Impact of Dietary Interventions on Depression in Adolescents and Young Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Selection Process
2.4. Data Collection Process
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
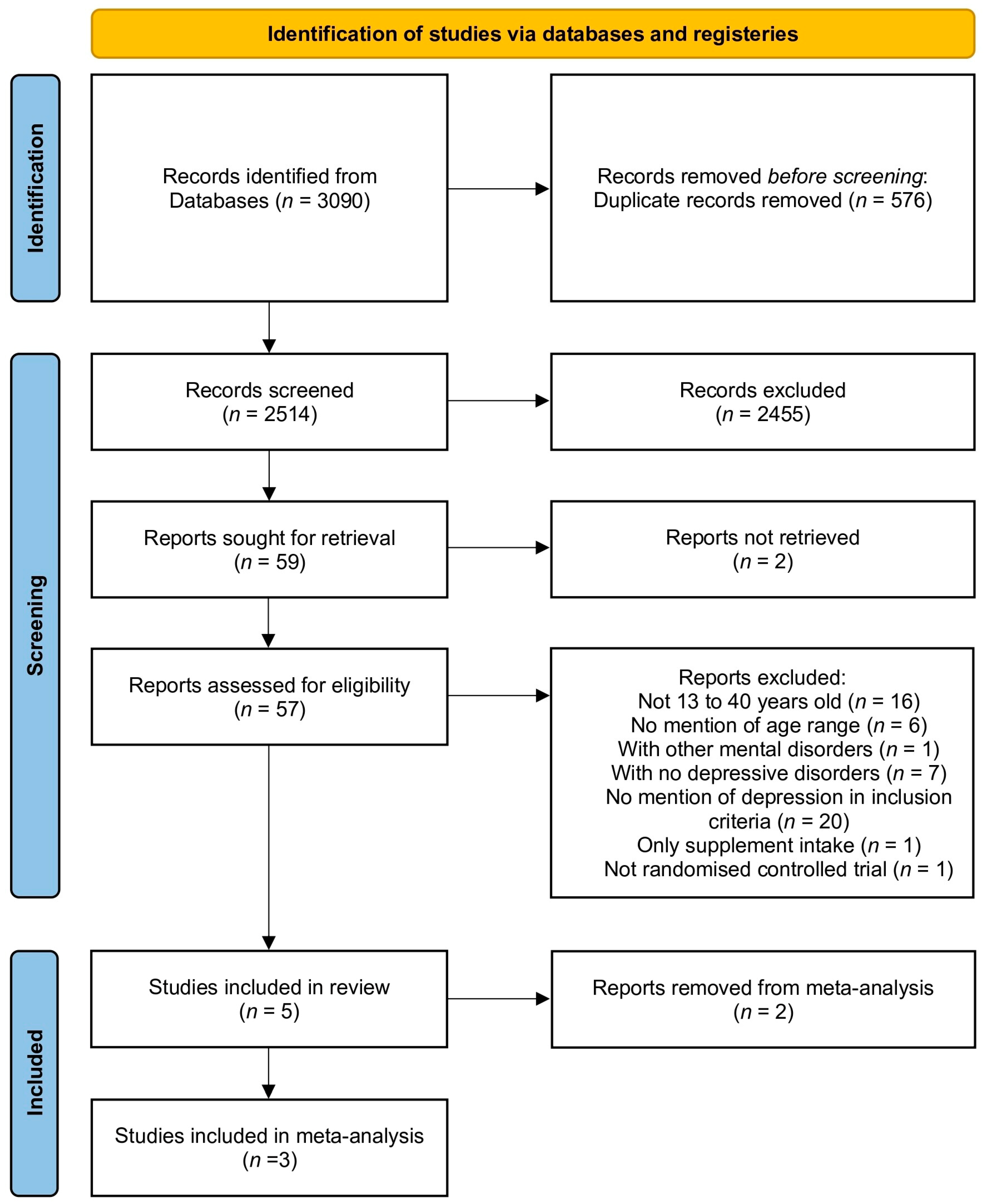
3.1. Study Selection (See Figure 1)
3.2. Study Characteristics
3.2.1. Overview
3.2.2. Dietary Intervention
3.2.3. Depressive Symptoms
3.2.4. Quality of Life
3.2.5. Outcomes Potentially Impacting Daily Life
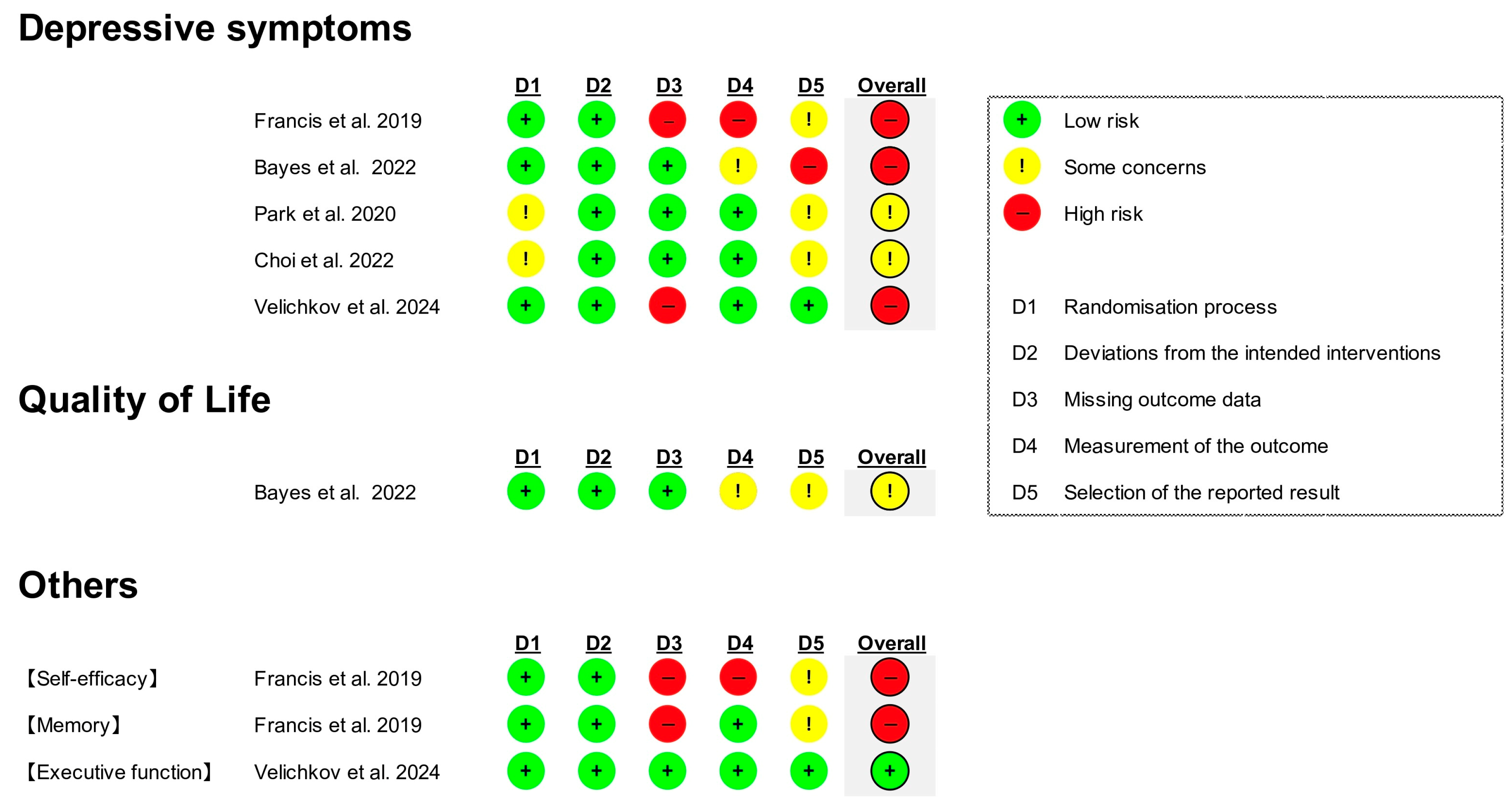
3.3. Risk of Bias in Studies
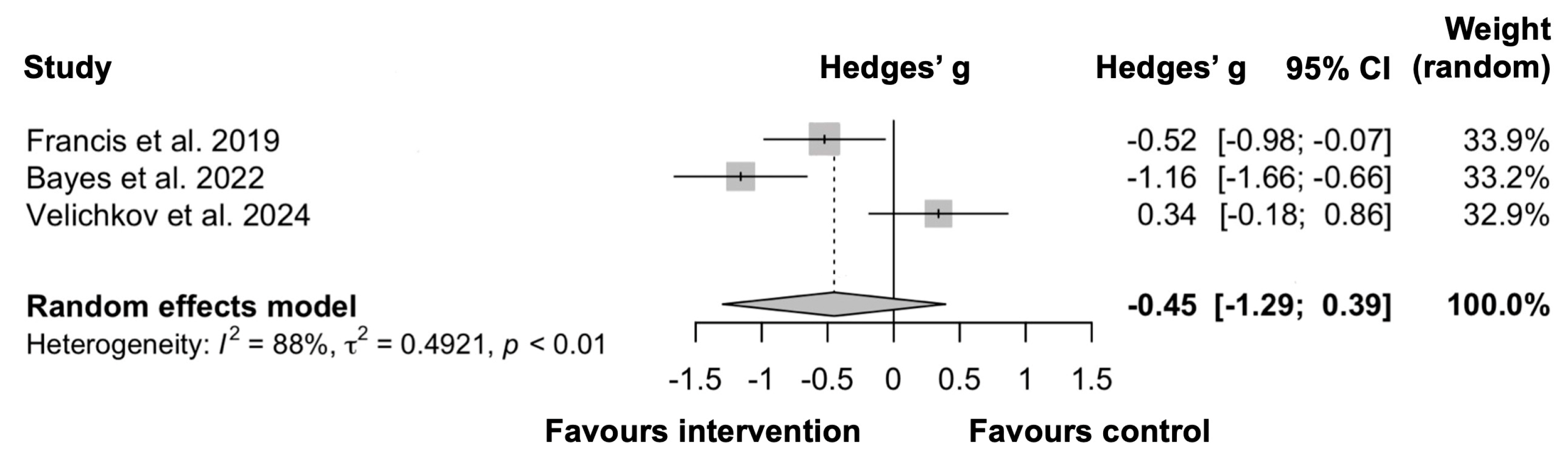
3.4. Meta-Analysis of Depressive Symptoms
4. Discussion
4.1. Summary of Main Findings
4.2. Effect on Depressive Symptoms
4.2.1. Possible Mechanisms Behind Symptom Improvement
4.2.2. Potential Reasons Limiting Improvement in Depressive Symptoms
4.2.3. Interpretation of Meta-Analysis Results
4.3. Effect on QOL
4.4. Impact on Daily Life
4.5. Implications for Future Research or Clinical Practice
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Depressive Disorder (Depression). 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 November 2024).
- Goodwin, R.D.; Dierker, L.C.; Wu, M.; Galea, S.; Hoven, C.W.; Weinberger, A.H. Trends in U.S. depression prevalence from 2015 to 2020: The widening treatment gap. Am. J. Prev. Med. 2022, 63, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Arnett, J.J.; Žukauskienė, R.; Sugimura, K. The new life stage of emerging adulthood at ages 18–29 years: Implications for mental health. Lancet Psychiatry 2018, 1, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Chung, D. Premodernity, modernity, postmodernity, and Eu-modernity as the four stages of civilizational developmental psychology: Comte’s parallel human-civilizational developments. Adv. Appl. Sociol. 2018, 10, 369–420. [Google Scholar] [CrossRef]
- Potterton, R.; Austin, A.; Robinson, L.; Webb, H.; Allen, K.L.; Schmidt, U. Identity development and social-emotional disorders during adolescence and emerging adulthood: A systematic review and meta-analysis. J. Youth Adolesc. 2022, 51, 16–29. [Google Scholar] [CrossRef]
- Cattelino, E.; Chirumbolo, A.; Baiocco, R.; Calandri, E.; Morelli, M. School achievement and depressive symptoms in adolescence: The role of self-efficacy and peer relationships at school. Child Psychiatry Hum. Dev. 2021, 52, 571–578. [Google Scholar] [CrossRef]
- Hong, R.Y.; Zainal, N.H.; Ong, X.P.L. Longitudinal associations between academic competence-building and depression symptoms in early adolescence. Dev. Psychopathol. 2023, 35, 2061–2072. [Google Scholar] [CrossRef]
- Evans-Lacko, S.; Knapp, M. Global patterns of workplace productivity for people with depression: Absenteeism and presenteeism costs across eight diverse countries. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 1525–1537. [Google Scholar] [CrossRef]
- Amick, H.R.; Gartlehner, G.; Gaynes, B.N.; Forneris, C.; Asher, G.N.; Morgan, L.C.; Coker-Schwimmer, E.; Boland, E.; Lux, L.J.; Gaylord, S.; et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: Systematic review and meta-analysis. BMJ 2015, 351, h6019. [Google Scholar] [CrossRef]
- Blier, P.; Ward, H.E.; Tremblay, P.; Laberge, L.; Hébert, C.; Bergeron, R. Combination of antidepressant medications from treatment initiation for major depressive disorder: A double-blind randomized study. Am. J. Psychiatry 2010, 167, 281–288. [Google Scholar] [CrossRef]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional therapies for mental disorders. Nutr. J. 2008, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Rossom, R.C.; Shortreed, S.; Coleman, K.J.; Beck, A.; Waitzfelder, B.E.; Stewart, C.; Ahmedani, B.K.; Zeber, J.E.; Simon, G.E. Antidepressant adherence across diverse populations and healthcare settings. Depress. Anxiety 2016, 33, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lebin, L.G.; Novick, A.M. Selective serotonin reuptake inhibitors (SSRIs) in pregnancy: An updated review on risks to mother, fetus, and child. Curr. Psychiatry Rep. 2022, 24, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bayes, J.; Schloss, J.; Sibbritt, D. Investigation into the diets and nutritional knowledge of young men with depression: The MENDDS survey. Nutrition 2020, 78, 110946. [Google Scholar] [CrossRef]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can. J. Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Campisi, S.C.; Zasowski, C.; Shah, S.; Bradley-Ridout, G.; Madigan, S.; Szatmari, P.; Korczak, D.J. Do healthy dietary interventions improve pediatric depressive symptoms? A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 2495–2507. [Google Scholar] [CrossRef]
- O’Neill, S.; Minehan, M.; Knight-Agarwal, C.R.; Turner, M. Depression, is it treatable in adults utilising dietary interventions? A systematic review of randomised controlled trials. Nutrients 2022, 14, 1398. [Google Scholar] [CrossRef]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Box 6.4.a: Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials in MEDLINE: Sensitivity-Maximizing Version (2008 Revision); PubMed Format. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (Updated March 2011). 2024. Available online: https://handbook-5-1.cochrane.org/chapter_6/box_6_4_a_cochrane_hsss_2008_sensmax_pubmed.htm (accessed on 7 November 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Crutzen, R. Adding effect sizes to a systematic review on interventions for promoting physical activity among European teenagers. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 29. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Obtaining Standard Deviations from Standard Errors and Confidence Intervals for Group Means. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. 2024. Available online: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm (accessed on 7 November 2024).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J.; Chambers, J.R.; Gupta, D.; Newey, B.; Lim, C.K. A brief diet intervention can reduce symptoms of depression in young adults—A randomised controlled trial. PLoS ONE 2019, 14, e0222768. [Google Scholar] [CrossRef] [PubMed]
- Bayes, J.; Schloss, J.; Sibbritt, D. The effect of a Mediterranean diet on the symptoms of depression in young males (the “AMMEND: A Mediterranean diet in men with depression” study): A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 572–580. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.H.; Park, M.; Lee, H.J. Effects of flavonoid-rich orange juice intervention on major depressive disorder in young adults: A randomized controlled trial. Nutrients 2022, 15, 145. [Google Scholar] [CrossRef]
- Velichkov, M.; Bezur, Z.; van Reekum, C.M.; Williams, C.M. A biphasic response to blueberry supplementation on depressive symptoms in emerging adults: A double-blind randomized controlled trial. Eur. J. Nutr. 2024, 63, 1071–1088. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Bendall, S.; Jackson, H.J.; Killackey, E.; Allot, K.; Johnson, T.; Harrigan, S.; Gleeson, J.; McGorryet, P.D. The credibility and acceptability of befriending as a control therapy in a randomized controlled trial of cognitive behaviour therapy for acute first episode psychosis. Behav. Cogn. Psychother. 2006, 34, 277–291. [Google Scholar] [CrossRef]
- WHO. WHOQOL-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment: Field Trial Version, December 1996. 2024. Available online: https://www.who.int/publications/i/item/WHOQOL-BREF (accessed on 25 November 2024).
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A modified Mediterranean dietary intervention for adults with major depression: Dietary protocol and feasibility data from the SMILES trial. Nutr. Neurosci. 2017, 21, 487–501. [Google Scholar] [CrossRef]
- Garcia-Toro, M.; Aguirre, I. Biopsychosocial model in depression revisited. Med. Hypotheses 2007, 68, 683–691. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- SAMHSA. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); Table 9, DSM-IV to DSM-5 Major Depressive Episode/Disorder Comparison; June 2016. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519712/table/ch3.t5/ (accessed on 7 November 2024).
- Usán, P.; Salavera, C.; Quílez-Robres, A. Self-efficacy, optimism, and academic performance as psychoeducational variables: Mediation approach in students. Children 2022, 9, 420. [Google Scholar] [CrossRef]
- Pignault, A.; Rastoder, M.; Houssemand, C. The relationship between self-esteem, self-efficacy, and career decision-making difficulties: Psychological flourishing as a mediator. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 1553–1568. [Google Scholar] [CrossRef]
- Yan, D.; Yang, X.; Zhang, H. Personality traits, self-efficacy, and friendship establishment: Group characteristics and network clustering of college students’ friendships. Front. Psychol. 2022, 13, 916938. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Klimova, B.; Novotny, M.; Schlegel, P.; Valis, M. The effect of mediterranean diet on cognitive functions in the elderly population. Nutrients 2021, 13, 2067. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.C.; Story, M.; Larson, N.I.; Neumark-Sztainer, D.; Lytle, L.A. Emerging adulthood and college-aged youth: An overlooked age for weight-related behavior change. Obesity 2008, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wall, M.; Larson, N.; Neumark-Sztainer, D.; Winpenny, E.M. Changes in diet quality across life transitions from adolescence to early adulthood: A latent growth analysis. Am J Clin Nutr. 2024, 120, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
| Author (Year), Country | Sample Size (% Female), Mean Age ± SD or SE Marked with * | Recruitment | Duration | Intervention | Comparison | Measure of Depressive Symptoms, Mean Score (Baseline → After Intervention) | Other Outcomes [Measure] | Summary of Findings |
|---|---|---|---|---|---|---|---|---|
| Francis et al. (2019), Australia [28] | I: n = 38 (63.2), 19.53 ± 2.1 C: n = 38 (63.2), 19.67 ± 2.8 | University community | 3 weeks |
| No intervention |
|
|
|
| Bayes et al. (2022), Australia [29] | I: n = 36 (0), 21.5 ± 2.9 C: n = 36 (0), 22.5 ± 2.5 | Community | 12 weeks |
| Befriending support on the same visit schedule and duration as the dietary intervention group |
|
|
|
| Park et al. (2020), Korea [30] | I: n = 20 (60.0), 22.20 ± 2.6 * C: n = 20 (60.0), 21.45 ± 2.3 * | Community | 8 weeks |
| Intake of a flavonoid-low orange cordial (190 mL each) twice a day (30–60 min before breakfast and dinner) |
| — |
|
| Choi et al. (2022), Korea [31] | I: n = 20 (70.0), 24.70 ± 2.8 C: n = 20 (70.0), 23.10 ± 2.6 | Community | 8 weeks |
| Intake of a flavonoid-low orange cordial (190 mL each) three times a day (30–60 min before breakfast, lunch, and dinner) |
| — |
|
| Velichkov et al. (2024), United Kingdom [32] | I: n = 30 (66.7), 19.9 ± 1.3 C: n = 30 (70.0), 20.1 ± 1.5 | University community | 6 weeks |
| Intake of a blueberry-flavoured placebo drink every morning |
|
| Acute effects (2 h)
Chronic effects (6 weeks)
|
| Accuracy | Reaction Time | |
|---|---|---|
| Acute effect | Significant difference favouring the intervention group | Significant difference favouring the control group |
| Chronic effect | No significant difference between groups | No significant difference between groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, H.; Ishihara, K.; Kanejima, Y.; Nakatani, M.; Izawa, K.P. Impact of Dietary Interventions on Depression in Adolescents and Young Adults: A Systematic Review and Meta-Analysis. Psychiatry Int. 2025, 6, 62. https://doi.org/10.3390/psychiatryint6020062
Koo H, Ishihara K, Kanejima Y, Nakatani M, Izawa KP. Impact of Dietary Interventions on Depression in Adolescents and Young Adults: A Systematic Review and Meta-Analysis. Psychiatry International. 2025; 6(2):62. https://doi.org/10.3390/psychiatryint6020062
Chicago/Turabian StyleKoo, Hanhwa, Kodai Ishihara, Yuji Kanejima, Miki Nakatani, and Kazuhiro P. Izawa. 2025. "Impact of Dietary Interventions on Depression in Adolescents and Young Adults: A Systematic Review and Meta-Analysis" Psychiatry International 6, no. 2: 62. https://doi.org/10.3390/psychiatryint6020062
APA StyleKoo, H., Ishihara, K., Kanejima, Y., Nakatani, M., & Izawa, K. P. (2025). Impact of Dietary Interventions on Depression in Adolescents and Young Adults: A Systematic Review and Meta-Analysis. Psychiatry International, 6(2), 62. https://doi.org/10.3390/psychiatryint6020062