Abstract
Suicidality, encompassing suicidal ideation, attempts, and completed suicide, continues to be a significant public health concern globally. Traditional research has emphasized genetic, neurobiological, and psychosocial factors; however, recent findings suggest that gut microbiota may play a crucial role in influencing suicidal behavior. The gut microbiota impacts neuroinflammation, neurotransmitter metabolism, and the hypothalamic–pituitary–adrenal (HPA) axis, all of which are associated with psychiatric disorders linked to suicidality. This review gathers current evidence on the gut–brain axis, investigating the role of microbiota in suicidality through mechanisms such as immune system modulation, serotonin regulation, and the stress response. We also consider the potential of microbiota-targeted interventions, such as probiotics and dietary changes, as innovative therapeutic strategies. Despite the accumulating evidence, research in this field remains limited, emphasizing the urgent need for further investigation to clarify the causal relationship between gut microbiota and suicidality.
1. Introduction
Suicide represents a significant global health challenge, accounting for approximately 700,000 fatalities annually, alongside a higher incidence of suicide attempts and suicidal ideation [1]. The phenomenon of suicidality is complex and influenced by a myriad of factors, including genetic, neurobiological, psychological, and environmental elements [2,3,4,5,6]. While traditional research has predominantly focused on neurotransmitter imbalances, neuroinflammation, and stress dysregulation, emerging evidence suggests that gut microbiota may play a crucial role in influencing suicidal behavior [7].
The gut microbiota, which comprises a complex ecosystem of bacteria, viruses, and fungi residing in the gastrointestinal tract, is recognized as a crucial modulator of brain function through the gut–brain axis. This bidirectional communication network includes the immune system, the vagus nerve, and microbial metabolites, such as short-chain fatty acids (SCFAs) and neurotransmitter precursors [8]. Dysbiosis, characterized by an imbalance in gut microbiota composition, has been associated with psychiatric disorders, particularly major depressive disorder (MDD), bipolar disorder (BD), schizophrenia (SCZ), and post-traumatic stress disorder (PTSD), all of which exhibit a strong correlation with an increased risk of suicide [9].
One proposed mechanism linking gut microbiota to suicidality involves neuroinflammation. Research indicates that individuals with a history of suicide attempts exhibit elevated levels of pro-inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) [10]. These inflammatory markers may be influenced by gut microbiota, which affects intestinal permeability, commonly referred to as “leaky gut,” and systemic immune responses [11]. Increased gut permeability permits microbial endotoxins, such as lipopolysaccharides (LPS), to enter the bloodstream, thereby triggering neuroinflammatory cascades associated with depressive symptoms and suicidality [12].
The gut microbiota plays a vital role in neurotransmitter metabolism, especially in the synthesis and regulation of serotonin (5-HT), gamma-aminobutyric acid (GABA), dopamine, and glutamate [13]. Up to 90% of serotonin is produced in the gut, with microbiota such as Lactobacillus and Bifidobacterium aiding in its synthesis [14]. Considering the established link between serotonin dysfunction and depression, as well as suicidal behavior, changes in gut microbiota may lead to serotonin depletion and an elevated risk of suicide [15]. However, it is important to acknowledge that the serotonergic hypothesis of mood disorders has been increasingly challenged in recent years. While serotonin remains a relevant factor in neurobiological models, current evidence suggests that mood disorders are multifactorial and that serotonergic dysfunction is likely just one component within a broader pathophysiological framework [16]. Likewise, microbial impacts on glutamate and GABA metabolism may influence impulsivity and stress responses, which are pertinent to suicide risk [12].
Chronic stress and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis have been identified as significant risk factors for suicidality [17]. The gut microbiota interacts with the HPA axis by influencing cortisol production and the body’s stress response. Research conducted on animal models indicates that dysbiotic microbiota can exacerbate stress-induced behaviors, potentially increasing vulnerability to suicide in humans [18]. Although these findings cannot be directly extrapolated to suicidality in humans, they provide important hypotheses that warrant further investigation in clinical settings. Preliminary evidence suggests that microbiota-targeted interventions, including probiotics and prebiotics, may help modulate stress responses and alleviate depressive symptoms, but their role in suicide prevention remains to be fully clarified. Probiotics and microbiota-targeted interventions, including prebiotics and fecal microbiota transplantation (FMT), have shown promise in modifying stress responses and alleviating depressive symptoms, suggesting a potential approach for suicide prevention strategies [19].
Although a few previous reviews have addressed the gut–brain axis in psychiatric disorders or the potential role of microbiota in depression and stress-related conditions [20,21], to our knowledge, no review has specifically focused on the association between gut microbiota and suicidality as a multidimensional construct encompassing ideation, attempts, and completed suicide. This review aims to fill this gap by integrating findings from both clinical and preclinical studies and by proposing a mechanistic framework that connects microbiota-related changes to neuroinflammatory pathways, neurotransmitter dysregulation, and HPA axis activation, all of which are relevant to suicide risk (Figure 1). This synthesis seeks to provide a novel perspective on potential preventive and therapeutic strategies targeting the gut–brain axis in individuals at risk for suicidality.
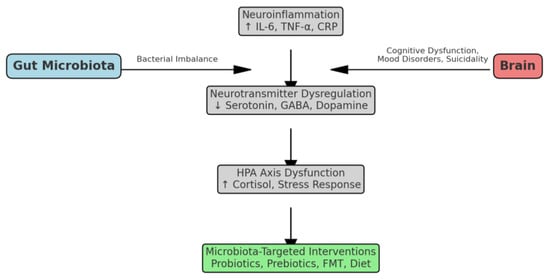
Figure 1.
Presents a conceptual overview of the influence of gut microbiota on suicidality, which is mediated through neuroinflammation, neurotransmitter dysregulation, and the HPA axis.
Objectives of This Review
Notwithstanding recent discoveries that associate gut microbiota with suicidality, the research within this domain remains restricted and disjointed. This narrative review seeks to consolidate and interpret the existing evidence on the link between gut microbiota and suicidality. Given the emerging and heterogeneous nature of the literature, we aim to provide an integrative synthesis of current findings, explore plausible biological mechanisms, and identify knowledge gaps to guide future research efforts, including systematic reviews.
More specifically, we will analyze:
- (I)
- The role of gut microbiota in neuroinflammation and immune dysfunction associated with suicidality;
- (II)
- The impact of microbial metabolites on neurotransmitter dysregulation;
- (III)
- The influence of gut microbiota on stress response and the HPA axis;
- (IV)
- The potential of microbiota-targeted therapies to modulate psychiatric symptoms associated with suicide risk.
By integrating findings from human studies, animal models, and psychiatric research, we aim to establish a comprehensive framework for understanding how gut microbiota influences suicidality and whether interventions targeting gut health could act as innovative therapeutic strategies.
2. Method
Given the limited and highly heterogeneous nature of available studies directly addressing the relationship between gut microbiota and suicidality, a narrative review approach was chosen. This methodology offers a broader and more flexible synthesis, integrating findings from both clinical and preclinical studies. While we acknowledge the potential risk of selection bias inherent in narrative reviews, we attempted to mitigate this by using multiple databases and thematically organizing the findings.
A non-systematic search was conducted in PubMed, Scopus, Web of Science, and PsycINFO to identify relevant publications. Keywords included “gut microbiota,” “suicide,” “suicidal ideation,” “neuroinflammation,” “HPA axis,” and “neurotransmitters.” The search included studies published from inception to February 2025 and limited to English-language articles.
We included original research articles (both observational and interventional) involving human participants, as well as preclinical studies in animal models that explored biologically plausible mechanisms relevant to suicidality. Studies were selected based on their relevance to the gut–brain axis and suicidal outcomes. We excluded commentaries, narrative reviews, editorials, and single case reports.
We also distinguished between studies reporting on direct indicators of suicidality (e.g., suicidal ideation, suicide attempts, or suicide mortality) and those focusing on indirect correlates, such as depressive or anxiety symptoms. The latter were included only when they provided mechanistic insights applicable to suicidality.
The findings were organized into four key thematic areas: neuroinflammation and immune dysfunction, neurotransmitter dysregulation, stress response and HPA axis activity, and microbiota-targeted interventions. Due to the heterogeneous methodologies, outcome measures, and populations of the included studies, a formal meta-analysis was not performed.
3. The Role of Gut Microbiota in Neuroinflammation and Immune Dysfunction Related to Suicidality
The intricate relationship between gut microbiota, neuroinflammation, and immune dysfunction establishes a compelling biological framework for understanding suicidality. Emerging evidence suggests that gut dysbiosis, characterized by an imbalance in microbial diversity and composition, contributes to systemic and neuroinflammatory processes that increase suicide risk. The gut–brain axis facilitates bidirectional communication between the gastrointestinal tract and the central nervous system, and disturbances in this axis have been linked to heightened neuroimmune activation and modified emotional regulation [22].
A key mechanism through which the gut microbiota influences neuroinflammation is the intestinal barrier, often called the “leaky gut” phenomenon. When intestinal integrity is compromised, bacterial endotoxins, particularly LPS, enter the bloodstream, triggering an innate immune response. This results in the activation of toll-like receptors (TLRs) on immune cells, releasing pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α [23]. These cytokines, while involved in normal immune regulation and tissue homeostasis, have also been implicated in the induction of microglial activation and neuroinflammatory processes, which have been observed in individuals with MDD, BD, and suicidality. However, their role in suicidality is likely to be non-specific and embedded within broader, complex systemic pathways [24]. Elevated peripheral inflammatory markers such as CRP have also been linked to increased suicide risk, further emphasizing the connection between systemic inflammation and suicidality [25].
Additionally, the gut microbiota influences immune tolerance and homeostasis by producing metabolites such as SCFAs, including butyrate, acetate, and propionate, which have anti-inflammatory effects and regulate microglial function [26]. A deficiency in SCFA-producing bacteria, often seen in individuals with psychiatric disorders, can lead to heightened inflammatory responses and increased oxidative stress, further disrupting neurotransmitter balance and cognitive function. These findings indicate that gut microbiota composition regulates immune function and influences stress responses and susceptibility to suicidal behavior.
Furthermore, gut microbiota interacts with the HPA axis, which is the body’s main stress response system. Chronic inflammation caused by gut dysbiosis has been shown to trigger HPA axis hyperactivity, resulting in excessive cortisol secretion. This, in turn, contributes to immune dysregulation, neuronal damage, and a heightened vulnerability to psychiatric disorders. Research indicates that individuals exhibiting suicidal behavior show increased cortisol reactivity, pointing to a maladaptive stress–inflammation cycle [27].
These findings collectively support the neuroimmune hypothesis of suicidality, where alterations in gut microbiota contribute to chronic neuroinflammation, increased activation of the HPA axis, and dysregulated immune signaling. All these factors converge to elevate suicide risk. Considering this growing body of evidence, future research should investigate the potential of microbiota-targeted therapies, including probiotics, prebiotics, dietary interventions, and fecal microbiota transplantation (FMT), as innovative strategies for reducing suicidality by modulating the immune system and restoring the gut–brain axis [28].
4. The Influence of Microbial Metabolites on Neurotransmitter Dysregulation
The gut microbiota profoundly influences neurotransmitter homeostasis through its production of bioactive microbial metabolites, which regulate mood, cognitive function, and stress responses. These metabolites include SCFAs, indole derivatives, kynurenine pathway metabolites, and polyamines, all of which have been implicated in neurotransmitter dysregulation associated with suicidality [29].
One of the best-characterized microbial pathways involves SCFAs, particularly butyrate, acetate, and propionate, which exert direct neuroactive effects by modulating neuroinflammation, neurotransmitter release, and blood–brain barrier integrity [30]. Butyrate, produced by Faecalibacterium prausnitzii and Roseburia species, boosts brain-derived neurotrophic factor (BDNF) expression, a vital element for synaptic plasticity and neuronal survival, often diminished in individuals with depression and suicidality [31]. SCFAs also affect dopaminergic and serotonergic signaling, specifically by modulating tryptophan metabolism, the precursor of serotonin [32]. A reduction in SCFA-producing bacteria has been noted in individuals with MDD and suicidal ideation, indicating a connection between gut dysbiosis, neurotransmitter imbalances, and heightened suicide risk [33].
Another significant microbial metabolic pathway impacting neurotransmitters is the kynurenine pathway, which is closely associated with neuroinflammation and glutamate excitotoxicity—two factors strongly linked to suicidality. The gut microbiota regulates the breakdown of tryptophan through the kynurenine pathway, which can divert metabolism from serotonin production toward the creation of neurotoxic metabolites such as quinolinic acid [34]. Quinolinic acid serves as a potent N-methyl-D-aspartate (NMDA) receptor agonist, leading to excessive glutamatergic activity that has been correlated with impulsivity, aggression, and suicidality [35]. Individuals displaying suicidal behavior often show elevated quinolinic acid-to-kynurenic acid ratios, indicating a shift toward neurotoxic inflammation instead of neuroprotective serotonin production [36].
In addition to SCFAs and kynurenine metabolites, gut bacteria also influence gamma-aminobutyric acid (GABA) and dopamine synthesis, both of which are crucial for impulse control and emotional regulation. Certain bacterial strains, such as Lactobacillus rhamnosus and Bifidobacterium breve, have been shown to enhance GABAergic activity, which has anxiolytic effects and may reduce stress-related suicidal behavior [37]. Conversely, gut dysbiosis has been linked to decreased GABA levels, contributing to heightened stress sensitivity and impulsive behaviors, which are core risk factors for suicidality [38]. Similarly, dopamine production is influenced by microbial species such as Bacillus and Escherichia, which regulate tyrosine hydroxylase activity, the rate-limiting enzyme in dopamine synthesis. Disruptions in gut microbiota that affect dopamine availability have been associated with anhedonia, reward-processing deficits, and suicidal behavior in mood disorders [39].
An additional area of increasing interest concerns the role of microbial polyamines, such as putrescine, spermidine, and spermine. These compounds are linked to neuronal signaling, oxidative stress regulation, and inflammation responses. Dysregulation of polyamines has been associated with major depressive disorders and suicidality. Certain studies suggest that an imbalance in gut-derived polyamines may lead to cognitive deficits, emotional dysregulation, and increased neuroinflammatory stress [40].
The collective findings clarify the role of microbial metabolites as critical modulators of neurotransmitter systems, notably influencing serotonergic, dopaminergic, glutamatergic, and GABAergic pathways. These pathways are essential for regulating mood, impulse control, and the risk of suicide. The possibility of restoring microbial balance through dietary interventions, probiotics, and psychobiotics offers a promising strategy for preventing and treating suicidality by modulating the gut–brain axis [41]. Future research efforts should focus on clinical trials that evaluate the effectiveness of microbiota-targeted therapies in establishing neurotransmitter balance and creating suicide prevention strategies.
5. The Impact of Gut Microbiota on Stress Response and the HPA Axis
The gut microbiota plays a fundamental role in stress regulation by directly influencing the HPA axis, which governs the body’s physiological response to stress. The HPA axis is activated in response to stressors, leading to the release of corticotropin-releasing hormone from the hypothalamus, which, in turn, stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH), ultimately triggering the adrenal glands to secrete cortisol [42]. While this mechanism is essential for survival, chronic stress can lead to dysregulation of the HPA axis, resulting in excessive cortisol secretion, neuroinflammation, and alterations in neurotransmitter function, all of which have been implicated in suicidality and psychiatric disorders.
One of the primary ways gut microbiota influences the HPA axis activity is through modulation of intestinal permeability. Stress-induced dysbiosis has been shown to compromise the integrity of the gut barrier, leading to increased permeability, commonly referred to as the “leaky gut” phenomenon [12]. This allows bacterial endotoxins such as LPS to enter the bloodstream, activating the immune system and triggering systemic inflammation, which further exacerbates HPA axis dysfunction. Elevated peripheral inflammatory markers such as IL-6 and TNF-α have been found in individuals with MDD, PTSD, and suicidal behavior, suggesting a strong link between gut-derived inflammation, HPA axis dysregulation, and suicidality [10].
Additionally, the gut microbiota influences the synthesis of key neuroactive compounds that regulate HPA axis function, including GABA, serotonin (5-HT), and dopamine [19]. Certain bacterial strains, such as Lactobacillus rhamnosus, have been shown to increase GABAergic signaling, reducing stress-induced HPA axis overactivation and promoting anxiolytic effects [34]. Similarly, gut-derived serotonin plays a role in regulating stress adaptation, and microbial alterations affecting serotonin production may contribute to exaggerated HPA axis responses and increased stress sensitivity [15].
Animal studies have provided further evidence of the gut microbiota’s impact on HPA axis function. Germ-free mice, which lack microbiota, exhibit exaggerated HPA axis responses to stress, characterized by heightened corticosterone and ACTH secretion, alongside increased anxiety-like behaviors. Interestingly, the introduction of specific bacterial strains, such as Bifidobacterium and Lactobacillus, can restore normal HPA axis regulation, suggesting that gut microbiota is essential for proper stress adaptation [36]. These findings have been extended to human studies, where individuals with depressive and anxiety disorders show increased cortisol reactivity, often in conjunction with altered gut microbial diversity, particularly a reduction in short-chain fatty acid (SCFA)-producing bacteria [14].
Furthermore, gut microbiota may influence epigenetic modifications that impact HPA axis sensitivity to stress. Recent studies have suggested that gut-derived metabolites, such as butyrate and propionate, can regulate gene expression related to HPA axis activity, potentially influencing long-term stress resilience or vulnerability [31]. This suggests that early-life microbial exposures may shape stress responses later in life, possibly predisposing individuals to increased suicide risk if gut microbial imbalances persist.
Given the strong link between HPA axis dysregulation and suicidality, microbiota-targeted interventions may offer novel therapeutic strategies for stress-related psychiatric disorders. Probiotic supplementation has been shown to reduce cortisol levels, attenuate inflammatory responses, and improve mood regulation, highlighting its potential role in suicide prevention. Specifically, in clinical populations, probiotics have shown potential benefits for mental health, particularly in improving depressive symptoms and emotional resilience [43]. Additionally, prebiotics, dietary modifications, and fecal microbiota transplantation (FMT) are emerging as promising strategies to restore gut microbial balance and mitigate stress-induced HPA axis dysfunction [21].
Overall, the interaction between gut microbiota and the HPA axis represents a key mechanism through which chronic stress may contribute to suicidality. Future research should focus on longitudinal human studies and clinical trials examining the effects of microbiota-based interventions on stress resilience and suicide risk, with the goal of identifying gut microbial markers and therapeutic targets for early intervention.
6. The Potential of Microbiota-Targeted Therapies in Reducing Suicide Risk
The potential of microbiota-targeted therapies in reducing suicide risk is an emerging area of interest, as research increasingly highlights the gut–brain axis as a key player in psychiatric disorders. Given the strong associations between gut dysbiosis, neuroinflammation, neurotransmitter imbalances, and HPA axis dysregulation, interventions aimed at restoring gut microbial balance may offer novel strategies for suicide prevention. Probiotics, which contain beneficial bacteria such as Lactobacillus and Bifidobacterium, have been shown to reduce systemic inflammation, enhance serotonin production, and modulate stress responses, leading to improved mood stability and emotional resilience [38]. Clinical studies have demonstrated that probiotic supplementation can lower cortisol levels, decrease depressive symptoms, and improve anxiety-related behaviors, suggesting that these interventions could play a role in reducing suicide risk [7]. Prebiotics, which serve as food sources for beneficial gut bacteria, have also been linked to enhanced stress resilience and reduced inflammation, further reinforcing their therapeutic potential [40].
Probiotics, defined as live microorganisms that confer health benefits when administered in adequate amounts, have been shown to modulate gut–brain axis activity, influencing neurotransmitter production, stress resilience, and immune function [38]. Specific strains such as Lactobacillus rhamnosus, Bifidobacterium longum, and Lactobacillus helveticus have demonstrated the ability to enhance serotonin and GABA production, lower cortisol levels, and reduce inflammation, leading to improvements in anxiety, depression, and stress-related behaviors [34]. Clinical studies suggest that probiotic supplementation can reduce depressive symptoms and improve cognitive function, potentially lowering suicide risk, especially in individuals with MDD, BD, and PTSD [15].
An emerging concept within this field is psychobiotics, which refers to probiotics that specifically exert positive effects on brain function and mental health. These psychotropic bacteria influence mood and cognition through their ability to alter gut microbiota composition, produce neuroactive compounds (e.g., SCFAs, serotonin precursors), and regulate neuroinflammatory pathways [39]. Psychobiotic therapy represents a promising adjunctive treatment for individuals at high risk for suicidality, particularly those with stress-induced gut dysbiosis and neuroinflammatory conditions.
Beyond probiotics, prebiotics—nondigestible dietary fibers that promote the growth of beneficial gut bacteria—have also been investigated for their role in reducing stress and improving emotional well-being. Prebiotic-rich diets, particularly those high in fructooligosaccharides (FOS) and galactooligosaccharides (GOS), have been found to increase the abundance of SCFA-producing bacteria, modulate HPA axis activity, and lower cortisol levels, all of which contribute to reduced anxiety and depressive symptoms [40].
Dietary interventions, particularly Mediterranean-style and high-fiber diets, have shown strong correlations with improved mental health outcomes, largely due to their ability to increase microbial diversity and promote anti-inflammatory gut bacteria [41]. Diets rich in polyphenols, fermented foods, and omega-3 fatty acids have been associated with increased levels of Lactobacillus and Bifidobacterium, reduced neuroinflammatory markers, and enhanced serotonin production, suggesting a protective role against suicidality [42].
FMT, which involves transferring gut microbiota from a healthy donor to an individual with dysbiosis, is gaining attention as a potential treatment for psychiatric disorders and suicidality. FMT has been successfully used to treat Clostridioides difficile infections, and recent studies suggest it may also improve mood, cognitive function, and emotional regulation in individuals with treatment-resistant depression (TRD) [43]. Case reports and preliminary trials indicate that restoring a balanced gut microbiota via FMT can alleviate depressive symptoms, regulate stress responses, and reduce inflammation, all of which are critical factors in suicide prevention [43]. However, more randomized controlled trials are needed to establish its efficacy in psychiatric populations.
In addition to probiotics, prebiotics, and FMT, researchers are exploring postbiotics, which are bioactive compounds produced by beneficial gut bacteria, such as SCFAs, bacteriocins, and neuroactive peptides [43]. Postbiotics can exert anti-inflammatory and neuroprotective effects without requiring live bacterial supplementation, making them a potentially safer alternative for individuals with immune dysfunction or severe psychiatric conditions. Future research is also focusing on personalized microbiome-targeted therapies, which involve sequencing an individual’s gut microbiota and designing customized interventions based on specific microbial imbalances linked to suicidality [7].
7. Challenges and Future Directions
While microbiota-targeted therapies hold great promise for reducing suicide risk, several challenges remain. The heterogeneity of psychiatric disorders, individual variations in gut microbiota composition, and the complexity of gut–brain interactions necessitate further research and clinical trials to determine optimal treatment protocols. Additionally, long-term studies are needed to assess the sustainability of microbiota interventions, as well as potential risks associated with FMT, psychobiotics, and postbiotic therapies. Furthermore, while microbiota research has focused heavily on serotonin-related pathways, future studies should aim to explore other neurobiological mechanisms beyond the serotonergic system, in line with recent critiques of the serotonin hypothesis.
An important limitation of current research on the gut microbiota and suicidality lies in the difficulty of distinguishing between causal mechanisms, risk factors, and correlational associations. Many of the available studies—especially those involving preclinical models—highlight biological plausibility and mechanistic hypotheses but do not allow for definitive conclusions about causation. In this review, while we have described associations between dysbiosis and suicidality-related dimensions (e.g., neuroinflammation, neurotransmitter dysregulation, and HPA axis hyperactivity), we recognize that these relationships are complex and potentially bidirectional, and that dysbiosis may be both a contributor to and a consequence of psychiatric symptoms.
Moreover, individuals affected by mood disorders often exhibit significant alterations in lifestyle and self-care behaviors, including poor diet quality, reduced physical activity, disturbed sleep, and increased use of substances such as alcohol or tobacco. These behaviors are not only central clinical features or consequences of mood dysregulation, but also recognized modulators of the gut microbiota. For example, a diet high in saturated fats and low in fiber has been associated with reduced microbial diversity and increased pro-inflammatory species, while regular physical exercise has been linked to enrichment of short-chain fatty acid–producing bacteria. Sleep fragmentation and circadian rhythm disruption can also alter microbial composition and gut permeability, thereby influencing systemic inflammation and stress sensitivity.
Despite their relevance, lifestyle-related factors are often underexplored or insufficiently controlled in microbiota research in psychiatry. Their inclusion in future study designs—either as covariates, mediators, or intervention targets—may help to clarify the directionality and clinical significance of the microbiota–suicidality link. Furthermore, interventions aimed at restoring a healthy gut microbiome in psychiatric populations may benefit from being embedded in multidimensional approaches that also address nutritional habits, sleep hygiene, and physical health, rather than relying solely on microbial manipulation through probiotics or prebiotics.
It is important to recognize that the expression of specific inflammatory or metabolic markers—such as IL-6 or SCFA-producing pathways—should not be interpreted as inherently pathological or causative in isolation. Their significance is highly context-dependent, influenced by host genetics, transcriptional regulation, circadian rhythms, environmental exposures, and measurement conditions. Future studies integrating transcriptomic, metabolomic, and chronobiological data will be essential to move beyond correlative findings and toward a systems-level understanding of psychoneuroimmune interactions.
In conclusion, growing evidence suggests that the gut microbiota may represent a previously underappreciated player in the complex pathophysiology of suicidality, acting through interconnected mechanisms involving immune modulation, neurotransmitter synthesis, and HPA axis regulation. While the associations identified to date are compelling, current findings remain largely correlational, and the field is still in its infancy in terms of establishing causality and identifying clinically actionable targets. The therapeutic potential of microbiota-directed interventions—ranging from probiotics and prebiotics to dietary modification and fecal microbiota transplantation—is undeniably promising; yet, it must be approached with scientific caution given the absence of robust clinical trials specifically addressing suicidality.
Given the current limitations of the evidence base, this narrative review should be interpreted not as a conclusive summary but rather as a conceptual roadmap to guide hypothesis generation and multidisciplinary investigation in this emerging field.
Future research should prioritize longitudinal and interventional designs, incorporate well-defined suicidality outcomes, and consider relevant confounders such as psychiatric comorbidities, medication use, and lifestyle factors. Integrating microbiome science into psychiatric research agendas may provide not only novel insights into suicide risk but also innovative, non-invasive avenues for prevention and treatment. Ultimately, advancing this field will require a rigorous, multidisciplinary effort that bridges psychiatry, neuroscience, microbiology, and systems biology to translate emerging knowledge into tangible clinical applications.
Author Contributions
Conceptualization, V.B. and M.C.; methodology, M.G.; software, G.S.; validation, V.B., M.G. and G.V.; formal analysis, V.B.; investigation, V.B.; resources, G.S.; data curation, M.G.; writing—original draft preparation, V.B.; writing—review and editing, M.G.; visualization, G.V.; supervision, D.D.R.; project administration, M.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data extracted and analyzed in this review are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Suicide Worldwide in 2019; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240026643 (accessed on 16 June 2021).
- Turecki, G.; Brent, D.A. Suicide and Suicidal Behaviour. Lancet 2016, 387, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Baldini, V.; Gnazzo, M.; Maragno, M.; Biagetti, R.; Stefanini, C.; Canulli, F.; Varallo, G.; Donati, C.; Neri, G.; Fiorillo, A.; et al. Suicidal Risk among Adolescent Psychiatric Inpatients: The Role of Insomnia, Depression, and Social-Personal Factors. Eur. Psychiatry 2025, 28, e42. [Google Scholar] [CrossRef]
- Baldini, V.; Gnazzo, M.; Rapelli, G.; Marchi, M.; Pingani, L.; Ferrari, S.; De Ronchi, D.; Varallo, G.; Starace, F.; Franceschini, C.; et al. Association between Sleep Disturbances and Suicidal Behavior in Adolescents: A Systematic Review and Meta-Analysis. Front. Psychiatry 2024, 15, 1341686. [Google Scholar] [CrossRef] [PubMed]
- Baldini, V.; Di Stefano, R.; Rindi, L.V.; Ahmed, A.O.; Koola, M.M.; Solmi, M.; Papola, D.; De Ronchi, D.; Barbui, C.; Ostuzzi, G. Association between Adverse Childhood Experiences and Suicidal Behavior in Schizophrenia Spectrum Disorders: A Systematic Review and Meta-Analysis. Psychiatry Res. 2023, 329, 115488. [Google Scholar] [CrossRef] [PubMed]
- Baldini, V.; Gnazzo, M.; Santangelo, G.; D’Agostino, A.; Varallo, G.; Scorza, M.; Ostuzzi, G.; Galeazzi, G.M.; De Ronchi, D.; Plazzi, G. Are Sleep Disturbances a Risk Factor for Suicidal Behavior in the First Episode of Psychosis? Evidence from a Systematic Review. J. Psychiatr. Res. 2025, 185, 186–193. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Ruan, B. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; Wolkowitz, O.M.; Mellon, S.H.; Yehuda, R.; Flory, J.D. Increased Pro-Inflammatory Milieu in Combat Related PTSD—A New Cohort Replication Study. Brain Behav. Immun. 2017, 59, 260–264. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Cryan, J.F. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM Responses against Gut Commensals in Chronic Depression: Further Evidence for Increased Bacterial Translocation or Leaky Gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Raes, J. The Neuroactive Potential of the Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X.; Fecchio, J.; Zhang, H.; Lu, Y.; et al. Fecal Microbiota Transplantation from Chronic Mild Stress Rats Induces Depressive-like Behaviors in Recipient Rats. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The Gut Microbiome and Diet in Psychiatry: Focus on Depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2019, 77, 430–443. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V. Interaction between Enteric Microbiota and Gut-Brain Axis: A New Clinical Frontier in Neuropsychiatric Disorders. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [CrossRef]
- Black, C.; Miller, B.J. Cytokines and Chemokines in Suicidality: Distinguishing Suicidal versus Nonsuicidal Patients—A Meta-Analysis. Biol. Psychiatry 2015, 78, 28–37. [Google Scholar] [CrossRef]
- Miola, A.; Dal Porto, V.; Tadmor, T.; Croatto, G.; Scocco, P.; Manchia, M.; Carvalho, A.F.; Maes, M.; Miola, M.; Maes, M. Increased C-Reactive Protein Levels in Major Depressive Disorder and Suicidal Behavior: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2023, 144, 537–552. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for Increased Microglial Priming and Macrophage Recruitment in the Dorsal Anterior Cingulate White Matter of Depressed Suicides. Brain Behav. Immun. 2014, 42, 50–59. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Green, J.A.; Ferguson, E.; O’Carroll, R.E.; O’Connor, R.C. Cortisol Reactivity and Suicidal Behavior: Investigating the Role of Hypothalamic-Pituitary-Adrenal Axis Responses to Stress in Suicide Attempters and Ideators. Psychoneuroendocrinology 2017, 75, 183–191. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-Endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, C.; Zhang, Q.; Lv, Q.; Liu, H.; Yuan, H.; Wang, C.; Meng, F.; Guo, Y.; Pei, J.; et al. Gut Microbiota Modulates Depressive-Like Behaviors Induced by Chronic Ethanol Exposure through Short-Chain Fatty Acids. J. Neuroinflamm. 2024, 21, 290. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial Genes, Brain & Behaviour—Epigenetic Regulation of the Gut–Brain Axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The Kynurenine Pathway and the Brain: Challenges, Controversies and Promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The New ‘5-HT’ Hypothesis of Depression: Cell-Mediated Immune Activation Induces Indoleamine 2,3-Dioxygenase, Leading to Lower Plasma Tryptophan and Increased Synthesis of Detrimental Tryptophan Catabolites (TRYCATs), Both Contributing to the Onset of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Saigusa, T.; Pacak, K. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2017, 69, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.G.; Fiori, L.M.; Moquin, L.; Gratton, A.; Mamer, O.; Mechawar, N.; Turecki, G. Evidence of Altered Polyamine Concentrations in Cerebral Cortex of Suicide Completers. Neuropsychopharmacology 2010, 35, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-Like Properties of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Hogenelst, K.; Krone, T.; Eveleens Maarse, B.; Warnke, I.; Snabel, J.; van den Broek, T.J.; Schuren, F.; Moerland, M.; Hoevenaars, F.P. A Prebiotic Intervention Improves Mood in Everyday Life in Healthy Women but Not in Men: Exploratory Results from a Larger Double-Blind Placebo-Controlled Cross-Over Study. Brain Behav. Immun. Health 2024, 43, 100918. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the ‘SMILES’ Trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A Meta-Analysis of the Use of Probiotics to Alleviate Depressive Symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).