Anxiety Disorder: Measuring the Impact on Major Depressive Disorder
Abstract
1. Introduction
2. Methods
2.1. Population Data
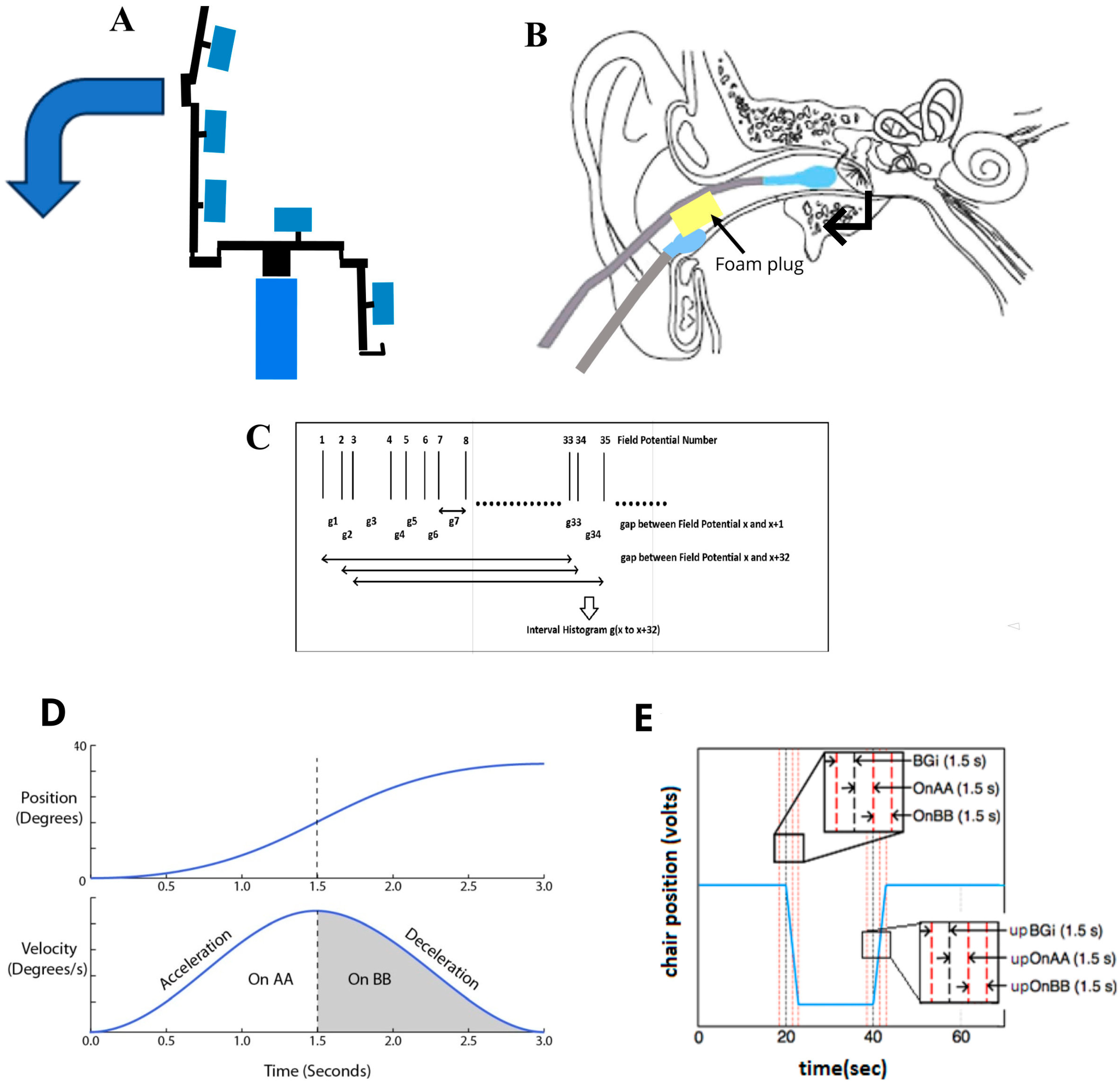
2.2. EVestG Recording and Feature Extraction
2.3. IH33 Curve Derivation
2.4. Data Analysis: Statistical Tests
3. Results
3.1. Feature Selection
3.2. Statistical Analysis
3.3. Classification
3.4. Medication
3.5. Depressive Severity
3.6. Anxiety Definition
4. Discussion
4.1. GABA in MDD
4.2. Hippocampal Theta
4.3. BD Versus MDD Anxiety
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fava, M.; Rankin, M.A.; Wright, E.C.; Alpert, J.E.; Nierenberg, A.A.; Pava, J.; Rosenbaum, J.F. Anxiety disorders in major depression. Compr. Psychiatry 2000, 41, 97–102. [Google Scholar] [CrossRef]
- Balaban, C.D.; Jacob, R.G.; Furman, J.M. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications. Expert. Rev. Neurother. 2011, 11, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, B.J.; Moussavi, Z. Measuring anxiety disorder in bipolar disorder using EVestG: Broad impact of medication groups. Front. Neurol. 2024, 14, 1303287. [Google Scholar] [CrossRef]
- McNaughton, N. What do you mean ‘anxiety’? Developing the first anxiety syndrome biomarker. J. R. Soc. N. Z. 2018, 48, 177–190. [Google Scholar] [CrossRef]
- Bosecke, C.; Ng, M.; Dastgheib, Z.; Lithgow, B.J. Perspective: Hippocampal theta rhythm as a potential vestibuloacoustic biomarker of anxiety. Eur. J. Neurosci. 2024, 61, e16641. [Google Scholar] [CrossRef]
- Lithgow, B.J.; Garrett, A.L.; Moussavi, Z.M.; Gurvich, C.; Kulkarni, J.; Maller, J.J.; Fitzgerald, P.B. Major Depression and Electrovestibulography. World J. Biol. Psychiatry 2015, 16, 334–350. [Google Scholar] [CrossRef]
- Lithgow, B.J.; Moussavi, Z.; Fitzgerald, P.B. Quantitative separation of the depressive phase of Bipolar Disorder and Major Depressive Disorder using Electrovestibulography. World J. Biol. Psychiatry 2019, 20, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, B.J.; Moussavi, Z.; Gurvich, C.; Maller, J.J.; Kulkarni, J.; Fitzgerald, P.B. Bipolar Disorder in the Balance. Europ. Arch. Psychiatry Clin. Neurosci. 2018, 269, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, B.J. A methodology for detecting field potentials from the external ear canal: NEER and EVestG. Ann. BME 2012, 40, 1835–1850. [Google Scholar] [CrossRef]
- Blakley, B.; Ashiri, M.; Moussavi, Z.; Lithgow, B.J. Verification EVestG recordings are Vestibuloacoustic signals. Laryngoscope Investig. Otolaryngol. 2022, 7, 1171–1177. [Google Scholar] [CrossRef]
- Moses, K.; Gayed, M.; Chuah, S.; Wootton, B.M. The Use of Evidence-Based Assessment for Anxiety Disorders in an Australian Sample. J. Anxiety Disord. 2020, 75, 102279. [Google Scholar] [CrossRef]
- Thomas, C.L.; Cassady, J.C. Validation of the State Version of the State-Trait Anxiety Inventory in a University Sample. SAGE Open 2021, 11, 21582440211031900. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press, Inc.: Palo Alto, CA, USA, 1983. [Google Scholar]
- Goldbloom, D.S.; Davine, J. Psychiatry in Primary Care: A Concise Canadian Pocket Guide, 2nd ed.; Davine, D.S.G.A.J., Ed.; CAMH: Toronto, ON, Canada, 2019; Volume 1, p. 546. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 20–57. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psych. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Austin, M.P.; Mitchell, P. Melancholia as a Neurological Disorder. In Melancholia: A Disorder of Movement and Mood; Parker, G., Hadzi-Pavloic, D., Eds.; Cambridge University Press: New York, NY, USA, 1996; pp. 223–236. [Google Scholar]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Heibert, D. Computer Models of the Vestibular Head Tilt Response, and Their Relationship to EVestG and Meniere’s Disease. Ph.D. Thesis, Monash University, Melbourne, Australia, 2010. [Google Scholar]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Gangemi, A.; Suriano, R.; Fabio, R.A. Longitudinal Exploration of Cortical Brain Activity in Cognitive Fog: An EEG Study in Patients with and without Anosmia. J. Integr. Neurosci. 2024, 23, 105. [Google Scholar] [CrossRef]
- Koller-Schlaud, K.; Ströhle, A.; Bärwolf, E.; Behr, J.; Rentzsch, J. EEG Frontal Asymmetry and Theta Power in Unipolar and Bipolar Depression. J. Affect. Disord. 2020, 276, 501–510. [Google Scholar] [CrossRef]
- Soza, A.; Barroilhet, S.; Vohringer, P. A vestibular biomarker of manic and depressive phase in bipolar disorder. Asia Pac. J. Clin. Trials Nerv. Syst. Dis. 2017, 2, 140. Available online: https://www.researchgate.net/publication/320731620 (accessed on 31 July 2025). [CrossRef]
- Soza Ried, A.M.; Aviles, M. Asymmetries of vestibular dysfunction in major depression. Neuroscience 2007, 144, 128–134. [Google Scholar] [CrossRef]
- Sachs, G.; Anderer, P.; Dantendorfer, K.; Saletu, B. EEG mapping in patients with social phobia. Psychiatry Res. 2004, 131, 237–247. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Hassan, M. Prediction of Depression Severity Scores Based on Functional Connectivity and Complexity of the EEG Signal. Clin. EEG Neurosci. 2021, 52, 52–60. [Google Scholar] [CrossRef]
- DeBattista, C.; Palmer, D.M.; Fitzgerald, P.B.; Harris, A.; deBeuss, R.; Gordon, E. Frontal and rostral anterior cingulate (rACC) theta EEG in depression: Implications for treatment outcome? Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 1190–1200. [Google Scholar] [CrossRef]
- Shao, F.; Fang, J.; Wang, S.; Qiu, M.; Xi, D.; Jin, X.; Liu, J.; Shao, X.; Shen, Z.; Liang, Y.; et al. Anxiolytic effect of GABAergic neurons in the anterior cingulate cortex in a rat model of chronic inflammatory pain. Mol. Brain 2021, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Luscher, B.E.; Shen, Q.; Sahir, N. The GABAergic Deficit Hypothesis of Major Depressive Disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Kuikka, J.; Rasanen, P.; Lepola, U.; Koponen, H.; Liuska, A.; Lehmusvaara, A.; Vainio, P.; Könönen, M.; Bergström, K.; et al. Cerebral benzodiazepine receptor binding and distribution in generalized anxiety disorder: A fractal analysis. Mol. Psychiatry 1997, 2, 463–471. [Google Scholar] [CrossRef]
- Bremner, J.D.; Innis, R.B.; Southwick, S.M.; Staib, L.; Zoghbi, S.; Charney, D.S. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am. J. Psychiatry 2000, 157, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, Y.; Sadeghi, S.G. Activation of GABA B receptors results in excitatory modulation of calyx terminals in rat semicircular canal cristae. J. Neurophysiol. 2020, 124, 962–972. [Google Scholar] [CrossRef]
- Jin, G.S.; Li, X.L.; Jin, Y.Z.; Kim, M.S.; Park, B.R. Role of peripheral vestibular receptors in the control of blood pressure following hypotension. Korean J. Physiol. Pharmacol. 2018, 22, 363–368. [Google Scholar] [CrossRef]
- Fergus, A.; Lee, K.S. GABAergic Regulation of Cerebral Microvascular Tone in the Rat. J. Cereb. Blood Flow. Metab. 1997, 17, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Kerman, I.A.; Thompson, R.C.; Jones, E.G.; Bunney, W.E.; Barchas, J.D.; Schatzberg, A.F.; Myers, R.M.; Akil, H.; Watson, S.J. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol. Psychiatry 2011, 16, 634–646. [Google Scholar] [CrossRef]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E., Jr.; Akil, H.; Watson, S.J.; et al. Altered cortical glutamatergic and GABA ergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653–15658. [Google Scholar] [CrossRef]
- Kocsis, B.; Vertes, R.P. Dorsal raphe neurons: Synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J. Neurophysiol. 1992, 68, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Balaban, C.D. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience 2006, 140, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Cuccurazzu, B.; Halberstadt, A.L. Projections from the vestibular nuclei and nucleus prepositus hypoglossi to dorsal raphe nucleus in rats. Neurosci. Lett. 2008, 439, 70–74. [Google Scholar] [CrossRef][Green Version]
- Plotnik, M.; Marlinski, V.; Goldberg, J.M. Efferent-mediated fluctuations in vestibular nerve discharge: A novel, positive-feedback mechanism of efferent control. J. Assoc. Res. Otolaryngol. 2005, 6, 311–323. [Google Scholar] [CrossRef][Green Version]
- Tai, S.K.; Ma, J.; Ossenkopp, K.; Leung, L.S. Activation of immobility-related hippocampal theta by cholinergic septohippocampal neurons during vestibular stimulation. Hippocampus 2012, 22, 914–925. [Google Scholar] [CrossRef]
| Diagnosis | Age | Years Since Diagnosis | MMSE Total | MADRS | CORE |
|---|---|---|---|---|---|
| MDD-R Asymptomatic or Mild n = 22 (10 males), 10 with anxiety disorder, MADRS ≤ 19 | 50.6 ± 13.3 | 15.8 ± 11.1 | 28.8 ± 1.3 | 12.3 ± 4.4 | 5.3 ± 4.3 |
| MDD-S, Moderate–Severe n = 20 (8 males), 16 with anxiety disorder, MADRS ≥ 20 | 44.1 ± 11.1 | 16.2 ± 6.7 | 29.1 ± 1.3 | 27.7 ± 6.3 | 9.4 ± 7.0 |
| MDD (MDD-R and MDD-S) n = 42 (18 males), 26 with anxiety disorder | 47.2 ± 12.7 | 16.0 ± 9.2 | 29.0 ± 1.3 | 20.4 ± 9.4 | 7.4 ± 6.4 |
| Definition | |
|---|---|
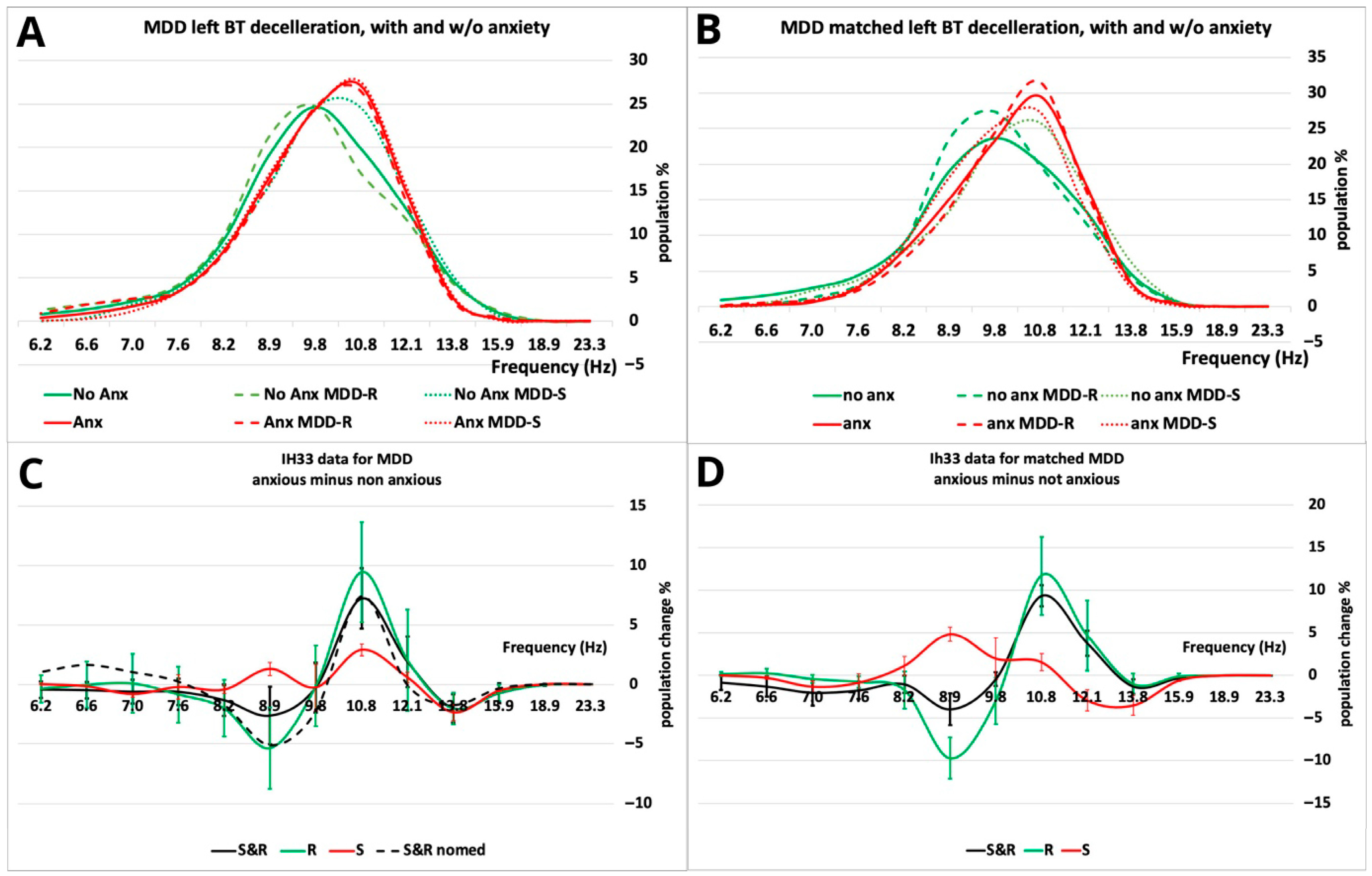
| F1 = BT left deceleration | Value of the 10.8 Hz bin during the deceleration phase of the backwards (BT, pitch) tilt (see Figure 2D). |
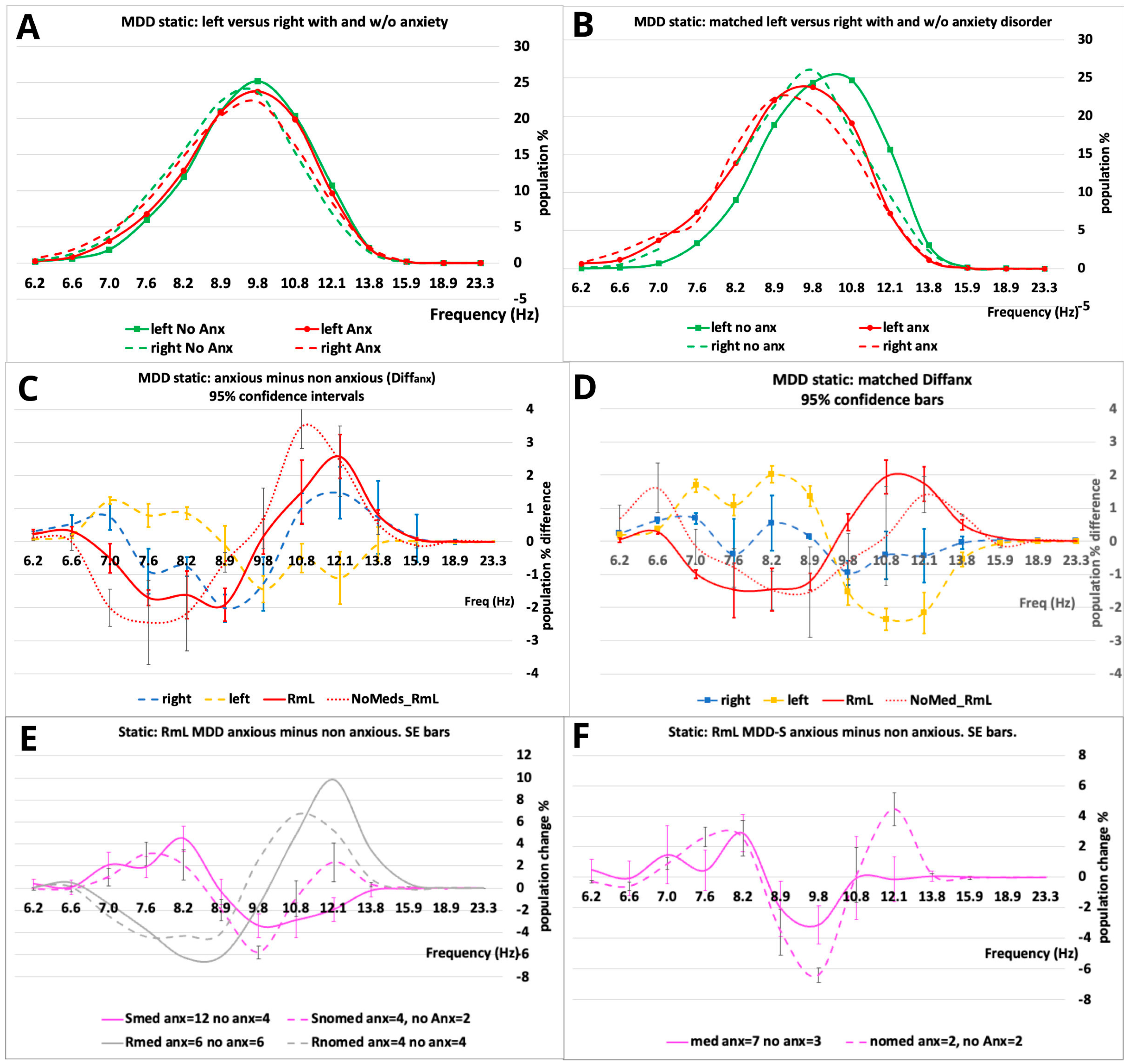
| F2 = RmL S non-medicated population static. | A total of 12.1 Hz minus 9.8 Hz bins (see Figure 3F). This feature emphasizes the large changes between the matched non-medicated and medicated S matched populations. |
| F3 = BT left deceleration S population. | A total of 10.8 Hz minus 13.1 Hz bin plus/minus the 8.9 Hz bin (S/R). Selected based on the main peaks/troughs in Figure 2D. |
| A. ROC | |||||||||
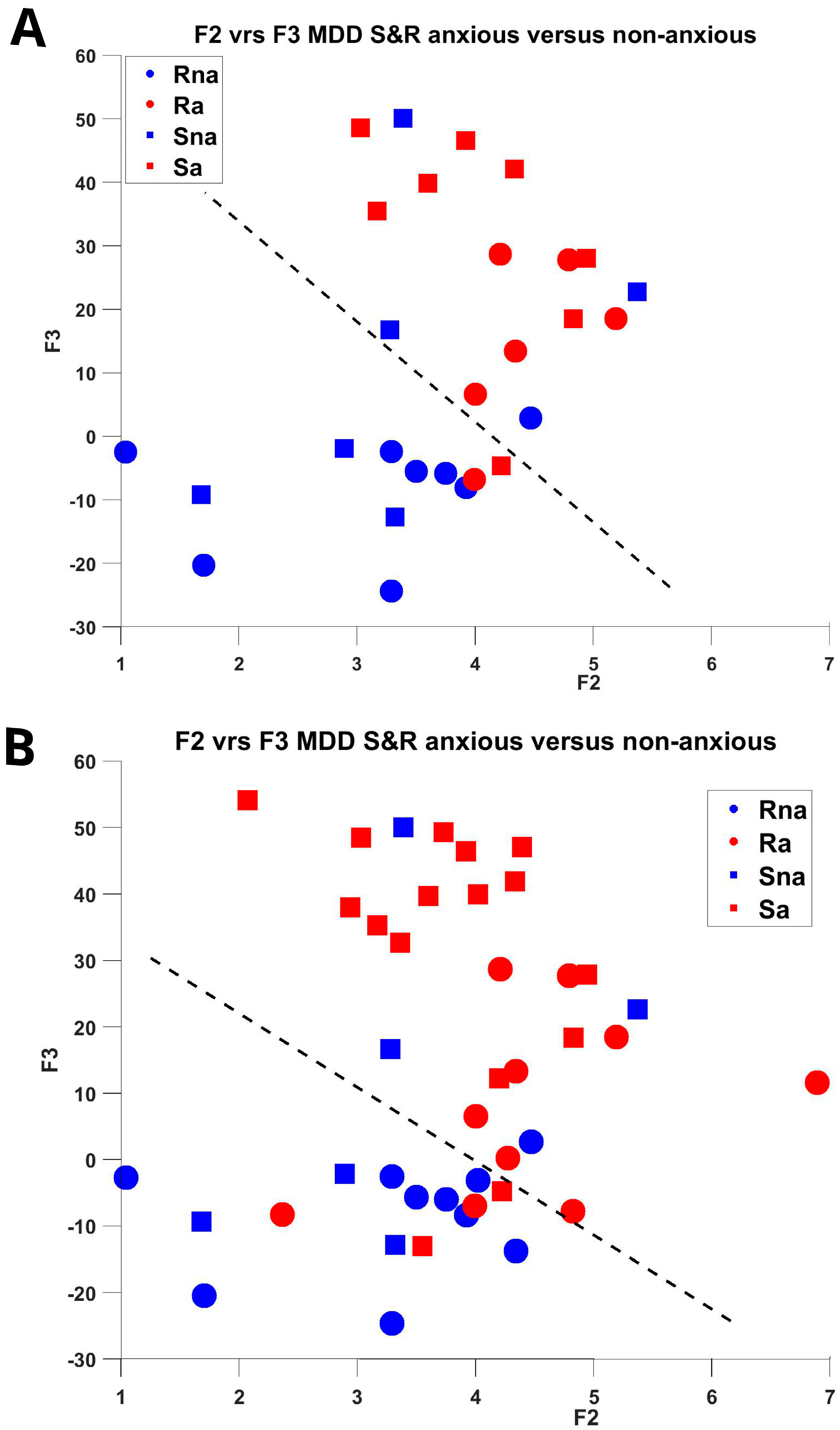
| (a = anxious, na = non-anxious) | F1 | F2 | F3 | ||||||
| S&R All (26,16), matched (14,14) | 0.74, 0.79 | 0.69, 0.79 | 0.73, 0.77 | ||||||
| R All (10,10), matched (6,8) | 0.77, 0.92 | 0.80, 0.92 | 0.81, 0.90 | ||||||
| S All (16,6), matched (8,6) | 0.59, 0.63 | 0.69, 0.71 | 0.64, 0.75 | ||||||
| B. Feature Significance (Matched populations) | |||||||||
| Feature | Quade ANCOVA | ||||||||
| F1: S&R matched (14,14) | F(1,26) = 6.52 | p = 0.017 | η2 = −0.20 | power = 0.691 | |||||
| R matched (6,8) | F(1,12) = 5.77 | 0.033 | 0.33 | 0.60 | |||||
| S matched (8,6) | F(1,12) = 0.76 | 0.399 | 0.06 | 0.13 | |||||
| F2: S&R matched (14,14) | F(1,26) = 6.72 | 0.008 | 0.24 | 0.78 | |||||
| R matched (6,8) | F(1,12) = 5.71 | 0.034 | 0.33 | 0.59 | |||||
| S matched (8,6) | F(1,12) = 3.65 | 0.080 | 0.23 | 0.42 | |||||
| F3: S&R matched (14,14) | F(1,26) = 10.15 | 0.004 | 0.28 | 0.87 | |||||
| R matched (6,8) | F(1,12) = 5.50 | 0.037 | 0.31 | 0.58 | |||||
| S matched (8,6) | F(1,12) = 1.710 | 0.215 | 0.13 | 0.23 | |||||
| Covariates: Age, Gender, MADRS | |||||||||
| C. Kernal Density Estimate of Distribution and Naïve Bayes Classifier | |||||||||
| All, Accuracy (%) | Matched, Accuracy (%) | ||||||||
| S&R (42) | R (20) | S (22) | S&R (28) | R (14) | S (14) | ||||
| F1 | 71.4 | 60.0 | 36.4 | 75.0 | 85.7 | 50.0 | |||
| F2 | 66.7 | 75.0 | 68.2 | 75.0 | 78.5 | 71.4 | |||
| F3 | 76.2 | 75.0 | 72.7 | 78.6 | 85.7 | 71.4 | |||
| F1, F2 | 69.0 | 80.0 | 59.1 | 78.6 | 85.7 | 64.3 | |||
| F2, F3 | 73.8 | 80.0 | 77.3 | 78.6 | 85.7 | 78.5 | |||
| F1, F3 | 69.0 | 75.0 | 72.7 | 78.6 | 92.9 | 64.3 | |||
| F1, F2, F3 | 71.4 | 75.0 | 72.7 | 78.6 | 85.7 | 71.4 | |||
| Accuracy = 1 − cross-validated error. Leave-one-out cross-validation was applied. | |||||||||
| F1 | F2 | F3 | MADRS | |
|---|---|---|---|---|
| F1 | 1 | 0.35 | 0.76 ** | 0.17 |
| F2 | 1 | 0.32 | −0.07 | |
| F3 | 1 | 0.50 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lithgow, B.J.; Garrett, A.; Moussavi, Z. Anxiety Disorder: Measuring the Impact on Major Depressive Disorder. Psychiatry Int. 2025, 6, 94. https://doi.org/10.3390/psychiatryint6030094
Lithgow BJ, Garrett A, Moussavi Z. Anxiety Disorder: Measuring the Impact on Major Depressive Disorder. Psychiatry International. 2025; 6(3):94. https://doi.org/10.3390/psychiatryint6030094
Chicago/Turabian StyleLithgow, Brian J., Amber Garrett, and Zahra Moussavi. 2025. "Anxiety Disorder: Measuring the Impact on Major Depressive Disorder" Psychiatry International 6, no. 3: 94. https://doi.org/10.3390/psychiatryint6030094
APA StyleLithgow, B. J., Garrett, A., & Moussavi, Z. (2025). Anxiety Disorder: Measuring the Impact on Major Depressive Disorder. Psychiatry International, 6(3), 94. https://doi.org/10.3390/psychiatryint6030094







