu, X. The Role of Tim-3 on dNK Cells Dysfunction During Abnormal Pregnancy With Toxoplasma gondii Infection. Front. Cell. Infect. Microbiol. 2021, 11, 587150. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Zhan, S.; Li, Z.; Liu, X.; Zhang, H.; Jiang, Y.; Hu, X. TGF-β1 improving abnormal pregnancy outcomes induced by Toxoplasma gondii infection: Regulating NKG2D/DAP10 and killer subset of decidual NK cells. Cell. Immunol. 2017, 317, 9–17. [Google Scholar] [CrossRef]
- Loverro, G.; Maiorano, E.; Napoli, A.; Selvaggi, L.; Marra, E.; Perlino, E. Transforming growth factor-beta 1 and insulin-like growth factor-1 expression in ovarian endometriotic cysts: A preliminary study. Int. J. Mol. Med. 2001, 7, 423–429. [Google Scholar]
- Mizumoto, Y. Changes in NK activities and TGF- beta concentrations in the peritoneal cavity in endometriosis and their interaction related with infertility. Nihon Sanka Fujinka Gakkai Zasshi 1996, 48, 379–385. [Google Scholar] [PubMed]
- Eriksson, M.; Meadows, S.K.; Wira, C.R.; Sentman, C.L. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J. Leukoc. Biol. 2004, 76, 667–675. [Google Scholar] [CrossRef]
- Chakraborty, D.; Rumi, M.A.; Soares, M.J. NK cells, hypoxia and trophoblast cell differentiation. Cell. Cycle. 2012, 11, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.; Raggi, F.; Cangelosi, D.; Manzini, C.; Balsamo, M.; Blengio, F.; Eva, A.; Varesio, L.; Pietra, G.; Moretta, L.; et al. Hypoxia Modifies the Transcriptome of Human NK Cells, Modulates Their Immunoregulatory Profile, and Influences NK Cell Subset Migration. Front. Immunol. 2018, 9, 2358. [Google Scholar] [CrossRef]
- Hawke, L.G.; Whitford, M.K.M.; Ormiston, M.L. The Production of Pro-angiogenic VEGF-A Isoforms by Hypoxic Human NK Cells Is Independent of Their TGF-β-Mediated Conversion to an ILC1-Like Phenotype. Front. Immunol. 2020, 25, 1903. [Google Scholar] [CrossRef]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef]
- Shirasuna, K.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef]
- Chou, Y.C.; Chen, C.H.; Chen, M.J.; Chang, C.W.; Chen, P.H.; Yu, M.H.; Chen, Y.J.; Tsai, E.M.; Yang, P.S.; Lin, S.Y.; et al. Killer cell immunoglobulin-like receptors (KIR) and human leukocyte antigen-C (HLA-C) allorecognition patterns in women with endometriosis. Sci. Rep. 2020, 10, 4897. [Google Scholar] [CrossRef]
- Lin, F.; Yang, C.; Feng, T.; Yang, S.; Zhou, R.; Li, H. The Maternal-Fetal Interface in Small-for-Gestational-Age Pregnancies Is Associated With a Reduced Quantity of Human Decidual NK Cells With Weaker Functional Ability. Front. Cell. Dev. Biol. 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Papuchova, H.; Kshirsagar, S.; Xu, L.; Bougleux Gomes, H.A.; Li, Q.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc. Natl. Acad. Sci. USA. 2020, 7, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, R.; Fujiki, Y.; Kishigami, H.; Kishi, H.; Kiyono, T.; Nakayama, S.; Sago, H.; Okamoto, A.; Umezawa, A. Endometrial regeneration with endometrial epithelium: Homologous orchestration with endometrial stroma as a feeder. Stem. Cell. Res. Ther. 2021, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Ouyang, N.; Dai, K.; Yuan, P.; Zheng, L.; Wang, W. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil. Steril. 2019, 112, 882–891. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, J.B. Perspectives of new therapies for endometriosis. Recent. Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 218–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Si, W.; Yang, B.; Lin, M.; Zheng, J.; Cui, L. Increased Peripheral NKG2A-NKG2D+CD3-CD16+CD56dim NK Cell Subset Was Positively Correlated with Antiphospholipid Antibodies in Patients of Obstetric Antiphospholipid Syndrome. Immunol. Invest. 2020, 26, 1–14. [Google Scholar] [CrossRef]
- Greenbaum, H.; Galper, B.L.; Decter, D.H.; Eisenberg, V.H. Endometriosis and autoimmunity: Can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun. Rev. 2021, 20, 102795. [Google Scholar] [CrossRef]
- Kliemann, N.; Viallon, V.; Murphy, N.; Beeken, R.J.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; van Roekel, E.H.; Schmidt, J.A.; Borch, K.B.; et al. Metabolic signatures of greater body size and their associations with risk of colorectal and endometrial cancers in the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2021, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.V.; Shen, Z.; Rodriguez-Garcia, M.; Usherwood, E.J.; Tafe, L.J.; Wira, C.R. Endometrial Cancer Suppresses CD8+ T Cell-Mediated Cytotoxicity in Postmenopausal Women. Front. Immunol. 2021, 12, 657326. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, L.; Zhang, J.; Song, W.; Li, M.; Wang, H. The landscape and prognostic value of immune characteristics in uterine corpus endometrial cancer. Biosci. Rep. 2021, 41, BSR20202321. [Google Scholar] [CrossRef] [PubMed]
- Bähr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, R.G.; Schutten, J.H.; van Cranenbroek, B.; ter Meer, M.; Donckers, J.; Scholten, R.R.; van der Heijden, O.W.; Spaanderman, M.E.; Joosten, I. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum. Reprod. 2014, 29, 303–314. [Google Scholar] [CrossRef]
- Tilburgs, T.; Scherjon, S.A.; Roelen, D.L.; Claas, F.H. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum. Immunol. 2009, 70, 96–100. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, Y.; Kang, G.H. Prognostic significance of CD103+ immune cells in solid tumor: A systemic review and meta-analysis. Sci. Rep. 2019, 9, 3808. [Google Scholar] [CrossRef]
- Degos, C.; Heinemann, M.; Barrou, J.; Boucherit, N.; Lambaudie, E.; Savina, A.; Gorvel, L.; Olive, D. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Marin, M.L.C.; Coelho, V.; Visentainer, J.E.L.; Alves, H.V.; Köhler, K.F.; Rached, M.R.; Abrão, M.S.; Kalil, J. Inhibitory KIR2DL2 Gene: Risk for Deep Endometriosis in Euro-descendants. Reprod. Sci. 2021, 28, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liang, J.; Liu, W.; Wang, F.; Li, C. Possible roles of HLA-G regulating immune cells in pregnancy and endometrial diseases via KIR2DL4. J. Reprod. Immunol. 2020, 142, 103176. [Google Scholar] [CrossRef]
- Xu, H. Expressions of natural cytotoxicity receptor, NKG2D and NKG2D ligands in endometriosis. J. Reprod. Immunol. 2019, 136, 102615. [Google Scholar] [CrossRef]
- Freitag, N.; Pour, S.J.; Fehm, T.N.; Toth, B.; Markert, U.R.; Weber, M.; Togawa, R.; Kruessel, J.S.; Baston-Buest, D.M.; Bielfeld, A.P. Are uterine natural killer and plasma cells in infertility patients associated with endometriosis, repeated implantation failure, or recurrent pregnancy loss? Arch. Gynecol. Obstet. 2020, 302, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Lu, M.Y.; Liu, B. Circulating CD56+ NKG2D+ NK cells and postoperative fertility in ovarian endometrioma. Sci. Rep. 2020, 10, 18598. [Google Scholar] [CrossRef] [PubMed]
- Tincani, A.; Nalli, C.; Khizroeva, J.; Bitsadze, V.; Lojacono, A.; Andreoli, L.; Shoenfeld, Y.; Makatsariya, A. Autoimmune diseases and pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101322. [Google Scholar] [CrossRef] [PubMed]
- Vomstein, K.; Feil, K.; Strobel, L.; Aulitzky, A.; Hofer-Tollinger, S.; Kuon, R.J.; Toth, B. Immunological Risk Factors in Recurrent Pregnancy Loss: Guidelines Versus Current State of the Art. J. Clin. Med. 2021, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Kwak-Kim, J.; Skariah, A.; Wu, L.; Salazar, D.; Sung, N.; Ota, K. Humoral and cellular autoimmunity in women with recurrent pregnancy losses and repeated implantation failures: A possible role of vitamin D. Autoimmun. Rev. 2016, 15, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Gridelet, V.; Perrier d’Hauterive, S.; Polese, B.; Foidart, J.M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotrophin: New Pleiotropic Functions for an "Old" Hormone During Pregnancy. Front. Immunol. 2020, 11, 343. [Google Scholar] [CrossRef]
- Jiang, M.; Shen, N.; Zhou, H.; Wang, Y.; Lin, S.; Wu, J.; Di, W. The enrichment of neutrophil extracellular traps impair the placentas of systemic lupus erythematosus through accumulating decidual NK cells. Sci. Rep. 2021, 11, 6870. [Google Scholar] [CrossRef]
- Wouters, K.; Kusters, Y.H.A.M.; Bijnen, M.; Wetzels, S.; Zhang, X.; Linssen, P.B.C.; Gaens, K.; Houben, A.J.H.M.; Joris, P.J.; Plat, J.; et al. NK cells in human visceral adipose tissue contribute to obesity-associated insulin resistance through low-grade inflammation. Clin. Transl. Med. 2020, 10, e192. [Google Scholar] [CrossRef]
- Fernø, J.; Strand, K.; Mellgren, G.; Stiglund, N.; Björkström, N.K. Natural Killer Cells as Sensors of Adipose Tissue Stress. Trends Endocrinol. Metab. 2020, 31, 3–12. [Google Scholar] [CrossRef]
- Spielmann, J.; Mattheis, L.; Jung, J.S.; Rauße, H.; Glaß, M.; Bähr, I.; Quandt, D.; Oswald, J.; Kielstein, H. Effects of obesity on NK cells in a mouse model of postmenopausal breast cancer. Sci. Rep. 2020, 10, 20606. [Google Scholar] [CrossRef]
- Valero-Pacheco, N.; Beaulieu, A.M. Transcriptional Regulation of Mouse Tissue-Resident Natural Killer Cell Development. Front. Immunol. 2020, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Braunschweig, A.; Poehlmann, T.G.; Busch, S.; Schleussner, E.; Markert, U.R. Signal transducer and activator of transcription 3 (STAT3) and Suppressor of Cytokine Signaling (SOCS3) balance controls cytotoxicity and IL-10 expression in decidual-like natural killer cell line NK-92. Am. J. Reprod. Immunol. 2011, 66, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sun, X.; Wang, Y.; Luan, H.; Zhang, R.; Hu, F.; Sun, X.; Li, X.; Guo, J. Role of IL-24 in NK cell activation and its clinical implication in systemic lupus erythematosus. Clin. Rheumatol. 2021, 40, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Nörenberg, J.; Meggyes, M.; Jakso, P.; Miko, E.; Barakonyi, A. TIM-3 and TIM-1 Could Regulate Decidual γδTCR Bright T Cells during Murine Pregnancy. J. Immunol. Res. 2019, 2019, 3836942. [Google Scholar] [CrossRef]
- Tohma, Y.A.; Musabak, U.; Gunakan, E.; Akilli, H.; Onalan, G.; Zeyneloglu, H.B. The Role of Analysis of NK Cell Subsets in Peripheral Blood and Uterine Lavage Samples in Evaluation of Patients with Recurrent Implantation Failure. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101793. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Marshall, N.E.; Messaoudi, I. Impact of pregravid obesity on maternal and fetal immunity: Fertile grounds for reprogramming. J. Leukoc. Biol. 2019, 106, 1035–1050. [Google Scholar] [CrossRef]
- Castellana, B.; Perdu, S.; Kim, Y.; Chan, K.; Atif, J.; Marziali, M.; Beristain, A.G. Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunol. Cell. Biol. 2018, 96, 805–819. [Google Scholar] [CrossRef]
- St-Germain, L.E.; Castellana, B.; Baltayeva, J.; Beristain, A.G. Maternal Obesity and the Uterine Immune Cell Landscape: The Shaping Role of Inflammation. Int. J. Mol. Sci. 2020, 21, 3776. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Fan, I.Y.; Gillen, J.B.; Chew, M.; Marcinko, K.; Steinberg, G.R.; Gibala, M.J.; Ashkar, A.A. High Intensity Interval Training Increases Natural Killer Cell Number and Function in Obese Breast Cancer-challenged Mice and Obese Women. J Cancer Prev. 2017, 22, 260–266. [Google Scholar] [CrossRef]
- Sappenfield, E.; Jamieson, D.J.; Kourtis, A.P. Pregnancy and susceptibility to infectious diseases. Infect. Dis. Obstet. Gynecol. 2013, 2013, 752852. [Google Scholar] [CrossRef]
- Cornish, E.F.; Filipovic, I.; Åsenius, F.; Williams, D.J.; McDonnell, T. Innate Immune Responses to Acute Viral Infection During Pregnancy. Front. Immunol. 2020, 11, 572567. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.L.; Silverstein, R.B.; Verma, S.; Mysorekar, I.U. Viral-Immune Cell Interactions at the Maternal-Fetal Interface in Human Pregnancy. Front. Immunol. 2020, 11, 522047. [Google Scholar] [CrossRef] [PubMed]
- Lu-Culligan, A.; Chavan, A.R.; Vijayakumar, P.; Irshaid, L.; Courchaine, E.M.; Milano, M.; Tang, Z.; Pope, S.D.; Song, E.; Vogels, C.B.F.; et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Medicine 2021, 2, 591–610. [Google Scholar] [CrossRef]
- Barber, E.M.; Pollard, J.W. The uterine NK cell population requires IL-15, but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J. Immunol. 2003, 171, 37–46. [Google Scholar] [CrossRef]
- Clayton, R.D.; Duffy, S.R.; Wilkinson, N.; Garry, R.; Jackson, A.M. Increase in peripheral blood mononuclear cell (PBMC)—And CD56+ cell-mediated killing of endometrial stromal cells by mycobacteria; a possible role in endometriosis immunotherapy? Hum. Reprod. 2004, 19, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Omosun, Y.O.; Blackstock, A.J.Ñ.; Gatei, W.; Hightower, A.; van Eijk, A.M.; Ayisi, J.; Otieno, J.; Lal, R.B.; Steketee, R.; Nahlen, B.; et al. Differential association of gene content polymorphisms of killer cell immunoglobulin-like receptors with placental malaria in HIV- and HIV+ mothers. PLoS ONE 2012, 7, e38617. [Google Scholar] [CrossRef][Green Version]
- Omosun, Y.O.; Blackstock, A.J.; Williamson, J.; van Eijk, A.M.; Ayisi, J.; Otieno, J.; Lal, R.B.; Ter Kuile, F.O.; Slutsker, L.; Shi, Y.P. Association of maternal KIR gene content polymorphisms with reduction in perinatal transmission of HIV-1. PLoS ONE 2018, 13, e0191733. [Google Scholar] [CrossRef] [PubMed]
- Damelang, T.; Aitken, E.H.; Hasang, W.; Lopez, E.; Killian, M.; Unger, H.W.; Salanti, A.; Shub, A.; McCarthy, E.; Kedzierska, K.; et al. Antibody-mediated activation of natural killer cells in malaria exposed pregnant women. Sci. Rep. 2021, 11, 4130. [Google Scholar] [CrossRef] [PubMed]
- Shmeleva, E.V.; Colucci, F. Maternal natural killer cells at the intersection between reproduction and mucosal immunity. Mucosal. Immunol. 2021, 26, 1–15. [Google Scholar]
- Colucci, F.; Traherne, J. Killer-cell immunoglobulin-like receptors on the cusp of modern immunogenetics. Immunology 2017, 152, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Huhn, O.; Chazara, O.; Ivarsson, M.A.; Retière, C.; Venkatesan, T.C.; Norman, P.J.; Hilton, H.G.; Jayaraman, J.; Traherne, J.A.; Trowsdale, J.; et al. High-Resolution Genetic and Phenotypic Analysis of KIR2DL1 Alleles and Their Association with Pre-Eclampsia. J. Immunol. 2018, 201, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Hof, L.J.; Schotvanger, N.; Haasnoot, G.W.; van der Keur, C.; Roelen, D.L.; Lashley, L.E.E.L.O.; Claas, F.H.J.; Eikmans, M.; van der Hoorn, M.P. Maternal-Fetal HLA Compatibility in Uncomplicated and Preeclamptic Naturally Conceived Pregnancies. Front. Immunol. 2021, 12, 673131. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Jiang, Y.; Lao, K.; Xu, X.; Zhan, S.; Wang, Y.; Hu, X. sHLA-G involved in the apoptosis of decidual natural killer cells following Toxoplasma gondii infection. Inflammation 2014, 37, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Tomac, J.; Mazor, M.; Lisnić, B.; Golemac, M.; Kveštak, D.; Bralić, M.; Bilić Zulle, L.; Brinkmann, M.M.; Dölken, L.; Reinert, L.S.; et al. Viral infection of the ovaries compromises pregnancy and reveals innate immune mechanisms protecting fertility. Immunity 2021. [Google Scholar] [CrossRef]
- Kujur, W.; Murillo, O.; Adduri, R.S.R.; Vankayalapati, R.; Konduru, N.V.; Mulik, S. Memory like NK cells display stem cell like properties after Zika virus infection. PLoS Pathog. 2020, 16, e1009132. [Google Scholar] [CrossRef]
- Glasner, A.; Oiknine-Djian, E.; Weisblum, Y.; Diab, M.; Panet, A.; Wolf, D.G.; Mandelboim, O. Zika Virus Escapes NK Cell Detection by Upregulating Major Histocompatibility Complex Class I Molecules. J. Virol. 2017, 91, e00785-17. [Google Scholar] [CrossRef]
- Schust, D.J.; Hill, A.B.; Ploegh, H.L. Herpes simplex virus blocks intracellular transport of HLA-G in placentally derived human cells. J. Immunol. 1996, 157, 3375–3380. [Google Scholar]
- Li, L.; Wang, L.; Huang, C.; Diao, L.; Zhang, Y.; Zhang, X.; Xu, J.; Zeng, Y. Chronic hepatitis B infection alters peripheral immune response in women with reproductive failure. Am. J. Reprod. Immunol. 2019, 81, e13083. [Google Scholar] [CrossRef] [PubMed]
- Corado, J.; Toro, F.; Rivera, H.; Bianco, N.E.; Deibis, L.; De Sanctis, J.B. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 1997, 109, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Shabrish, S.; Karnik, N.; Gupta, V.; Bhate, P.; Madkaikar, M. Impaired NK cell activation during acute dengue virus infection: A contributing factor to disease severity. Heliyon 2020, 6, e04320. [Google Scholar] [CrossRef] [PubMed]
- Wagstaffe, H.R.; Susannini, G.; Thiébaut, R.; Richert, L.; Lévy, Y.; Bockstal, V.; Stoop, J.N.; Luhn, K.; Douoguih, M.; Riley, E.M.; et al. Durable natural killer cell responses after heterologous two-dose Ebola vaccination. NPJ Vaccines. 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2021, 11, 1–12. [Google Scholar]
- Piersma, S.J.; Brizić, I. Natural Killer cell effector functions in antiviral defense. FEBS J. 2021. [Google Scholar] [CrossRef]
- Ścieżyńska, A.; Komorowski, M.; Soszyńska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef] [PubMed]
- Dizaji Asl, K.; Velaei, K.; Rafat, A.; Tayefi Nasrabadi, H.; Movassaghpour, A.A.; Mahdavi, M.; Nozad Charoudeh, H. The role of KIR positive NK cells in diseases and its importance in clinical intervention. Int. Immunopharmacol. 2021, 92, 107361. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, B.; Hassouneh, F.; Delgado, E.; Casado, J.G.; Tarazona, R. Natural killer cells in recurrent miscarriage: An overview. J. Reprod. Immunol. 2020, 142, 103209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, J.; Wang, Y.; Sun, J.; Li, Z.; Sui, L.; Fan, J.; Liu, C.; Shang, Y.; Kong, L.; et al. Progesterone Regulates Glucose Metabolism Through Glucose Transporter 1 to Promote Endometrial Receptivity. Front. Physiol. 2020, 11, 543148. [Google Scholar] [CrossRef]
- Ata, B.; Telek, S.B. Assisted reproductive technology for women with endometriosis, a clinically oriented review. Curr. Opin. Obstet. Gynecol. 2021, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Y.; Yi, M.; Gu, C.; Zhu, Y.; Hu, G. Involvement of natural killer cells in the pathogenesis of endometriosis in patients with pelvic pain. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1747. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Schindler, A.E. Progestogens and immunology. Best Pract. Res Clin. Obstet. Gynaecol. 2019, 60, 17–23. [Google Scholar] [CrossRef]
- Miko, E.; Halasz, M.; Jericevic-Mulac, B.; Wicherek, L.; Arck, P.; Arató, G.; Skret Magierlo, J.; Rukavina, D.; Szekeres-Bartho, J. Progesterone-induced blocking factor, (PIBF) and trophoblast invasiveness. J. Reprod. Immunol. 2011, 90, 50–57. [Google Scholar] [CrossRef]
- Wilsher, S.; Newcombe, J.R.; Allen, W.R.T. The immunolocalisation of Galectin-1 and Progesterone-Induced Blocking Factor (PIBF) in equine trophoblast: Possible roles in trophoblast invasion and the immunological protection of pregnancy. Placenta 2019, 85, 32–39. [Google Scholar] [CrossRef]
- Murata, H.; Taaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, H.; Li, B.; Liu, M.; Liu, D.; Deng, M.; Wang, Y.; Xia, X.; Jiang, Q.; Chen, D. Effects of human chorionic gonadotropin, estradiol, and progesterone on interleukin-18 expression in human decidual tissues. Gynecol. Endocrinol. 2017, 33, 265–269. [Google Scholar] [CrossRef]
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the IL15 gene in human endometrial stromal cells. J. Biol. Chem. 2020, 295, 9596–9605. [Google Scholar] [CrossRef]
- Thomsen, L.H.; Kesmodel, U.S.; Erb, K.; Bungum, L.; Pedersen, D.; Hauge, B.; Elbæk, H.O.; Povlsen, B.B.; Andersen, C.Y.; Humaidan, P. The impact of luteal serum progesterone levels on live birth rates-a prospective study of 602 IVF/ICSI cycles. Hum. Reprod. 2018, 33, 1506–1516. [Google Scholar] [CrossRef]
- Martínez, M.C.; Rodríguez-Varela, C.; Demur, E.L. New concepts and difficulties with progesterone supplementation in the luteal phase. Curr. Opin.Obstet. Gynecol. 2021, 33, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kook, H.; Kang, S.; Lee, J. Study of immune-tolerised cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semiallograft immune tolerance during pregnancy. J. Extracell. Vesicles. 2020, 9, 1795364. [Google Scholar] [CrossRef]
- Johann, S.; Zoller, C.; Haas, S.; Blümel, G.; Lipp, M.; Förster, R. Sulfated polysaccharide anticoagulants suppress natural killer cell activity in vitro. Thromb. Haemost. 1995, 74, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.; Kajihara, T.; Mizuno, Y.; Takano, N.; Tochigi, H.; Sato, T.; Ishihara, O. Heparin prevents oxidative stress-induced apoptosis in human decidualised endometrial stromal cells. Med. Mol. Morphol. 2019, 52, 209–216. [Google Scholar] [CrossRef]
- Dias, A.T.B.; Modesto, T.B.; Oliveira, S.A. Effectiveness of the use of Low Molecular Heparin in patients with repetition abortion history: Systematic review and meta-analysis. JBRA Assist. Reprod. 2021, 25, 10–27. [Google Scholar]
- Bruno, V.; Svensson-Arvelund, J.; Rubér, M.; Berg, G.; Piccione, E.; Jenmalm, M.C.; Ernerudh, J. Effects of low molecular weight heparin on the polarisation and cytokine profile of macrophages and T helper cells in vitro. Sci. Rep. 2018, 8, 4166. [Google Scholar] [CrossRef]
- Rossi, G.R.; Gonçalves, J.P.; McCulloch, T.; Delconte, R.B.; Hennessy, R.J.; Huntington, N.D.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. The Antitumor Effect of Heparin is not Mediated by Direct NK Cell Activation. J. Clin. Med. 2020, 9, 2666. [Google Scholar] [CrossRef]
- Lee, E.E.; Jun, J.K.; Lee, E.B. Management of Women with Antiphospholipid Antibodies or Antiphospholipid Syndrome during Pregnancy. J. Korean Med. Sci. 2021, 36, e24. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef]
- Thum, M.Y.; Bhaskaran, S.; Abdalla, H.I.; Ford, B.; Sumar, N.; Bansal, A. Prednisolone suppresses NK cell cytotoxicity in vitro in women with a history of infertility and elevated NK cell cytotoxicity. Am. J. Reprod. Immunol. 2008, 59, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.F.; Elkholy, A.G.; El-Said, M.M.; Abdel-Salam, N.E. Combined oral prednisolone and heparin versus heparin: The effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double-blind placebo randomised controlled trial. Arch. Gynecol. Obstet. 2014, 290, 757–762. [Google Scholar] [CrossRef]
- Wdowiak, A.; Raczkiewicz, D.; Janczyk, P.; Bojar, I.; Makara-Studzińska, M.; Wdowiak-Filip, A. Interactions of Cortisol and Prolactin with Other Selected Menstrual Cycle Hormones Affecting the Chances of Conception in Infertile Women. Int. J. Environ. Res. Public Health 2020, 17, 7537. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Lai, Y.; He, G.; He, G. Expression and significance of PD-1 and PD-L1 in patients with recurrent spontaneous abortion: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25444. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lv, C.; Su, Y.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol. Endocrinol. 2019, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Aston, W.J.; Hope, D.E.; Cook, A.M.; Boon, L.; Dick, I.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Dexamethasone differentially depletes tumour and peripheral blood lymphocytes and can impact the efficacy of chemotherapy/checkpoint blockade combination treatment. Oncoimmunology 2019, 8, e1641390. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghaebi, M.; Abdolmohammadi-Vahid, S.; Abbaspour-Aghdam, S.; Hamdi, K.; Abdollahi-Fard, S.; Danaii, S.; Mosapour, P.; Koushaeian, L.; Dolati, S.; et al. NK cell frequency and cytotoxicity in correlation to pregnancy outcome and response to IVIG therapy among women with recurrent pregnancy loss. J. Cell. Physiol. 2019, 234, 9428–9437. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Ahmadi, M.; Dolati, S.; Aghebati-Maleki, L.; Eghbal-Fard, S.; Kamrani, A.; Behrad, B.; Roshangar, L.; Jadidi-Niaragh, F.; Yousefi, B.; et al. Intravenous immunoglobulin G treatment increases live birth rate in women with recurrent miscarriage and modulates regulatory and exhausted regulatory T cells frequency and function. J. Cell. Biochem. 2019, 120, 5424–5434. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Kolte, A.M.; Krog, M.C.; Nielsen, H.S.; Egerup, P. Treatment with intravenous immunoglobulin in patients with recurrent pregnancy loss: An update. J. Reprod. Immunol. 2019, 133, 37–42. [Google Scholar] [CrossRef]
- Abdolmohammadi-Vahid, S.; Pashazadeh, F.; Pourmoghaddam, Z.; Aghebati-Maleki, L.; Abdollahi-Fard, S.; Yousefi, M. The effectiveness of IVIG therapy in pregnancy and live birth rate of women with recurrent implantation failure (RIF): A systematic review and meta-analysis. J. Reprod. Immunol. 2019, 134–135, 28–33. [Google Scholar] [CrossRef]
- Roussev, R.G.; Ng, S.C.; Coulam, C.B. Natural killer cell functional activity suppression by intravenous immunoglobulin, intralipid and soluble human leukocyte antigen-G. Am. J. Reprod. Immunol. 2007, 57, 262–269. [Google Scholar] [CrossRef]
- Meng, L.; Lin, J.; Chen, L.; Wang, Z.; Liu, M.; Liu, Y.; Chen, X.; Zhu, L.; Chen, H.; Zhang, J. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2016, 294, 29–39. [Google Scholar] [CrossRef]
- Lédée, N.; Vasseur, C.; Petitbarat, M.; Chevrier, L.; Vezmar, K.; Dray, G.; Chenière, S.; Lobersztajn, A.; Vitoux, D.; Cassuto, G.N.; et al. Intralipid® may represent a new hope for patients with reproductive failures and simultaneously an over-immune endometrial activation. J. Reprod. Immunol. 2018, 130, 18–22. [Google Scholar] [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of intralipid’s suppressive effect on NK cell’s functional activity. Am. J. Reprod. Immunol. 2008, 60, 258–263. [Google Scholar] [CrossRef]
- Canella, P.R.B.C.; Barini, R.; Carvalho, P.O.; Razolli, D.S. Lipid emulsion therapy in women with recurrent pregnancy loss and repeated implantation failure: The role of abnormal natural killer cell activity. J. Cell. Mol. Med. 2021, 25, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Kolanska, K.; Alijotas-Reig, J.; Cohen, J.; Cheloufi, M.; Selleret, L.; d’Argent, E.; Kayem, G.; Valverde, E.E.; Fain, O.; Bornes, M.; et al. Endometriosis with infertility: A comprehensive review on the role of immune deregulation and immunomodulation therapy. Am. J. Reprod. Immunol. 2021, 85, e13384. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Blanca, I.; Bianco, N.E. Expression of different lipoprotein receptors in natural killer cells and their effect on natural killer proliferative and cytotoxic activity. Immunology 1995, 86, 399–407. [Google Scholar] [PubMed]
- De Sanctis, J.B.; Dumut, D.C.; Radzioch, D.; Hajdúch, M. Functionally Relevant Differences in Plasma Fatty Acid Composition and Expression of Cytotoxic and Inhibitory NK Cell Receptors between Healthy Young and Healthy Elder Adults. Nutrients 2020, 12, 3641. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.N.; Nguyen, L.; Chan, J.; Innes, B.A.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol. Reprod. 2006, 75, 816–822. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Jeffery, L.E.; Susarla, R.; Lissauer, D.M.; Coort, S.L.; Garcia, A.M.; Knoblich, K.; Fletcher, A.L.; Bulmer, J.N.; Kilby, M.D.; et al. Transcriptomic analysis of vitamin D responses in uterine and peripheral NK cells. Reproduction 2019, 158, 211–221. [Google Scholar] [CrossRef]
- Ota, K.; Dambaeva, S.; Kim, M.W.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015, 45, 3188–3199. [Google Scholar] [CrossRef]
- Chen, X.; Yin, B.; Lian, R.C.; Zhang, T.; Zhang, H.Z.; Diao, L.H.; Li, Y.Y.; Huang, C.Y.; Liang, D.S.; Zeng, Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016, 76, 432–438. [Google Scholar] [CrossRef]
- Ota, K.; Takahashi, T.; Han, A.; Damvaeba, S.; Mizunuma, H.; Kwak-Kim, J. Effects of MTHFR C677T polymorphism on vitamin D, homocysteine and natural killer cell cytotoxicity in women with recurrent pregnancy losses. Hum. Reprod. 2020, 35, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, X.; Yang, X. A novel update on vitamin D in recurrent pregnancy loss. Mol. Med. Rep. 2021, 23, 382. [Google Scholar] [CrossRef]
- Zhang, W.B.; Cheng, M.J.; Huang, Y.T.; Jiang, W.; Cong, Q.; Zheng, Y.F.; Xu, C.J. A study in vitro on differentiation of bone marrow mesenchymal stem cells into endometrial epithelial cells in mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 185–190. [Google Scholar] [CrossRef]
- Tempest, N.; Maclean, A.; Hapangama, D.K. Endometrial Stem Cell Markers: Current Concepts and Unresolved Questions. Int. J. Mol. Sci. 2018, 19, 3240. [Google Scholar] [CrossRef]
- Rezaei Kahmini, F.; Shahgaldi, S.; Moazzeni, S.M. Mesenchymal stem cells alter the frequency and cytokine profile of natural killer cells in abortion-prone mice. J. Cell. Physiol. 2020, 235, 7214–7223. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, H.; Huang, W.; Wang, L.; Xia, X.; Fang, X. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics 2020, 12, 1193–1213. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fang, X.; Huang, H.; Huang, W.; Wang, L.; Xia, X. Construction and topological analysis of an endometriosis-related exosomal circRNA-miRNA-mRNA regulatory network. Aging 2021, 26, 13. [Google Scholar]
- Xu, P.; Ma, Y.; Wu, H.; Wang, Y.L. Placenta-Derived MicroRNAs in the Pathophysiology of Human Pregnancy. Front. Cell. Dev. Biol. 2021, 9, 646326. [Google Scholar] [CrossRef]
- Kharazi, U.; Badalzadeh, R. A review on the stem cell therapy and an introduction to exosomes as a new tool in reproductive medicine. Reprod. Biol. 2020, 20, 447–459. [Google Scholar] [CrossRef]
- Rodriguez-Caro, H.; Dragovic, R.; Shen, M.; Dombi, E.; Mounce, G.; Field, K.; Meadows, J.; Turner, K.; Lunn, D.; Child, T.; et al. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565262. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, X.; Zhang, Q.; Guo, S.W. Platelets induce increased estrogen production through NF-κB and TGF-β1 signaling pathways in endometriotic stromal cells. Sci. Rep. 2020, 10, 1281. [Google Scholar] [CrossRef]
- Pourakbari, R.; Ahmadi, H.; Yousefi, M.; Aghebati-Maleki, L. Cell therapy in female infertility-related diseases: Emphasis on recurrent miscarriage and repeated implantation failure. Life Sci. 2020, 258, 118181. [Google Scholar] [CrossRef] [PubMed]
- Strunz, B.; Bister, J.; Jönsson, H.; Filipovic, I.E.; Sleiers, N.; Dumitrescu, B.; Brännström, M.; Lentini, A.; Reinius, B.; Brnillet, M.; et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 2021, 6, eabb7800. [Google Scholar] [CrossRef]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl. Oncol. 2020, 13, 100825. [Google Scholar] [CrossRef] [PubMed]
- Okoye, I.S.; Xu, L.; Walker, J.; Elahi, S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol. Immunother. 2020, 69, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Malinowski, B.; Puk, O.; Wartęga, M.; Stankiewicz, M.; Kazdepka-Ziemińska, A.; Wiciński, M. The Role of NF-κB in Uterine Spiral Arteries Remodeling, Insight into the Cornerstone of Preeclampsia. Int. J. Mol. Sci. 2021, 22, 704. [Google Scholar] [CrossRef]
- Yang, J.H.; Chen, C.D.; Wu, M.Y.; Chao, K.H.; Yang, Y.S.; Ho, H.N. Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T-cell subpopulation or interferon-gamma production in postmenopausal women. Fertil. Steril. 2000, 74, 261–267. [Google Scholar] [CrossRef]
- Waiyaput, W.; Wattanakamolchai, K.; Tingthanatikul, Y.; Lertvikool, S.; Tantanavipas, S.; Dittharot, K.; Sroyraya, M.; Sophonsritsuk, A. Effect of combined contraceptive pill on immune cell of ovarian endometriotic tissue. J. Ovarian. Res. 2021, 14, 66. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Shi, J.W.; Shen, H.H.; Wang, Y.; Chang, K.K.; Zhang, T.; Ye, J.F.; Sun, J.S.; Qiu, X.M.; et al. Protopanaxadiol improves endometriosis associated infertility and miscarriage in sex hormones receptors-dependent and independent manners. Int. J. Biol. Sci. 2021, 17, 1878–1894. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Sun, Y.; Tao, Y.; Piao, H.; Wang, X.; Luan, X.; Du, M.; Li, D. Involvement of the JAK-STAT pathway in collagen regulation of decidual NK cells. Am. J. Reprod. Immunol. 2017, 78, 119. [Google Scholar] [CrossRef] [PubMed]
- Vian, L.; Le, M.T.; Gazaniga, N.; Kieltyka, J.; Liu, C.; Pietropaolo, G.; Dell’Orso, S.; Brooks, S.R.; Furumoto, Y.; Thomas, C.J.; et al. JAK Inhibition Differentially Affects NK Cell and ILC1 Homeostasis. Front. Immunol. 2019, 10, 2972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wu, P.; Chen, D.; Ning, F.; Lu, Q.; Qiu, X.; Hewison, M.; Tamblyn, J.A.; Kilby, M.D.; Lash, G.E. Vitamin D Promotes Trophoblast Cell Induced Separation of Vascular Smooth Muscle Cells in Vascular Remodeling via Induction of G-CSF. Front. Cell. Dev. Biol. 2020, 8, 601043. [Google Scholar] [CrossRef] [PubMed]
| Type of NK Cell | Markers | Cytokine Response |
|---|---|---|
| Circulating tolerogenic | CD16low CD56bright CD94bright, NKG2A(C or E)/CD94, CD161. | IL10, TGFβ |
| Circulating cytotoxic | CD16bright CD56dim, CD57+, CD94dim, NKG2D/CD94, NKp30+, NKp46+, CD62L med | IFNγ |
| cNK | CD16high CD56dim, CD49a−, DX5+, NKG2D/CD94, NKp30+, NKp46+, CD62Lmed | IFNγ |
| uNK | CD56bright, CD16low, NKG2A/CD94, CD49a+, NKp46+, integrin β7+, CD117+, DX5− | |
| uNK1 | CD49ahigh, CD103high, CD69+ | IFNγ |
| uNK2 | CD49ahigh, CD103med, CD69+, KIRhigh inhibitory | TGFβ/VEGF |
| uNK3 | CD56high CD49a+, KIRhigh inhibitory, CD69med | IL-4 |
| dNK | CD56high CD16low, CD94high, NKp46+ | |
| dNK 1 | CD103low, CD9+, CD39+, CD69low, CYP26A1, B4GALNT1+, Jag1+, TIM3high, KIR2DL1+, KIR2DL2+, KIR2DL3+, KIR2DS1+, KIR2DS4+, LIRB1+, NKG2A+, NKG2C+, NKG2E+ | IFNγ, TGFβ |
| dNK 2 | CD103high, CD9low, CD69high, Jag1+, CD83+, KIRhigh inhibitory, ANXA1+, ITGB2+, TGIT+, TIM3low, NKG2A+, NKG2C+, NKG2E+ | TGFβ/VEGF |
| dNK 3 | CD103low, KIRhigh inhibitory, ITGβ7+, CD74+, CD160+, KLRB1+, ITGB2+, TGIT+ | TGFβ/IL-4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia, J.V.; De Sanctis, J.B. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno 2021, 1, 174-193. https://doi.org/10.3390/immuno1030011
Garmendia JV, De Sanctis JB. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno. 2021; 1(3):174-193. https://doi.org/10.3390/immuno1030011
Chicago/Turabian StyleGarmendia, Jenny Valentina, and Juan Bautista De Sanctis. 2021. "A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation" Immuno 1, no. 3: 174-193. https://doi.org/10.3390/immuno1030011
APA StyleGarmendia, J. V., & De Sanctis, J. B. (2021). A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno, 1(3), 174-193. https://doi.org/10.3390/immuno1030011







