Biological Rhythms and Psychosocial Functioning in Depression: An Exploratory Analysis Informed by a Mediation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Participants
2.2. Inclusion Criteria
- A diagnosis of moderate to severe Major Depressive Disorder (MDD) according to DSM-5 criteria, with a Hamilton Depression Rating Scale (HAM-D) score > 17.
- Age between 18 and 65 years.
- Provision of written informed consent.
2.3. Exclusion Criteria
- A history of intellectual disability or any condition that could significantly impair cognitive performance.
- Comorbidity with a psychotic disorder.
- Electroconvulsive therapy (ECT) within the 12 months prior to the neuropsychological assessment.
2.4. Neuropsychological Assessment
2.5. Affective Domain
- Beck Depression Inventory-Second Edition (BDI-II), Italian Version [21]: It is a 21-item self-administered instrument to detect the severity of depression in adults and adolescents from age 13 onward. Scores 0–13 indicate no depressive content; scores 14–19: mild depression; scores 20–29: moderate depression; scores 30–63: severe depression. The Italian validation data confirm the existence of two sides of depression, the mental and the somatic, as in the original edition. The internal consistency calculated through Cronbach’s alpha results in 0.86 for the first factor and 0.65 for the second factor.
2.6. Psychosocial Domain
- Functioning Assessment Short Test (FAST) [22]: It was used as a primary outcome of psychosocial risk at the study endpoints to identify predictors for specific domains of function, such as autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships, and leisure. The higher the score, the worse the patient’s functional impairment. The cut-off scores for the FAST scale derived from this equation were as follows: scores from 0 to 11 included patients with no impairment. Scores from 12 to 20 represented the category of mild impairment. Moderate impairment comprised scores from 21 to 40. Finally, scores above 40 represented severe functional impairment [23]. Cronbach’s alpha for the five components was 0.96, 0.88, 0.88, 0.91, 0.92, respectively, and for the total was 0.93.
2.7. Sleep Domain
- Pittsburgh Sleep Quality Index (PSQI) [24] is a self-assessment scale about sleep quality that can be easily compiled by the subject. It consists of 19 items that can be summarized into seven evaluation domains: (1) subjective quality of sleep, (2) sleep latency, (3) duration of sleep, (4) usual efficiency of sleep, (5) sleep disorders, (6) drugs used for sleep, and (7) daytime malfunction. A PSQI score >5 highlights sleep quality problems.
- Biological Rhythms Interview of Assessment in Neuropsychiatry (BRIAN) [25] is a self-report questionnaire consisting of 21 items, referring to the 15 days immediately preceding completion. The subject is asked to report how often he or she experienced sleep disturbances at different times during that time period. The four domains considered, which are related to circadian rhythm disorders, are (1) sleep, (2) general activity, (3) social rhythms, and (4) nutrition. The fifth scope (items 19–21), not included in the total score, involves the evaluation of the subject’s chronotype. Each of these domains represents a potential factor in the onset and worsening of affective states, psychosocial functioning, and clinical functioning. Each item is evaluated on a 4-point Likert scale (from “no difficulty” to “severe difficulty”) the sum of the final score (18 to 72), where the highest intervals indicate a more serious subjective impairment of the circadian rhythm. The Italian clinical mean score is 22.22 (SD = 11.19) [25]. In this sense, the BRIAN scale offers a rapid self-reported measurement of the biological rhythm dysregulation in individuals with depression.
2.8. Data Analysis
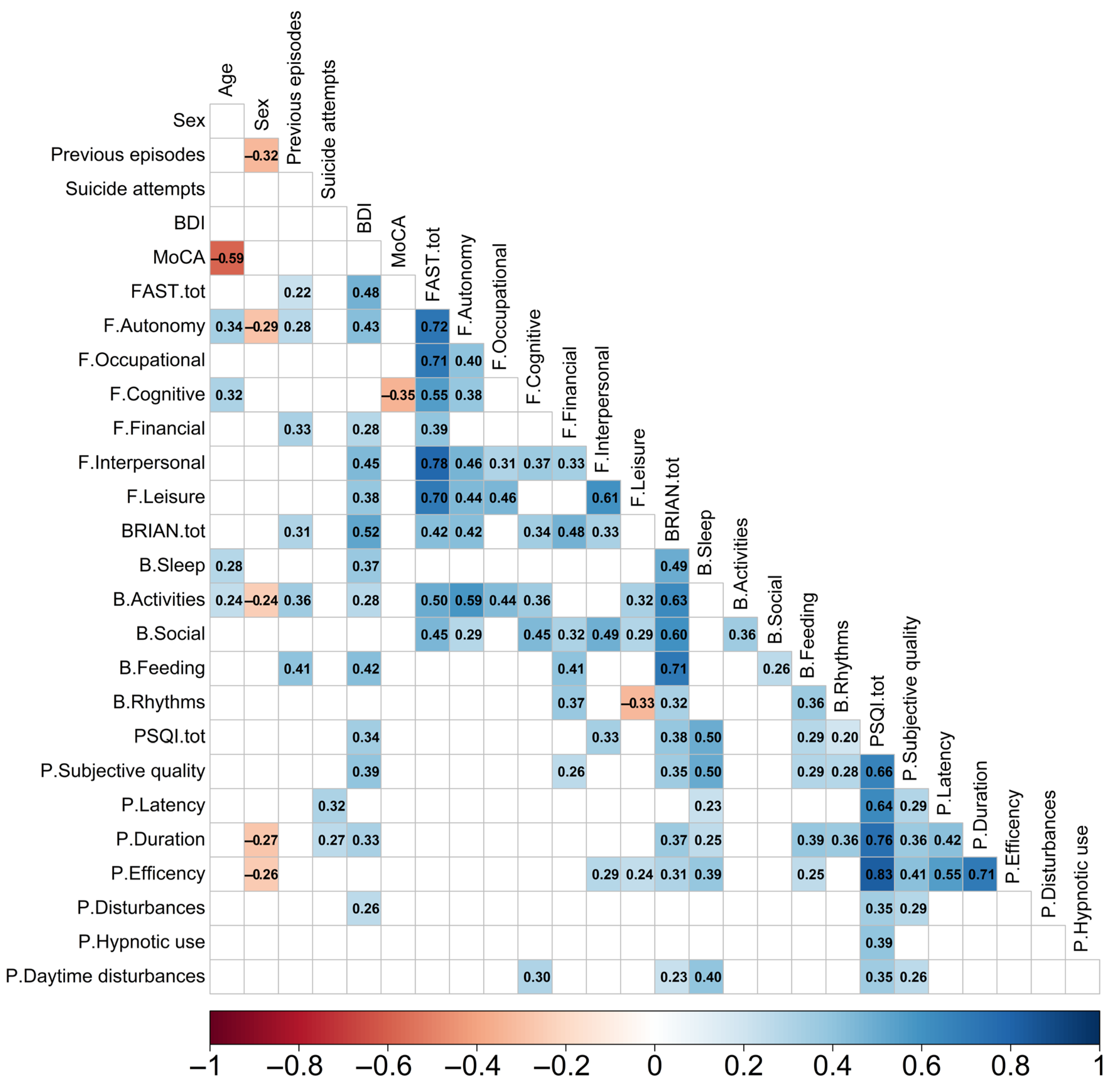
3. Results
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. DSM-5-TR—Manuale Diagnostico e Statistico dei Disturbi Mentali; Cortina, R., Ed.; American Psychiatric Association: Washington, DC, USA, 2023; ISBN 978-88-3285-517-3. [Google Scholar]
- Platania, G.A.; Varrasi, S.; Castellano, S.; Godos, J.; Pirrone, C.; Petralia, M.C.; Cantarella, R.A.; Tascedda, F.; Guerrera, C.S.; Buono, S.; et al. Biological and Neuropsychological Markers of Cognitive Dysfunction in Unipolar vs Bipolar Depression: What Evidence Do We Have? Life Span Disabil. 2020, 23, 239–281. [Google Scholar]
- IHME—GHDx Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx)—MDD. Available online: https://vizhub.healthdata.org/gbd-results (accessed on 26 March 2025).
- Bitter, I.; Szekeres, G.; Cai, Q.; Feher, L.; Gimesi-Orszagh, J.; Kunovszki, P.; El Khoury, A.C.; Dome, P.; Rihmer, Z. Mortality in Patients with Major Depressive Disorder: A Nationwide Population-Based Cohort Study with 11-Year Follow-Up. Eur. Psychiatry 2024, 67, e63. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafò, A.; Torrisi, S.A.; Leggio, G.M.; et al. Neurobiological Links between Depression and AD: The Role of TGF-Β1 Signaling as a New Pharmacological Target. Pharmacol. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef]
- Krittanawong, C.; Maitra, N.S.; Qadeer, Y.K.; Wang, Z.; Fogg, S.; Storch, E.A.; Celano, C.M.; Huffman, J.C.; Jha, M.; Charney, D.S.; et al. Association of Depression and Cardiovascular Disease. Am. J. Med. 2023, 136, 881–895. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Y.; Qiao, D.; Wen, Y.; Zhao, T.; Wang, X.; Liang, T.; Li, X.; Liu, Z. Abnormal Sleep Features in Adolescent MDD and Its Potential in Diagnosis and Prediction of Early Efficacy. Sleep Med. 2023, 106, 116–122. [Google Scholar] [CrossRef]
- Tonon, A.C.; Constantino, D.B.; Amando, G.R.; Abreu, A.C.; Francisco, A.P.; de Oliveira, M.A.B.; Pilz, L.K.; Xavier, N.B.; Rohrsetzer, F.; Souza, L.; et al. Sleep Disturbances, Circadian Activity, and Nocturnal Light Exposure Characterize High Risk for and Current Depression in Adolescence. Sleep 2022, 45, zsac104. [Google Scholar] [CrossRef]
- Guerrera, C.S.; Boccaccio, F.M.; Varrasi, S.; Platania, G.A.; Coco, M.; Pirrone, C.; Castellano, S.; Caraci, F.; Ferri, R.; Lanza, G. A Narrative Review on Insomnia and Hypersomnolence within Major Depressive Disorder and Bipolar Disorder: A Proposal for a Novel Psychometric Protocol. Neurosci. Biobehav. Rev. 2024, 158, 105575. [Google Scholar] [CrossRef]
- Hutka, P.; Krivosova, M.; Muchova, Z.; Tonhajzerova, I.; Hamrakova, A.; Mlyncekova, Z.; Mokry, J.; Ondrejka, I. Association of Sleep Architecture and Physiology with Depressive Disorder and Antidepressants Treatment. Int. J. Mol. Sci. 2021, 22, 1333. [Google Scholar] [CrossRef]
- Solelhac, G.; Imler, T.; Strippoli, M.-P.F.; Marchi, N.A.; Berger, M.; Haba-Rubio, J.; Raffray, T.; Bayon, V.; Lombardi, A.S.; Ranjbar, S.; et al. Sleep Disturbances and Incident Risk of Major Depressive Disorder in a Population-Based Cohort. Psychiatry Res. 2024, 338, 115934. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Ma, Y.; Du, L.-T.; Wang, K.; Li, Z.; Zhu, W.; Sun, Y.-H.; Lu, L.; Bao, Y.-P.; Li, S.-X. Sleep Disorders and Non-Sleep Circadian Disorders Predict Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Neurosci. Biobehav. Rev. 2022, 134, 104532. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, Insomnia and Mental Health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Monti, J.M.; Burman, D.; Karthikeyan, R.; BaHammam, A.S.; Spence, D.W.; Brown, G.M.; Narashimhan, M. Clarifying the Role of Sleep in Depression: A Narrative Review. Psychiatry Res. 2020, 291, 113239. [Google Scholar] [CrossRef] [PubMed]
- Meaklim, H.; Saunders, W.J.; Byrne, M.L.; Junge, M.F.; Varma, P.; Finck, W.A.; Jackson, M.L. Insomnia Is a Key Risk Factor for Persistent Anxiety and Depressive Symptoms: A 12-Month Longitudinal Cohort Study during the COVID-19 Pandemic. J. Affect. Disord. 2023, 322, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, M.; Ding, L.; Huang, J.; Wang, Y.; Su, Y.; Chen, Z.; Cai, Y.; He, S.; Peng, D. Relationship between Biological Rhythm Dysregulation and Suicidal Ideation in Patients with Major Depressive Disorder. BMC Psychiatry 2024, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, M.; Sahbaz, C. Clinical Evaluation of Biological Rhythm Domains in Patients with Major Depression. Rev. Bras. Psiquiatr. Sao Paulo Braz. 1999 2020, 42, 258–263. [Google Scholar] [CrossRef]
- Song, Y.M.; Jeong, J.; Reyes, A.A.d.l.; Lim, D.; Cho, C.-H.; Yeom, J.W.; Lee, T.; Lee, J.-B.; Lee, H.-J.; Kim, J.K. Causal Dynamics of Sleep, Circadian Rhythm, and Mood Symptoms in Patients with Major Depression and Bipolar Disorder: Insights from Longitudinal Wearable Device Data. eBioMedicine 2024, 103, 105094. [Google Scholar] [CrossRef]
- Baltacioğlu, M.; Puşuroğlu, M. Investigation of the Relationship between Biological Rhythm Pattern and Eating Attitude in Patients Diagnosed with Bipolar Disorder. J. Affect. Disord. 2025, 379, 136–142. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics 2025, 15, 327. [Google Scholar] [CrossRef]
- Sica, C.; Ghisi, M. The Italian Versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric Properties and Discriminant Power. In Leading-Edge Psychological Tests and Testing Research; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Barbato, A.; Bossini, L.; Calugi, S.; D’Avanzo, B.; Fagiolini, A.; Koukouna, D.; Parabiaghi, A.; Rapisarda, F.; Rucci, P.; Vallarino, M. Validation of the Italian Version of the Functioning Assessment Short Test (FAST) for Bipolar Disorder. Epidemiol. Psychiatr. Sci. 2013, 22, 187–194. [Google Scholar] [CrossRef]
- Bonnín, C.M.; Martínez-Arán, A.; Reinares, M.; Valentí, M.; Solé, B.; Jiménez, E.; Montejo, L.; Vieta, E.; Rosa, A.R. Thresholds for Severity, Remission and Recovery Using the Functioning Assessment Short Test (FAST) in Bipolar Disorder. J. Affect. Disord. 2018, 240, 57–62. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Moro, M.F.; Carta, M.G.; Pintus, M.; Pintus, E.; Melis, R.; Kapczinski, F.; Vieta, E.; Colom, F. Validation of the Italian Version of the Biological Rhythms Interview of Assessment in Neuropsychiatry (BRIAN): Some Considerations on Its Screening Usefulness. Clin. Pract. Epidemiol. Ment. Health CP EMH 2014, 10, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 7th ed.; Always Learning; Pearson: New York, NY, USA, 2019; ISBN 978-0-13-479054-1. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2025. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. CRAN: Contributed Packages. The R Foundation. 2014. Available online: https://github.com/tidyverse/dplyr (accessed on 10 April 2025).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. CRAN: Contributed Packages. The R Foundation. 2019. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 10 April 2025).
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix. CRAN: Contributed Packages. The R Foundation. 2010. Available online: https://github.com/taiyun/corrplot (accessed on 10 April 2025).
- Harrell, J.; Frank, E. Hmisc: Harrell Miscellaneous. CRAN: Contributed Packages. The R Foundation. 2003. Available online: https://hbiostat.org/R/Hmisc/ (accessed on 10 April 2025).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Van Den Brand, T. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. CRAN: Contributed Packages. The R Foundation. 2007. Available online: https://github.com/tidyverse/ggplot2 (accessed on 10 April 2025).
- Mangiafico, S.; Rcompanion: Functions to Support Extension Education Program Evaluation. CRAN: Contributed Packages. The R Foundation. 2016. Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 10 April 2025).
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K.; Trinh, M.; Wong, W. Mediation: Causal Mediation Analysis. CRAN: Contributed Packages. The R Foundation. 2009. Available online: https://imai.princeton.edu/projects/mechanisms.html (accessed on 10 April 2025).
- Baron, R.M.; Kenny, D.A. The Moderator–Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Hayes, A.F. Asymptotic and Resampling Strategies for Assessing and Comparing Indirect Effects in Multiple Mediator Models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Bailey, M.; Silver, R. Sex Differences in Circadian Timing Systems: Implications for Disease. Front. Neuroendocrinol. 2014, 35, 111–139. [Google Scholar] [CrossRef]
- Zeng, L.-N.; Zong, Q.-Q.; Yang, Y.; Zhang, L.; Xiang, Y.-F.; Ng, C.H.; Chen, L.-G.; Xiang, Y.-T. Gender Difference in the Prevalence of Insomnia: A Meta-Analysis of Observational Studies. Front. Psychiatry 2020, 11, 577429. [Google Scholar] [CrossRef]
- Iannone, R.; Roy, O. DiagrammeR: Graph/Network Visualization. CRAN: Contributed Packages. The R Foundation. 2015. Available online: https://github.com/rich-iannone/DiagrammeR (accessed on 10 April 2025).
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive Impairment in Depression: A Systematic Review and Meta-Analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Dollish, H.K.; Tsyglakova, M.; McClung, C.A. Circadian Rhythms and Mood Disorders: Time to See the Light. Neuron 2024, 112, 25–40. [Google Scholar] [CrossRef]
- Guerrera, C.S.; Platania, G.A.; Boccaccio, F.M.; Sarti, P.; Varrasi, S.; Colliva, C.; Grasso, M.; De Vivo, S.; Cavallaro, D.; Tascedda, F.; et al. The Dynamic Interaction between Symptoms and Pharmacological Treatment in Patients with Major Depressive Disorder: The Role of Network Intervention Analysis. BMC Psychiatry 2023, 23, 885. [Google Scholar] [CrossRef]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017, 2017, 1504507. [Google Scholar] [CrossRef] [PubMed]
- Coco, M.; Buscemi, A.; Guerrera, C.S.; Licitra, C.; Pennisi, E.; Vettor, V.; Rizzi, L.; Bovo, P.; Fecarotta, P.; Dell’Orco, S.; et al. Touch and Communication in the Institutionalized Elderly. In Proceedings of the 2019 10th IEEE International Conference on Cognitive Infocommunications (CogInfoCom), Naples, Italy, 23–25 October 2019; pp. 451–458. [Google Scholar] [CrossRef]
- Calatayud, E.; Marcén-Román, Y.; Rodríguez-Roca, B.; Salavera, C.; Gasch-Gallen, A.; Gómez-Soria, I. Sex Differences on Anxiety and Depression in Older Adults and Their Relationship with Cognitive Impairment. Med. Fam. SEMERGEN 2023, 49, 101923. [Google Scholar] [CrossRef] [PubMed]
- Rydberg Sterner, T.; Gudmundsson, P.; Sigström, R.; Ahlner, F.; Seidu, N.; Zettergren, A.; Kern, S.; Östling, S.; Waern, M.; Skoog, I. Depression and Neuroticism Decrease among Women but Not among Men between 1976 and 2016 in Swedish Septuagenarians. Acta Psychiatr. Scand. 2019, 139, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Sarti, P.; Colliva, C.; Varrasi, S.; Guerrera, C.S.; Platania, G.A.; Boccaccio, F.M.; Castellano, S.; Pirrone, C.; Pani, L.; Tascedda, F.; et al. A Network Study to Differentiate Suicide Attempt Risk Profiles in Male and Female Patients with Major Depressive Disorder. Clin. Psychol. Psychother. 2024, 31, e2924. [Google Scholar] [CrossRef]
- Christensen, M.C.; Grande, I.; Rieckmann, A.; Chokka, P. Efficacy of Vortioxetine versus Desvenlafaxine in the Treatment of Functional Impairment in Patients with Major Depressive Disorder: Results from the Multinational VIVRE Study. CNS Spectr. 2024, 29, 423–432. [Google Scholar] [CrossRef]
- Schwarz, R.; Miskowiak, K.W.; Kessing, L.V.; Vinberg, M. Clinical and Personal Predictors of Functioning in Affective Disorders: Exploratory Results from Baseline and 6-Month Follow-up of a Randomised Controlled Trial. J. Psychiatr. Res. 2024, 175, 386–392. [Google Scholar] [CrossRef]
- Vieira, I.S.; Ferrugem, S.C.R.; Reyes, A.N.; Branco, J.C.; Mondin, T.C.; Cardoso, T.D.A.; Kapczinski, F.; Souza, L.D.D.M.; Jansen, K.; Da Silva, R.A.; et al. Effects of Depression and Excess Body Weight on Cognition and Functioning in Young Adults: A Population-Based Study. J. Affect. Disord. 2021, 282, 401–406. [Google Scholar] [CrossRef]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; et al. Insomnia as a Predictor of Mental Disorders: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef]
- Park, E.-H.; Jung, M.H. The Impact of Major Depressive Disorder on Adaptive Function: A Retrospective Observational Study. Medicine 2019, 98, e18515. [Google Scholar] [CrossRef]
- Yang, H.; Gao, S.; Li, J.; Yu, H.; Xu, J.; Lin, C.; Yang, H.; Teng, C.; Ma, H.; Zhang, N. Remission of Symptoms Is Not Equal to Functional Recovery: Psychosocial Functioning Impairment in Major Depression. Front. Psychiatry 2022, 13, 915689. [Google Scholar] [CrossRef]
- De Leeuw, M.; Verhoeve, S.I.; Van Der Wee, N.J.A.; Van Hemert, A.M.; Vreugdenhil, E.; Coomans, C.P. The Role of the Circadian System in the Etiology of Depression. Neurosci. Biobehav. Rev. 2023, 153, 105383. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Benedetti, F. Perspectives in Affective Disorders: Clocks and Sleep. Eur. J. Neurosci. 2020, 51, 346–365. [Google Scholar] [CrossRef]
- Menculini, G.; Verdolini, N.; Brufani, F.; Pierotti, V.; Cirimbilli, F.; Di Buò, A.; Spollon, G.; De Giorgi, F.; Sciarma, T.; Tortorella, A.; et al. Comorbidities, Depression Severity, and Circadian Rhythms Disturbances as Clinical Correlates of Duration of Untreated Illness in Affective Disorders. Medicina 2021, 57, 459. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Chelminski, I.; Young, D.; Dalrymple, K.; Hrabosky, J.; Zimmerman, M. Severe Insomnia Is Associated with More Severe Presentation and Greater Functional Deficits in Depression. J. Psychiatr. Res. 2011, 45, 1101–1105. [Google Scholar] [CrossRef]
- Scott, M.R.; McClung, C.A. Circadian Rhythms in Mood Disorders. In Circadian Clock in Brain Health and Disease; Engmann, O., Brancaccio, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1344, pp. 153–168. ISBN 978-3-030-81146-4. [Google Scholar]
- Geoffroy, P.A.; Palagini, L. Biological Rhythms and Chronotherapeutics in Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110158. [Google Scholar] [CrossRef]
| Variable | Mean | SD | Shapiro–Wilk Value |
|---|---|---|---|
| Age | 45.75 | 13.32 | 0.94 * |
| Previous episodes | 3.02 | 1.15 | 0.80 *** |
| Suicide attempts | 0.38 | 0.80 | 0.54 *** |
| BDI-II | 34.33 | 10.20 | 0.97 |
| MoCA | 23.23 | 4.64 | 0.94 ** |
| FAST.tot | 36.57 | 14.66 | 0.98 |
| BRIAN.tot | 49.75 | 9.28 | 0.98 |
| PSQI.tot | 11.20 | 4.33 | 0.98 |
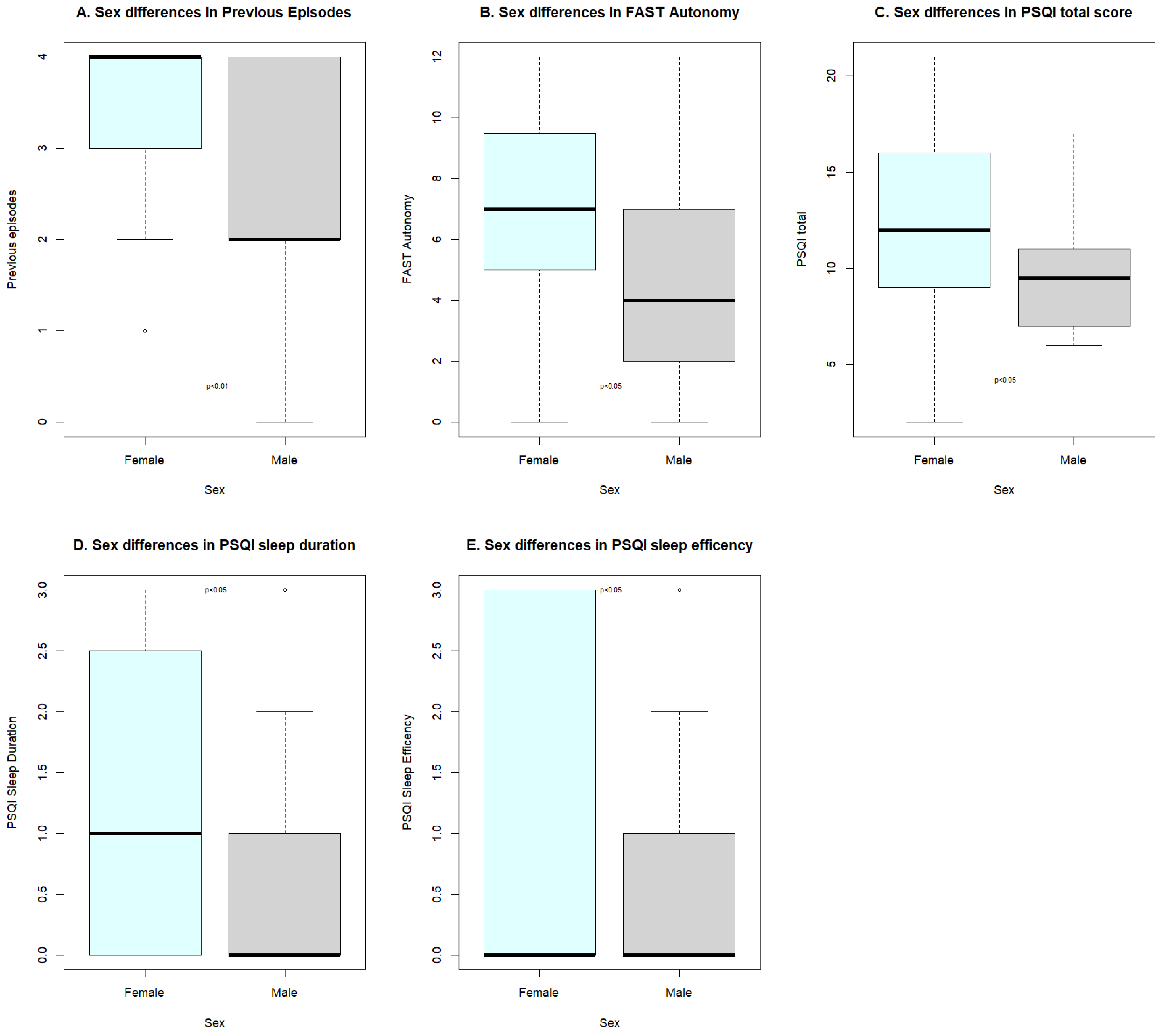
| Variable | Groups | Mean (DS) | W | rsb | p Value |
|---|---|---|---|---|---|
| Previous episodes | 1 | 3.31 (0.98) | 585 | 0.323 | <0.01 |
| 2 | 2.50 (1.26) | ||||
| F.Autonomy | 1 | 6.87 (3.40) | 576 | 0.284 | <0.05 |
| 2 | 4.68 (3.80) | ||||
| PSQI.tot | 1 | 12.00 (4.80) | 560 | 0.252 | <0.05 |
| 2 | 9.77 (2.93) | ||||
| P.Duration | 1 | 1.26 (1.27) | 559 | 0.273 | <0.05 |
| 2 | 0.55 (0.96) | ||||
| P.Efficiency | 1 | 1.10 (1.31) | 545.5 | 0.254 | <0.05 |
| 2 | 0.41 (0.80) |
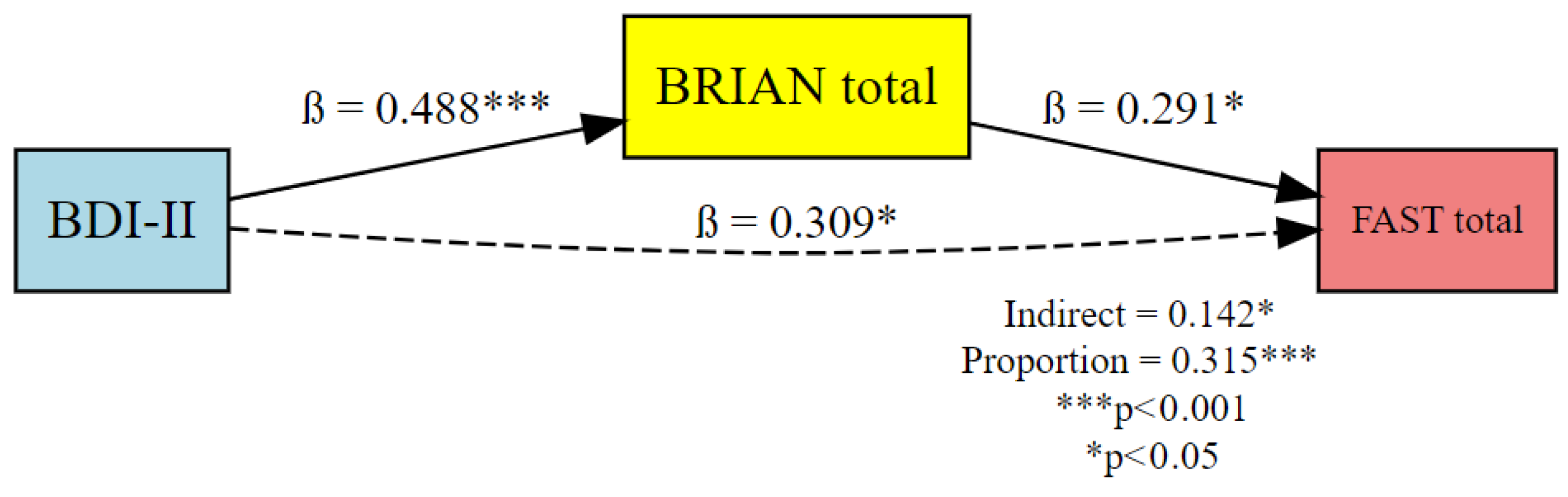
| Effect | Predictor (X) | Mediator (M) | Outcome (Y) | β (Standardized) | 95% CI (Lower) | 95% CI (Upper) | p-Value |
|---|---|---|---|---|---|---|---|
| Indirect Effect (ACME) | BDI-II | BRIAN.tot | FAST.tot | 0.142 | 0.017 | 0.38 | <0.05 |
| Direct Effect (ADE) | BDI-II | — | FAST.tot | 0.309 | 0.048 | 0.51 | <0.05 |
| Total Effect | BDI-II | — | FAST.tot | 0.451 | 0.260 | 0.63 | <0.001 |
| Proportion Mediated | BDI-II | BRIAN.tot | FAST.tot | 0.315 | 0.045 | 0.93 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrera, C.S.; Boccaccio, F.M.; D’Antoni, R.A.; Riggio, F.; Varrasi, S.; Platania, G.A.; Torre, V.; Pesimena, G.; Gangemi, A.; Pirrone, C.; et al. Biological Rhythms and Psychosocial Functioning in Depression: An Exploratory Analysis Informed by a Mediation Model. Psychiatry Int. 2025, 6, 85. https://doi.org/10.3390/psychiatryint6030085
Guerrera CS, Boccaccio FM, D’Antoni RA, Riggio F, Varrasi S, Platania GA, Torre V, Pesimena G, Gangemi A, Pirrone C, et al. Biological Rhythms and Psychosocial Functioning in Depression: An Exploratory Analysis Informed by a Mediation Model. Psychiatry International. 2025; 6(3):85. https://doi.org/10.3390/psychiatryint6030085
Chicago/Turabian StyleGuerrera, Claudia Savia, Francesco Maria Boccaccio, Rosa Alessia D’Antoni, Febronia Riggio, Simone Varrasi, Giuseppe Alessio Platania, Vittoria Torre, Gabriele Pesimena, Amelia Gangemi, Concetta Pirrone, and et al. 2025. "Biological Rhythms and Psychosocial Functioning in Depression: An Exploratory Analysis Informed by a Mediation Model" Psychiatry International 6, no. 3: 85. https://doi.org/10.3390/psychiatryint6030085
APA StyleGuerrera, C. S., Boccaccio, F. M., D’Antoni, R. A., Riggio, F., Varrasi, S., Platania, G. A., Torre, V., Pesimena, G., Gangemi, A., Pirrone, C., Caraci, F., & Castellano, S. (2025). Biological Rhythms and Psychosocial Functioning in Depression: An Exploratory Analysis Informed by a Mediation Model. Psychiatry International, 6(3), 85. https://doi.org/10.3390/psychiatryint6030085












