Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists for Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Searches and Inclusion Criteria
2.2. Study Screening
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Search Results and Baseline Characteristics of Included Trials
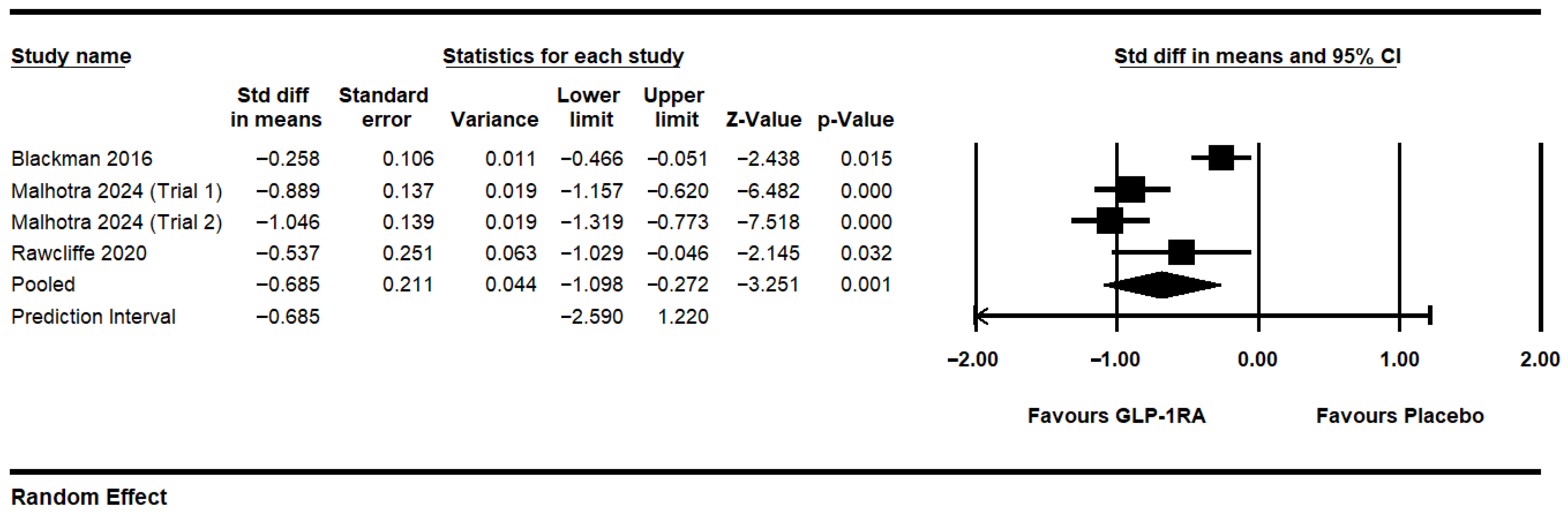
3.2. GLP-1RAs Overall
3.3. Risk of Bias
3.4. Liraglutide
3.5. Tirzepatide
3.6. Serious Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea–hypopnea index |
| BMI | Body mass index |
| CI | Confidence interval |
| CMA | Comprehensive meta-analysis v4 |
| CPAP | Continuous positive airway pressure |
| ESS | Epworth sleepiness scale |
| FDA | Food and Drug Administration |
| GI | Gastrointestinal |
| GLP1-RA | Glucagon-like peptide-1 receptor agonists |
| HKSJ | Hartung–Knapp–Sidik–Jonkman |
| OAT | Oral appliance therapy |
| ODI | Oxygen desaturation index |
| OSA | Obstructive sleep apnea |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSG | Polysomnography |
| RCT | Randomized controlled trial |
| RDI | Respiratory disturbance index |
| ROB2 | Risk of Bias 2 (tool by Cochrane) |
| RR | Risk ratio |
| SAE | Serious adverse events |
| SMD | Standardized mean difference |
| T2DM | Type 2 diabetes mellitus |
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- de Araujo Dantas, A.B.; Gonçalves, F.M.; Martins, A.A.; Alves, G.Â.; Stechman-Neto, J.; de Castro Corrêa, C.; Santos, R.S.; Nascimento, W.V.; de Araujo, C.M.; Taveira, K.V.M. Worldwide prevalence and associated risk factors of obstructive sleep apnea: A meta-analysis and meta-regression. Sleep Breath. 2023, 27, 2083–2109. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep Apnea and 20-Year Follow-Up for All-Cause Mortality, Stroke, and Cancer Incidence and Mortality in the Busselton Health Study Cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Leung, R.S.; Tomlinson, G. Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study. PLOS Med. 2014, 11, e1001599. [Google Scholar] [CrossRef]
- Stranks, E.K.; Crowe, S.F. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-analysis. Arch. Clin. Neuropsychol. 2016, 31, 186–193. [Google Scholar] [CrossRef]
- Borsoi, L.; Armeni, P.; Donin, G.; Costa, F.; Ferini-Strambi, L. The invisible costs of obstructive sleep apnea (OSA): Systematic review and cost-of-illness analysis. PLoS ONE 2022, 17, e0268677. [Google Scholar] [CrossRef]
- Léger, D.; Stepnowsky, C. The economic and societal burden of excessive daytime sleepiness in patients with obstructive sleep apnea. Sleep Med. Rev. 2020, 51, 101275. [Google Scholar] [CrossRef]
- Watson, N.F. Health Care Savings: The Economic Value of Diagnostic and Therapeutic Care for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2016, 12, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J.C.; Yang, H.; King, T.S.; Sawyer, D.A.; Rizzo, A.; Sawyer, A.M. Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung. 2015, 44, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Dunbar-Jacob, J.; Aloia, M.S.; Martire, L.M.; Buysse, D.J.; Strollo, P.J. Patient and Partner Experiences with Obstructive Sleep Apnea and CPAP Treatment: A Qualitative Analysis. Behav. Sleep Med. 2016, 14, 67–84. [Google Scholar] [CrossRef]
- Billings, M.E.; Auckley, D.; Benca, R.; Foldvary-Schaefer, N.; Iber, C.; Redline, S.; Rosen, C.L.; Zee, P.; Kapur, V.K. Race and Residential Socioeconomics as Predictors of CPAP Adherence. Sleep 2011, 34, 1653–1658. [Google Scholar] [CrossRef]
- Janson, C.; Nöges, E.; Svedberg-brandt, S.; Lindberg, E. What characterizes patients who are unable to tolerate continuous positive airway pressure (CPAP) treatment? Respir. Med. 2000, 94, 145–149. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head Neck Surg. 2016, 45, 43. [Google Scholar] [CrossRef]
- Kent, D.; Stanley, J.; Aurora, R.N.; Levine, C.; Gottlieb, D.J.; Spann, M.D.; Torre, C.A.; Green, K.; Harrod, C.G. Referral of adults with obstructive sleep apnea for surgical consultation: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Kapen, S.; Lee-Chiong, T.; Alessi, C.; Boehlecke, B.; Brown, T.; Coleman, J.; Friedman, L.; Kapur, V.; Owens, J.; et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 2006, 29, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Bednarik, J.; Chakladar, S.; Dunn, J.P.; Weaver, T.; Grunstein, R.; Fietze, I.; Redline, S.; Azarbarzin, A.; Sands, S.A.; et al. Tirzepatide for the treatment of obstructive sleep apnea: Rationale, design, and sample baseline characteristics of the SURMOUNT-OSA phase 3 trial. Contemp. Clin. Trials 2024, 141, 107516. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef] [PubMed]
- Commissioner of the FDA. Approves New Drug Treatment for Chronic Weight Management First Since, 2.0.1.4.FDA. 21 June 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management (accessed on 5 April 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Veritas Health Information. Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 13 July 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, Version 4; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar] [CrossRef]
- Chapter 10: Analysing Data and Undertaking Meta-Analyses. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 5 April 2024).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Malhotra, A.; Grunstein, R.R.; Fietze, I.; Weaver, T.E.; Redline, S.; Azarbarzin, A.; Sands, S.A.; Schwab, R.J.; Dunn, J.P.; Chakladar, S.; et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N. Engl. J. Med. 2024, 391, 1193–1205. [Google Scholar] [CrossRef]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef]
- O’Donnell, C.; Crilly, S.; O’Mahony, A.; O’Riordan, B.; Traynor, M.; Gitau, R.; McDonald, K.; Ledwidge, M.; O’Shea, D.; Murphy, D.J.; et al. Continuous Positive Airway Pressure but Not GLP1-mediated Weight Loss Improves Early Cardiovascular Disease in Obstructive Sleep Apnea: A Randomized Proof-of-Concept Study. Ann. Am. Thorac. Soc. 2024, 21, 464–473. [Google Scholar] [CrossRef]
- Jiang, W.; Li, W.; Cheng, J.; Li, W.; Cheng, F. Efficacy and safety of liraglutide in patients with type 2 diabetes mellitus and severe obstructive sleep apnea. Sleep Breath. 2023, 27, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- EudraCT Number 2014-000988-41—Clinical Trial Results—EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-000988-41/results (accessed on 17 November 2024).
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Rotty, M.-C.; Suehs, C.M.; Mallet, J.-P.; Martinez, C.; Borel, J.-C.; Rabec, C.; Bertelli, F.; Bourdin, A.; Molinari, N.; Jaffuel, D. Mask side-effects in long-term CPAP-patients impact adherence and sleepiness: The InterfaceVent real-life study. Respir. Res. 2021, 22, 17. [Google Scholar] [CrossRef]
- Bachour, A.; Vitikainen, P.; Virkkula, P.; Maasilta, P. CPAP interface: Satisfaction and side effects. Sleep Breath. 2013, 17, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chai, S.; Yu, K.; Quan, X.; Yang, Z.; Wu, S.; Zhang, Y.; Ji, L.; Wang, J.; Shi, L. Gastrointestinal Adverse Events of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetes Technol. Ther. 2015, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, Z.; Lyu, X.; Xu, H.; Zhu, H.; Pan, H.; Wang, L.; Yang, H.; Gong, F. The Antiobesity Effect and Safety of GLP-1 Receptor Agonist in Overweight/Obese Patients Without Diabetes: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2022, 54, 458–471. [Google Scholar] [CrossRef]
- Alves, C.; Batel-Marques, F.; Macedo, A.F. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res. Clin. Pract. 2012, 98, 271–284. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, X.; Fu, S.; Liu, P.; Long, C.; Wang, Y.; Li, Z.; Xie, Q.; Chen, Q. Efficacy and safety of tirzepatide, dual GLP-1/GIP receptor agonists, in the management of type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2023, 15, 222. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by The American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 EXECUTIVE SUMMARY. Endocr. Pract. 2016, 22, 84–113. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef]
- Dankner, R.; Murad, H.; Agay, N.; Olmer, L.; Freedman, L.S. Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients with Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2350408. [Google Scholar] [CrossRef]
- Elashoff, M.; Matveyenko, A.V.; Gier, B.; Elashoff, R.; Butler, P.C. Pancreatitis, Pancreatic, and Thyroid Cancer with Glucagon-Like Peptide-1–Based Therapies. Gastroenterology 2011, 141, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2015, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, R.; Cheng, R.; Liu, C.; Guo, R.; Tang, W.; Zhang, J.; Zhao, Q.; Li, X.; Liu, J. Use of GLP-1 Receptor Agonists and Occurrence of Thyroid Disorders: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 927859. [Google Scholar] [CrossRef] [PubMed]

| Study (Country, Trial #, and Sponsor) | Study Design and Duration of Trial | Treatment Participants (n, Mean Age, Sex) | GLP-1RA Investigated, Dosing, and Duration | Control Participants (n, Mean Age, Sex) | Type of Control |
|---|---|---|---|---|---|
| Blackman 2016 [32] (Canada and USA, NCT01557166, Novo Nordisk A/S) | Double-blind, placebo-controlled parallel-group (32 week and 2 weeks post-trial) | n = 180 Mean age: 48.6 ± 9.9 Female: 51 | Liraglutide starting at 0.6 mg/day and increased over 4 weeks to 3 mg, which was maintained for 28 weeks with diet and exercise counseling | n = 179 Mean age: 48.4 ± 9.5 Female: 50 | Placebo dose-volume equivalent with diet and exercise counseling |
| Jiang 2023 [34] (China, 20180329, unknown) | Non-blinded randomized controlled trial (12 weeks) | n = 44 Mean age: 55.7 ± 7.4 Female: 10 Participants all had comorbid diabetes mellitus type 2 | CPAP + liraglutide starting at 0.6 mg/day up to 1.2–1.8 mg/day at week two. | n = 45 Mean age: 54.8 ± 5.5 Female: 16 Participants all had comorbid diabetes mellitus type 2 | CPAP therapy standalone |
| Malhotra 2024 [31] (USA, NCT05412004. Eli Lilly) | Double-blind, randomized placebo-controlled, parallel-group (52 weeks) | Trial 1: n = 114 Mean age: 47.2 ± 11.0 Female: 36 Trial 2: n = 120 Mean age: 50.8 ± 10.7 Female: 33 | Tirzepatide starting at 2.5 mg/week and was increased by 2.5 mg every 4 weeks until the participant reached the maximum tolerated dose of 10 mg or 15 mg in week 20. | Trial 1: n = 120 Mean age: 48.4 ± 11.9 Female: 41 Trial 2: n = 115 Mean age: 52.7 ± 11.3 Female: 32 | Subcutaneous placebo |
| O’Donnell 2023 [33] (Ireland, NCT04186494, St Vincent’s University Hospital) | Open-label, randomized placebo-controlled, parallel-group (24 weeks) | n = 10 Mean age: 50 ± 7 Female: 6 CPAP + liraglutide: n = 10 Mean age: 50 ± 5 Female: 3 | Liraglutide standalone starting at 0.6 mg/day with a dose increase each week up to 3.0 mg at week 5. CPAP + liraglutide is the same as above. | n = 10 Mean age: 51 ± 8 Female: 1 | CPAP therapy standalone |
| Rawcliffe 2020 [35] (United Kingdom, EudraCT # 2014-000988-41, University of Liverpool) [Trial completed with data available but not currently published] | Non-blinded, randomized, placebo-controlled (26 weeks) | Arm C: n = 33 Median age: 55 (45 to 61) Female: 19 Arm D: n = 33 Median age: 54 (46 to 61) Female: 11 Participants all had comorbid diabetes mellitus type 2 | Arm C: liraglutide standalone starting at 0.6 mg/day with a dose increase each week up to 1.8 mg at week 3. Arm D: CPAP + liraglutide is the same as above | Arm A: n = 33 Median age: 52 (48 to 57) Female: 17 Arm B: n = 33 Median age: 57 (49 to 60) Female: 20 Participants all had comorbid diabetes mellitus type 2 | Arm A: No treatment Arm B: CPAP therapy standalone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.; Fabiano, N.; Zhou, C.; Luu, B.; Shorr, R.; Slassi, S.; Solmi, M.; Husain, I.; Mak, M.S.B. Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists for Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychiatry Int. 2025, 6, 111. https://doi.org/10.3390/psychiatryint6030111
Wong S, Fabiano N, Zhou C, Luu B, Shorr R, Slassi S, Solmi M, Husain I, Mak MSB. Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists for Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychiatry International. 2025; 6(3):111. https://doi.org/10.3390/psychiatryint6030111
Chicago/Turabian StyleWong, Stanley, Nicholas Fabiano, Carl Zhou, Brandon Luu, Risa Shorr, Sarah Slassi, Marco Solmi, Ishrat Husain, and Michael S. B. Mak. 2025. "Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists for Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Psychiatry International 6, no. 3: 111. https://doi.org/10.3390/psychiatryint6030111
APA StyleWong, S., Fabiano, N., Zhou, C., Luu, B., Shorr, R., Slassi, S., Solmi, M., Husain, I., & Mak, M. S. B. (2025). Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists for Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychiatry International, 6(3), 111. https://doi.org/10.3390/psychiatryint6030111






