Abstract
Coronavirus 2019 (COVID-19) lockdown periods had a significant negative impact on people’s lives and psychological well-being. However, the impact of lockdowns differed between individuals. This study aimed to identify vulnerable groups and investigated the relationship between mood and perceived immune fitness and the number and severity of coronavirus 2019 (COVID-19) symptoms during the first COVID-19 lockdown in the Netherlands. In addition, the impact of emotion regulation and other preventive measures was considered. The aim of the study was to identify possible differences according to sex, age, and the presence of underlying disease. A two-part online survey among N = 1415 individuals of the Dutch population (18 to 94 years old) was conducted in the summer of 2020. N = 541 of these participants also completed part 2 of the survey. A series of questionnaires was completed on mood, quality of life, lifestyle, immune fitness, and the number and severity of COVID-19 symptoms. Retrospectively, the period before the first lockdown (15 January–14 March 2020) was compared with the first lockdown (15 March–11 May 2020). The analysis revealed that the lockdown period was associated with significantly poorer mood, poorer immune fitness, and reduced quality of life. Poorer mood was associated with a significantly reduced immune fitness and a significant increase in the number and severity of COVID-19 symptoms. Mood changes did not differ significantly between men and women. Some mood effects were significantly more pronounced for individuals with underlying diseases (depression, fatigue, and stress) and younger individuals (depression and loneliness). Regarding lifestyle factors, no significant lockdown effects were seen according to underlying disease status. During the lockdown period, women reported a decline in nutrition scores, which was not seen in men, whereas they reported receiving more support from family and friends than men. Regarding age, younger individuals reported a significantly greater negative impact on physical activity and being active than the older participants. No differential effects for the groups were found for health correlates. In conclusion, significant negative lockdown effects on mood, quality of life, and immune fitness were observed across the population. The effects were significantly more pronounced among young individuals and those with underlying disease.
Keywords:
COVID-19; lockdown; immune fitness; mood; quality of life; coping; underlying disease; age; sex 1. Introduction
There is a growing scientific literature pointing at the negative consequences of the 2019 coronavirus disease (COVID-19) pandemic lockdowns for public health and psychological well-being [1,2,3,4,5,6,7,8]. However, the impact of lockdowns differed between individuals. Various factors have been suggested to influence the magnitude of the impact of lockdowns on mood and wellbeing, including but not limited to the duration of the lockdown, the extent of disruption of daily routines [6], the level of social isolation [9], the lack of psychological or physical coping strategies [10], financial and career consequences [6], and the impact of changes in lifestyle such as sleep, substance use, and nutrition [6,11].
Most governments around the world took preventive measures to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including lockdowns. In the Netherlands, the first lockdown was enforced from 15th March to 11th May 2020, including the closure of schools, bars, restaurants, and all businesses except supermarkets and pharmacies. Except for those with essential occupations, such as the police and health workers, people were advised to work from home where possible. Leaving home was only allowed for essential activities, and meetings with more than three people were not allowed.
The first Dutch lockdown period was associated with a significant reduction in positive COVID-19 cases [12,13]. However, lockdown periods had a significant negative impact on people’s lives and psychological well-being. For example, lockdown periods have been associated with increased reporting of stress, anxiety, and depression [4,5,6,7,8,11]. However, several factors impacted the level at which individuals were affected by lockdown measures, including but not limited to a lack of coping strategies, financial burdens (e.g., job loss), lifestyle changes (e.g., sleep and dietary patterns), and disruption of daily routines [6].
Of particular concern are vulnerable populations of individuals who are at increased risk when becoming infected with SARS-CoV-2, such as the elderly [14], and those with underlying diseases, such as diabetes, asthma, and cardiovascular diseases [15]. As these vulnerable populations may be more susceptible to lockdown-associated stress, this may lead to relapse or worsening of the course of their illness [16,17]. A systematic review [18] confirmed that the psychological impact of the COVID-19 pandemic on the mental health of vulnerable populations was significantly greater compared to the general population.
The negative impact of mood changes during the COVID-19 pandemic among vulnerable populations should not be underestimated. For example, a study found that anxiety was the second highest risk factor of death among individuals with underlying diseases [15]. When considering the impact of age on psychological well-being during the COVID-19 pandemic, one should take into account that the elderly more often have underlying diseases than younger adults. Several studies have described the impact of age on mental health responses during the COVID-19 pandemic. Studies revealed that older adults tend to report greater emotional well-being than younger people [19,20] and better coping strategies [21]. The latter may be due to the fact that the ability of young people to cope with distressed situations and emotions may not be fully developed yet [22,23]. Research further showed that the elderly had less negativity towards the COVID-19 crisis than younger adults [24]. The loss of social and work-related activities during lockdown periods may have had a greater negative impact on mental health among young adults. This could be related to the fact that the elderly more often already lived alone or did not participate in economic activities. However, other studies found increased levels of anxiety and depression among the elderly [25,26,27,28]. This was related to increased levels of social disconnectedness among the elderly and reduced access to health services [29,30,31,32]. Taken together, studies show mixed results regarding the impact of age on COVID-19 lockdown effects. Depending on the sociodemographic and health characteristics of individuals, increased levels of psychological distress during the COVID-19 pandemic [33], or the absence thereof [34], has been reported for both young adults and the elderly, and more research is needed on what factors make individuals more or less resilient to the health effects associated with COVID-19 lockdowns [35].
Previous research revealed that immune fitness plays a central role in health and disease [36] and also significantly impacted an individual’s vulnerability during the COVID-19 pandemic [37]. Immune fitness can be defined as the body’s capacity to respond to health challenges (such as infections) by activating an appropriate immune response, which is essential to maintain health and prevent disease [36]. Previous research revealed that poorer immune fitness in 2019 was the strongest predictor of reporting a greater number of COVID-19 symptoms and higher symptom severity ratings during the start of the pandemic [37]. Therefore, in the current study, immune fitness was also assessed.
The first aim of the current study was to investigate the effects of the first COVID-19 lockdown in the Netherlands on mood, lifestyle factors, and coping strategies and whether these were different among vulnerable groups. To this extent, analyses were conducted to contrast subsamples according to sex, age, and having underlying diseases. A second aim of the study was to relate these findings with experienced immune fitness and the number and severity of reported COVID-19 symptoms. Taken together, the analysis aimed to identify vulnerable groups at risk that may need additional support from health professionals and service managers.
2. Methods
Directly after the first lockdown period in the Netherlands (between the 24th of June and the 26th of July 2020), an online survey was conducted among the Dutch general population [38]. Participants were approached via Facebook advertisement, and the survey was designed in SurveyMonkey. Individuals could participate if they were aged 18 years and older. There were no exclusion criteria. The Ethics Committee of the Faculty of Social and Behavioral Sciences of Utrecht University approved the study (approval code: FETC17-061, approval date: 8 June 2017), and electronic informed consent was obtained from all participants. The survey comprised two parts. In Part I, demographics, mood, health correlates, and the number and severity of COVID-19 symptoms were assessed. In Part II, lifestyle factors and coping were assessed. A detailed description of the study methodology is published elsewhere [38].
For the current analysis, demographic information on sex and age were considered. In addition, participants reported whether they had chronic health conditions, including the most common chronic diseases and conditions in Dutch adults and the elderly [39]. These comprised cardiovascular diseases or hypertension, diabetes, liver disease, neurological diseases, immune disorders, allergy, kidney disease, pulmonary diseases, anxiety, depression, sleep disorders, or “other”. The open answers in the category “other” were reviewed by the study physician (J.B.), and if warranted, these were allocated to the appropriate disease category.
The assessments of mood, quality of life, lifestyle, immune fitness, and the number and severity of COVID-19 symptoms were made retrospectively for the period before the lockdown and for the first lockdown period.
Mood was assessed via single-item scales, with scores ranging from 0 (absent) to 10 (extreme). The assessed items included stress, anxiety, depression, fatigue, hostility, loneliness, and happiness. Quality of life was assessed using a single-item scale ranging from 0 (poor) to 10 (excellent). The mood and quality of life single-item scales have been validated previously and have a high test-retest reliability [40,41,42]. Lifestyle factors of importance for coping and emotion regulation were assessed using a modified version of the FANTASTIC Lifestyle Checklist [38,43,44,45,46]. For the domains ‘support of family and friends’, ‘physical activity level’, ‘nutrition’, and ‘coping with stress’ sum scores were computed, whereas single item scores were used for the items sleep (“I sleep well and feel rested”) and optimism (“I am a positive or optimistic thinker”). Higher scores on items or domains correspond to a better or healthier lifestyle. Finally, ‘being active’ was assessed with a 1-item scale, ranging from 0 (extremely inactive) to 10 (very active) [42].
Immune fitness was assessed with a single-item scale ranging from 0 (poor) to 10 (excellent) [36]. The number and severity of COVID-19 symptoms were assessed with a scale including the items: sneezing, running nose, sore throat, cough, malaise/feeling sick, high temperature (up to 38 Celsius), fever (38 Celsius and higher), shortness of breath, and chest pain [38]. The items were rated as none (0), mild (1), moderate (2) or severe (3). The COVID-19 symptom severity score was computed as the sum score of the nine items (range 0 to 27), with higher scores implying a greater severity. The number of COVID-19 symptoms was the sum of items with a severity score greater than zero.
Data Analysis
The data were analyzed with SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY, USA, IBM Corp.). Mean and standard deviation (SD) were computed for all variables, and distributions were checked for normality with the Kolmogorov–Smirnov test and by visual inspection. Demographics for men and women were compared using the Independent Samples Mann–Whitney U Test. Sex differences were considered significant if p < 0.05. Differences between ‘before COVID-19′ and ‘during the first COVID-19 lockdown’ were analyzed with the Related-Samples Wilcoxon Signed Ranks Test. Differences were considered significant if p < 0.00625 (applying a Bonferroni’s correction for multiple comparisons). For demographics and health correlates, the difference was considered significant if p < 0.05. Difference scores (Δ, during lockdown—before COVID-19) were computed for all variables. Spearman’s correlations were computed between these variables. Correlations were considered significant if p < 0.00625 (applying a Bonferroni’s correction for multiple comparisons).
3. Results
N = 1415 participants, with an age range of 18 to 94 years old, completed Part I of the survey. Their demographics are summarized in Table 1. N = 514 participants also completed Part II of the survey, which included the assessments of lifestyle factors and coping. Significantly more women (64.5%) than men completed the survey. Women were significantly younger and had a lower BMI than men. The study outcomes are summarized in Table 2.

Table 1.
Demographics.

Table 2.
Mood, countermeasures, and health correlates.
For the lockdown period, significantly poorer mood and lifestyle ratings were reported, immune fitness was significantly poorer, and COVID-19 symptoms severity ratings were significantly higher.
Table 3 summarizes the correlations of the difference scores (during lockdown—before COVID-19) for mood, lifestyle factors, and health correlates with immune fitness and the number and severity of COVID-19 symptoms. The analysis revealed significant negative correlations between mood and immune fitness and significant positive correlations between mood and the number and severity of COVID-19 symptoms.

Table 3.
Correlations between mood, immune fitness, and experiencing COVID-19 symptoms.
Significant correlations were found between changes in mood, immune fitness, and the number and severity of COVID-19 symptoms (see Table 3). Most lifestyle factors also correlated significantly with immune fitness. Sleep, being active, and physical activity correlated significantly with the number and severity of COVID-19 symptoms. Coping with stress correlated significantly with the number of COVID-19 symptoms, but not with the severity score. Finally, reduced quality of life was associated with poorer immune fitness and reporting greater COVID-19 symptom severity.
3.1. Sex
The data for men (N = 503, 35.5%) and women (N = 912, 64.5%) are summarized in Table A1. Significant sex differences were observed, both before COVID-19 and during lockdown. Women reported poorer mood than men, both before COVID-19 and during lockdown. Immune fitness was poorer in women, and women reported more COVID-19 symptoms than men. However, the COVID-19 severity scores were significantly lower among women. Women reported poorer coping with stress and poorer sleep than men. On the other hand, women experienced greater support from family and friends than men. During lockdown, women were significantly less optimistic than men, reported poorer sleep, and had a significantly poorer ability to cope with stress.
Data on the difference scores (during lockdown—before COVID-19) are visualized in Figure 1. The analysis revealed that the lockdown did not differentially affect men and women with regard to mood, health correlates, and most lifestyle factors. Significant sex differences were found only for nutrition and support of family and friends (i.e., a healthier diet and more support in women), but the magnitude of these differences was small.
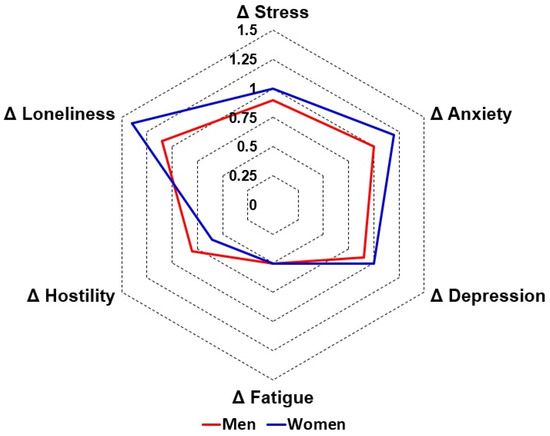
Figure 1.
Effects of the first COVID-19 lockdown in the Netherlands on mood in men and women. Difference scores (Δ, during lockdown—before COVID-19) are shown for men (red line) and women (blue line).
3.2. Age
To evaluate possible age effects, participants were allocated to one of the following age groups: ‘young adults’ (18–35 years old, N = 678, 35.5%), ‘adults’ (36–65 years old, N = 901, 47.2%), or ‘elderly’ (>65 years old, N = 331, 17.3%). The data for the age groups are summarized in Table A2 and Table A3. Compared to before COVID-19, all age groups reported poorer mood, lifestyle, and quality of life during lockdown. Age differences in these measures were already present before COVID-19. That is, mood was poorest among young adults and best among the elderly (See Table A2). The ability to cope with stress increases with increasing age, while physical activity and being active decreases with increasing age.
With regard to the lockdown effect, the difference scores (during lockdown—before COVID-19) were compared between the age groups. The lockdown effects on mood are summarized in Figure 2. Compared to the older age groups, for young adults the lockdown had a significantly greater negative impact on depression and loneliness, and was associated with a significant decrease in being active and physical activity. Compared to the elderly, young adults reported a significantly greater reduction in quality of life during the lockdown. No age differences were found for the assessed health correlates.
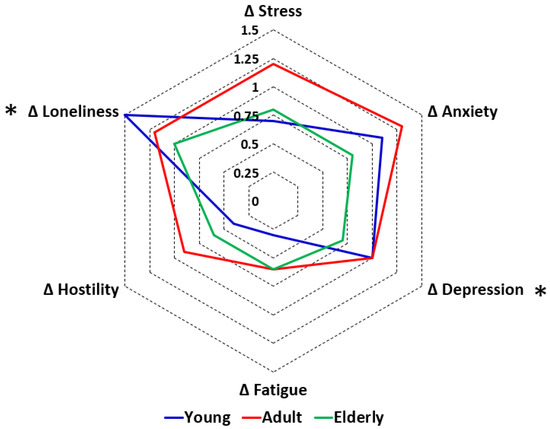
Figure 2.
Effects of the first COVID-19 lockdown in the Netherlands on mood according to age. Difference scores (Δ, during lockdown—before COVID-19) are shown for young adults (blue line), adults (red line), and the elderly (green line). Significant differences between age groups (p < 0.017, after Bonferroni’s correction for multiple comparisons) are indicated by *.
3.3. Underlying Disease
Of the sample, N = 484 (34.5%) reported no underlying diseases. N = 920 (65.5%) reported having one or more underlying diseases (See Table 4).

Table 4.
Type and occurrence of underlying disease.
Of the participants who reported underlying diseases, more than half reported having one underlying disease (N = 518, 56.3%), and the other participants reported a combination of two or more underlying diseases. The most frequently reported combinations were allergy and lung diseases (N = 26), diabetes and cardiovascular diseases (N = 21), sleep disorders and allergy (N = 16), allergy and cardiovascular diseases (N = 14), and lung diseases and cardiovascular diseases (N = 13).
The impact of underlying disease status is summarized in Table A4. Both before COVID-19 and during the lockdown, participants with underlying diseases reported significantly poorer mood, lifestyle, and health correlates compared to participants without underlying diseases. When directly comparing the difference scores (during lockdown—before COVID-19) of the group with and without underlying diseases, the analysis revealed no significant differences in lifestyle or health correlates. For mood, significantly greater negative changes were found for depression, stress, and fatigue among participants with underlying diseases (see Figure 3).
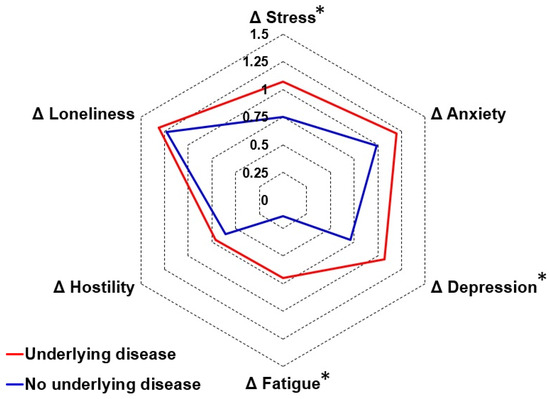
Figure 3.
Effects of the first COVID-19 lockdown in the Netherlands on mood in participants with and without underlying diseases. Difference scores (Δ, during lockdown—before COVID-19) are shown for participants with underlying diseases (red line) and individuals without underlying diseases (blue line). Significant differences (p < 0.05) are indicated by *.
4. Discussion
The first COVID-19 lockdown in the Netherlands was associated with significant negative effects on mood and lifestyle, reduced quality of life, and poorer immune fitness. These effects were accompanied by a significant increase in the number and severity of reported COVID-19 symptoms. Lifestyle factors, such as support of family and friends, coping with stress, adequate sleep, and attaining a healthy diet, reduced the negative lockdown effects on mood, immune fitness, and quality of life. However, the impact of lifestyle factors varied greatly between individuals.
Factors that differentially influenced the lockdown effects on mood included age and having underlying diseases, whereas no differential lockdown effects according to sex were observed. With regard to age, the lockdown had a significantly greater negative impact on ratings of depression and loneliness among younger individuals. This highlights the vulnerability of younger individuals in the face of prolonged social restrictions and limited opportunities for social interaction. For individuals with underlying diseases, the lockdown effects on depression, fatigue, and stress were significantly more pronounced compared to individuals without underlying diseases. This suggests that individuals with pre-existing health conditions may face additional challenges adapting to the lockdown measures and managing their psychological well-being.
Differential effects for lifestyle factors were found for sex and age. Women reported a significantly greater increase in support of family and friends than men. Whereas men reported significantly poorer nutrition during lockdown, the positive difference score in women suggested a small improvement in nutrition. These findings emphasize the importance of considering sex-specific needs and challenges when implementing public health measures. With regard to age, younger individuals reported a significantly greater impact of the lockdown on being active and physical activity compared to older individuals. The magnitude of changes in lifestyle factors did not significantly differ between participants with and without underlying diseases.
In a previous article, we reported that immune fitness before the COVID-19 pandemic was the strongest predictor of the presence and severity of COVID-19 symptoms during the first lockdown in the Netherlands [37]. The results reported here show that this relationship was not differentially impacted by sex, age, or having underlying diseases. This observation is in line with the regression analyses presented by Kiani et al. [37], which yielded models that did not include these variables as significant predictors of the number or severity of COVID-19 symptoms. Studies from the Netherlands and Germany conducted among young adults also reported poorer immune fitness during lockdown periods, which significantly correlated with negative mood changes and reduced quality of life [47,48]. The current study confirmed these findings for the general Dutch population.
A strength of the study is its adequate sample size to allow the planned comparisons according to age, sex, and underlying disease status. Although, as expected, the elderly were underrepresented, the sample size was adequate for age group comparisons and had a considerable age range. There was a good diversity of underlying diseases among the sample. However, the sample was a convenience sample, which may impact generalizability. A limitation of the study included the fact that all data were collected retrospectively and were self-reported. Given the latter, recall bias may have influenced the study outcomes. The survey was conducted after the first COVID-19 lockdown in the Netherlands. At that time, knowledge on COVID-19 was limited, and there were no standardized questionnaires available to assess COVID-19 severity. The scale used in the current study was developed in the first quarter of 2020 and included symptoms that at that time were known for the alpha variant of SARS-CoV-2, as listed by the National Institute for Public Health and the Environment. The scale mostly comprised respiratory symptoms. However, it should be noted that COVID-19 is not limited to respiratory symptoms: as many other organ systems may be affected, extra-pulmonary symptoms (e.g., loss of smell and taste) may also be experienced [49]. Although it was asked whether participants were tested for infection with SARS-CoV-2, at the beginning of the COVID-19 pandemic, self-tests for COVID-19 were not available. Therefore, the actual COVID-19 status of the majority of the participants of the current study was not confirmed. The study consisted of a primary survey (Part 1) and a second survey (Part 2). After completion of Part 1, participants were invited to also complete Part 2. As expected from previous research [50], the sample that participated in Part 2 was much smaller than the number of participants that completed Part 1. Nevertheless, the sample size of Part 2 was appropriate to conduct the presented analyses with sufficient statistical power.
5. Conclusions
Notwithstanding its limitations, the results of our study are in line with previous findings that the COVID-19 lockdown had negative effects on mood, lifestyle, and health correlates. The effects on depression, loneliness, and being active were most pronounced among younger individuals. The differential impact of the lockdown measures among vulnerable groups supports the need for targeted support and interventions. In general, the findings stress that individuals’ well-being and mental health should not be underestimated and taken into consideration when designing public health strategies for future pandemics.
Author Contributions
Conceptualization, P.K., P.A.H., G.B., J.G. and J.C.V.; methodology, J.C.V., N.R.S. and A.S.M.S., formal analysis, J.C.V. and J.B.; writing—original draft preparation, P.K., J.B. and J.C.V.; writing—review and editing, P.K., J.B., J.G., P.A.H., N.R.S., A.S.M.S., G.B. and J.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Social and Behavioral Sciences of Utrecht University (an ‘umbrella’ approval to conduct a series of survey studies on the topic ‘immune fitness and health’, approval code: FETC17-061, approval date: 8 June 2017).
Informed Consent Statement
Electronic informed consent was obtained from all participants.
Data Availability Statement
The data are available upon request from the corresponding author.
Conflicts of Interest
P.K. is CEO of PanGenix. Over the past 3 years, J.C.V. has acted as a consultant/advisor for Eisai, KNMP, Red Bull, Sen-Jam Pharmaceutical, and Toast! J.G. is part-time employee of Nutricia Research and received research grants from Nutricia research foundation, Top Institute Pharma, Top Institute Food and Nutrition, GSK, STW, NWO, Friesland Campina, CCC, Raak-Pro, and EU. The other authors have no potential conflict of interest to disclose.
Appendix A

Table A1.
Assessments for before and during lockdown, according to sex.
Table A1.
Assessments for before and during lockdown, according to sex.
| Before Lockdown | During Lockdown | Difference Score 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Men | Women | p-Value | Men | Women | p-Value | Men | Women | p-Value |
| Mood | |||||||||
| Anxiety | 1.3 (2.0) | 1.8 (2.5) | 0.001 S | 2.2 (2.7) * | 3.1 (3.1) * | <0.001 S | 1.0 (2.0) | 1.2 (2.5) | 0.048 |
| Depression | 1.3 (2.2) | 1.8 (2.5) | 0.001 S | 2.2 (2.9) * | 2.7 (3.1) * | 0.004 S | 0.9 (2.0) | 1.0 (2.2) | 0.423 |
| Loneliness | 1.8 (2.6) | 2.1 (2.6) | 0.026 | 2.9 (3.2) * | 3.5 (3.4) * | 0.001 S | 1.1 (2.3) | 1.4 (2.5) | 0.020 |
| Fatigue | 3.2 (2.8) | 4.2 (3.0) | <0.001 S | 3.7 (2.9) * | 4.7 (3.1) * | <0.001 S | 0.5 (2.1) | 0.5 (2.6) | 0.800 |
| Hostility | 1.0 (2.0) | 0.9 (1.8) | 0.018 | 1.8 (2.7) * | 1.5 (2.5) * | 0.010 | 0.8 (2.0) | 0.6 (1.8) | 0.210 |
| Happiness | 6.9 (2.3) | 6.8 (2.3) | 0.122 | 6.0 (2.5) * | 5.9 (2.5) * | 0.228 | −0.9 (2.0) | −0.9 (2.3) | 0.955 |
| Stress | 2.2 (2.6) | 3.2 (2.9) | <0.001 S | 3.1 (3.1) * | 4.2 (3.1) * | 0.001 S | 0.9 (2.4) | 1.0 (2.8) | 0.658 |
| Lifestyle factors and coping | |||||||||
| Optimism | 3.2 (0.9) | 3.0 (1.0) | 0.039 | 3.0 (1.0) | 2.7 (1.1) * | 0.005 S | −0.2 (0.7) | −0.3 (0.8) | 0.152 |
| Coping with stress | 6.5 (1.5) | 5.7 (1.8) | <0.001 S | 6.2 (1.7) | 5.4 (2.0) * | <0.001 S | −0.3 (1.1) | −0.3 (1.3) | 0.557 |
| Support of family and friends | 5.6 (2.1) | 6.2 (1.8) | 0.001 S | 5.8 (1.9) | 6.3 (1.7) | 0.014 | 0.2 (0.9) | 0.1 (1.0) | 0.004 S |
| Nutrition | 7.5 (3.0) | 7.9 (2.7) | 0.268 | 7.6 (2.8) | 7.5 (2.8) * | 0.518 | 0.1 (1.4) | −0.4 (1.6) | <0.001 S |
| Being active | 6.5 (2.6) | 6.5 (2.5) | 0.780 | 5.6 (2.8) * | 5.4 (2.7) * | 0.148 | −0.9 (2.5) | −1.1 (2.8) | 0.438 |
| Physical activity level | 4.8 (2.1) | 5.2 (2.0) | 0.047 | 4.5 (2.0) | 4.7 (2.1) * | 0.785 | −0.3 (1.6) | −0.6 (1.8) | 0.071 |
| Sleep | 2.8 (1.1) | 2.5 (1.1) | 0.003 S | 2.8 (1.1) | 2.4 (1.2) * | 0.001 S | −0.1 (0.7) | −0.1 (0.8) | 0.333 |
| Health correlates | |||||||||
| Quality of life | 7.2 (2.2) | 7.1 (2.1) | 0.222 | 6.2 (2.4) * | 6.3 (2.4) * | 0.906 | −1.0 (2.0) | −0.9 (2.2) | 0.525 |
| Immune fitness | 7.6 (1.7) | 7.2 (1.9) | <0.001 S | 7.4 (1.8) * | 6.9 (2.1) * | <0.001 S | −0.2 (1.2) | −0.3 (1.3) | 0.582 |
| COVID-19 Symptom—presence | 2.4 (2.1) | 3.9 (4.0) | 0.081 | 2.4 (2.1) | 4.3 (4.4) | 0.010 S | 0.0 (1.8) | 0.1 (2.1) | 0.354 |
| COVID-19 Symptom—severity | 3.3 (3.4) | 2.7 (2.2) | 0.030 S | 3.4 (3.7) | 2.8 (2.3) | 0.001 S | 0.1 (3.4) | 0.4 (4.3) | 0.306 |
Mean, standard deviation (SD, between brackets), and p-values are shown. Significant sex differences (p < 0.05) are indicated by S. Significant differences between before and during lockdown are indicated by *. Differences in mood and lifestyle factors were considered significant if p < 0.00625, after Bonferroni’s correction for multiple comparisons, and if p < 0.05 for health correlates. 1 Difference score = during lockdown—before lockdown.

Table A2.
Assessments for before and during lockdown, according to age.
Table A2.
Assessments for before and during lockdown, according to age.
| Time Period | Before Lockdown | During Lockdown | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | p-Value Paired Comparison | Mean (SD) | p-Value Paired Comparison | |||||||||
| Age Range | Young (18–35) | Adult (36–65) | Elderly (>65) | Young—Adult | Adult—Elderly | Young—Elderly | Young (18–35) | Adult (36–65) | Elderly (>65) | Young—Adult | Adult—Elderly | Young—Elderly |
| Mood | ||||||||||||
| Anxiety | 2.1 (2.5) | 1.5 (2.4) | 0.9 (1.9) | <0.001 A | 0.002 A | <0.001 A | 3.1 (2.9) * | 2.8 (3.2) * | 1.7 (2.6) * | 0.002 A | <0.001 A | <0.001 A |
| Depression | 2.0 (2.4) | 1.5 (2.4) | 0.9 (1.8) | <0.001 A | 0.001 A | <0.001 A | 3.1 (3.0) * | 2.5 (3.1) * | 1.6 (2.7) * | <0.001 A | <0.001 A | <0.001 A |
| Loneliness | 2.1 (2.4) | 1.9 (2.8) | 1.9 (2.8) | <0.001 A | 0.559 | 0.001 A | 3.7 (3.1) * | 3.1 (3.5) * | 2.9 (3.4) * | <0.001 A | 0.577 | <0.001 A |
| Fatigue | 4.5 (2.7) | 3.7 (3.1) | 2.3 (2.5) | <0.001 A | <0.001 A | <0.001 A | 4.8 (2.7) | 4.4 (3.2) * | 2.9 (2.8) * | 0.016 A | <0.001 A | <0.001 A |
| Hostile | 1.5 (2.4) | 0.9 (1.9) | 0.6 (1.6) | 0.012 A | 0.004 A | <0.001 A | 2.1 (2.4) * | 1.8 (2.8) * | 1.2 (2.4) * | 0.505 | <0.001 A | 0.002 A |
| Happy | 7.0 (1.9) | 6.8 (2.5) | 6.7 (2.6) | - | - | - | 6.1 (2.1) * | 5.8 (2.7) * | 5.9 (2.8) * | - | - | - |
| Stress | 4.1 (2.7) | 2.3 (2.7) | 1.2 (2.1) | <0.001 A | <0.001 A | <0.001 A | 4.8 (2.7) * | 3.6 (3.3) * | 2.0 (2.9) * | <0.001 A | <0.001 A | <0.001 A |
| Lifestyle factors and coping | ||||||||||||
| Optimism | 3.0 (1.0) | 3.1 (1.0) | 3.4 (0.9) | 0.100 | 0.008 A | <0.001 A | 2.7 (1.0) * | 2.9 (1.1) * | 3.2 (0.9) | 0.011 A | 0.008 A | <0.001 A |
| Coping with stress | 5.5 (1.7) | 6.2 (1.7) | 6.8 (1.3) | <0.001 A | 0.005 A | <0.001 A | 5.2 (1.8) * | 5.9 (2.0) * | 6.6 (1.5) * | 0.001 A | 0.010 A | 0.001 A |
| Support of family and friends | 6.3 (1.8) | 5.8 (2.0) | 5.6 (1.9) | 0.018 | 0.263 | 0.003 A | 6.4 (1.6) | 5.9 (1.9) * | 5.9 (1.7) * | - | - | - |
| Nutrition | 8.3 (2.8) | 7.1 (2.7) | 8.1 (2.7) | <0.001 A | 0.007 A | 0.588 | 7.9 (2.8) * | 7.0 (2.8) | 7.9 (2.7) | 0.001 A | 0.006 A | 0.900 |
| Being active | 6.7 (2.2) | 6.5 (2.7) | 6.2 (2.8) | - | - | - | 5.5 (2.4) * | 5.5 (2.9) * | 5.6 (2.9) * | - | - | - |
| Physical activity level | 5.4 (2.0) | 5.0 (2.1) | 4.4 (2.1) | 0.018 | 0.042 | <0.001 A | 4.6 (2.1) * | 4.7 (2.1) * | 4.4 (2.1) | - | - | - |
| Sleep | 2.7 (1.0) | 2.6 (1.2) | 2.9 (1.2) | - | - | - | 2.6 (1.0) | 2.5 (1.3) | 2.7 (1.2) | - | - | - |
| Health correlates | ||||||||||||
| Quality of life | 7.3 (1.8) | 7.1 (2.3) | 7.2 (2.4) | - | - | - | 6.3 (2.1) * | 6.2 (2.7) * | 6.5 (2.5) * | - | - | - |
| Immune fitness | 7.4 (1.7) | 7.2 (2.1) | 7.5 (1.7) | - | - | - | 7.2 (1.8) | 6.9 (2.2) * | 7.2 (2.0) * | - | - | - |
| COVID-19 Symptom—presence | 2.8 (2.2) | 2.4 (2.2) | 2.4 (1.8) | 0.002 A | 0.634 | 0.063 | 2.8 (2.2) | 2.6 (2.4) * | 2.3 (1.8) | - | - | - |
| COVID-19 Symptom—severity | 4.0 (3.8) | 3.5 (3.9) | 3.5 (3.3) | 0.011 A | 0.476 | 0.242 | 4.1 (4.2) | 4.1 (4.5) * | 3.5 (3.5) | - | - | - |
Mean and standard deviation (SD, between brackets) are shown. Paired comparisons were conducted if the main effect of mood or lifestyle factors was p < 0.00625 (after Bonferroni’s correction) and p < 0.05 for health correlates. Significant differences between the age groups (p < 0.017, after Bonferroni’s correction) are indicated by A. Differences between ‘before lockdown’ and ‘during lockdown’ for mood and lifestyle factors were considered significant if p < 0.00625, after Bonferroni’s correction for multiple comparisons, and if p < 0.05 for health correlates. Significant differences between before and during lockdown are indicated by *.

Table A3.
Lockdown effects according to age.
Table A3.
Lockdown effects according to age.
| Difference Score 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Age Range | Young (18–35) | Adult (36–65) | Elderly (>65) | Overall | Young—Adult | Adult—Elderly | Young—Elderly |
| Mood | |||||||
| Anxiety | 1.1 (2.3) | 1.3 (2.6) | 0.8 (1.7) | 0.009 | - | - | - |
| Depression | 1.0 (2.2) | 1.0 (2.2) | 0.7 (1.7) | 0.004 A | 0.002 A | 0.183 | 0.014 |
| Loneliness | 1.5 (2.4) | 1.2 (2.5) | 1.0 (2.3) | <0.001 A | <0.001 A | 0.790 | <0.001 A |
| Fatigue | 0.3 (2.7) | 0.6 (2.3) | 0.6 (1.7) | 0.249 | - | - | - |
| Hostility | 0.4 (1.5) | 0.9 (2.2) | 0.6 (1.8) | 0.017 | - | - | - |
| Happiness | −0.9 (2.1) | −1.0 (2.3) | -0.8 (1.9) | 0.288 | - | - | - |
| Stress | 0.7 (2.8) | 1.2 (2.7) | 0.8 (2.0) | 0.030 | - | - | - |
| Lifestyle factors and coping | |||||||
| Optimism | −0.3 (0.9) | −0.2 (0.6) | −0.1 (0.6) | 0.071 | - | - | - |
| Coping with stress | −0.3 (1.5) | −0.3 (1.0) | −0.3 (0.9) | 0.759 | - | - | - |
| Support of family and friends | 0.1 (1.1) | 0.1 (1.0) | 0.3 (0.6) | 0.140 | - | - | - |
| Nutrition | −0.4 (2.0) | −0.1 (1.4) | −0.1 (0.8) | 0.214 | - | - | - |
| Being active | −1.2 (3.0) | −1.0 (2.6) | −0.6 (2.1) | 0.005 A | 0.023 | 0.142 | 0.002 A |
| Physical activity level | −0.8 (2.1) | −0.3 (1.5) | 0.0 (1.2) | <0.001 A | 0.002 A | 0.108 | <0.001 A |
| Sleep | −0.1 (1.0) | −0.1 (0.6) | −0.1 (0.5) | 0.445 | - | - | - |
| Health correlates | |||||||
| Quality of life | −1.0 (1.9) | −0.9 (2.4) | −0.7 (1.9) | 0.013 A | 0.092 | 0.087 | 0.004 A |
| Immune fitness | −0.2 (1.3) | −0.2 (1.3) | −0.3 (1.2) | 0.422 | - | - | - |
| COVID-19 Symptom—presence | 0.0 (2.1) | 0.2 (2.0) | 0.0 (1.1) | 0.194 | - | - | - |
| COVID-19 Symptom—severity | 0.1 (4.2) | 0.5 (4.2) | 0.0 (2.4) | 0.332 | - | - | - |
Mean and standard deviation (SD, between brackets) are shown. Paired comparisons were conducted if the main effect of mood or lifestyle factors was p < 0.00625 (after Bonferroni’s correction) and p < 0.05 for health correlates. Significant differences between the age groups (p < 0.017, after Bonferroni’s correction) are indicated by A. Differences between ‘before lockdown’ and ‘during lockdown’ for mood and lifestyle factors were considered significant if p < 0.00625, after Bonferroni’s correction for multiple comparisons, and if p < 0.05 for health correlates. No significant differences between before and during lockdown were found. 1 Difference score = during lockdown—before lockdown.

Table A4.
Assessments according to underlying disease status.
Table A4.
Assessments according to underlying disease status.
| Before Lockdown | During Lockdown | Difference Score 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Underlying Disease | Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value |
| Mood | |||||||||
| Anxiety | 2.0 (2.6) | 1.0 (1.8) | <0.001 U | 3.2 (3.1) * | 2.0 (2.6) * | <0.001 U | 1.2 (2.5) | 1.0 (2.0) | 0.086 |
| Depression | 1.9 (2.6) | 1.0 (1.7) | <0.001 U | 3.0 (3.2) * | 1.7 (2.4) * | <0.001 U | 1.1 (2.2) | 0.7 (1.9) | 0.003 U |
| Loneliness | 2.3 (2.8) | 1.3 (2.1) | <0.001 U | 3.7 (3.5) * | 2.6 (3.0) * | <0.001 U | 1.3 (2.5) | 1.2 (2.4) | 0.427 |
| Fatigue | 4.2 (3.0) | 3.1 (2.7) | <0.001 U | 4.9 (3.0) * | 3.2 (2.7) | <0.001 U | 0.7 (2.5) | 0.1 (2.3) | <0.001 U |
| Hostility | 1.0 (2.0) | 0.7 (1.6) | 0.001 U | 1.8 (2.7) * | 1.3 (2.4) * | <0.001 U | 0.7 (1.9) | 0.6 (1.8) | 0.232 |
| Happiness | 6.7 (2.4) | 7.2 (2.1) | <0.001 U | 5.7 (2.5) * | 6.4 (2.3) * | <0.001 U | −1.0 (2.3) | −0.8 (1.9) | 0.027 |
| Stress | 3.0 (2.9) | 2.5 (2.6) | 0.003 U | 4.1 (3.2) * | 3.2 (2.9) * | <0.001 U | 1.1 (2.8) | 0.8 (2.5) | 0.006 U |
| Lifestyle factors and coping | |||||||||
| Optimism | 3.0 (1.0) | 3.3 (0.8) | 0.001 U | 2.7 (1.1) * | 3.0 (0.9) * | 0.013 | −0.2 (0.7) | −0.43 (0.7) | 0.218 |
| Coping with stress | 5.8 (1.8) | 6.4 (1.6) | <0.001 U | 5.5 (2.0) * | 6.1 (1.6) | 0.001 U | −0.3 (1.2) | −0.3 (1.3) | 0.440 |
| Support of family and friends | 5.8 (1.9) | 6.3 (1.9) | 0.004 U | 6.0 (1.7) * | 6.3 (1.9) | 0.028 | 0.2 (1.0) | 0.0 (1.0) | 0.015 |
| Nutrition | 7.6 (2.8) | 8.1 (2.8) | 0.047 | 7.2 (2.8) * | 8.1 (2.7) | 0.001 U | −0.4 (1.7) | 0.0 (1.4) | 0.030 |
| Being active | 6.4 (2.6) | 6.8 (2.5) | 0.003 U | 5.3 (2.7) * | 5.9 (2.7) * | <0.001 U | −1.1 (2.8) | −0.9 (2.5) | 0.151 |
| Physical activity level | 4.9 (2.0) | 5.4 (2.1) | 0.009 | 4.4 (2.1) * | 4.9 (2.1) * | 0.017 | −0.5 (1.8) | −0.5 (1.8) | 0.658 |
| Sleep | 2.5 (1.2) | 3.0 (0.9) | <0.001 U | 2.4 (1.2) | 2.9 (0.9) | <0.001 U | −0.1 (0.7) | −0.1 (0.8) | 0.914 |
| Health correlates | |||||||||
| Quality of life | 7.0 (2.2) | 7.5 (2.0) | <0.001 U | 6.0 (2.5) * | 6.8 (2.3) * | <0.001 U | −1.0 (2.2) | −0.8 (1.9) | 0.106 |
| Immune fitness | 7.0 (1.9) | 7.9 (1.5) | <0.001 U | 6.7 (2.1) * | 7.8 (1.6) * | <0.001 U | −0.3 (1.4) | −0.1 (1.1) | 0.244 |
| COVID-19 Symptom—presence | 2.9 (2.2) | 2.0 (2.0) | <0.001 U | 3.0 (2.3) | 2.0 (1.9) | <0.001 U | 0.2 (2.0) | −0.1 (1.9) | 0.331 |
| COVID-19 Symptom—severity | 4.3 (4.0) | 2.6 (3.0) | <0.001 U | 4.7 (4.5) * | 2.6 (3.4) | <0.001 U | 0.4 (4.2) | 0.0 (3.5) | 0.167 |
Mean and standard deviation (SD, between brackets), and p-values are shown. Significant differences between before and during lockdown are indicated by *. Significant differences between having underlying diseases and not having underlying diseases are indicated by U. Differences for mood and lifestyle factors were considered significant if p < 0.00625, after Bonferroni’s correction for multiple comparisons, and if p < 0.05 for health correlates. 1 Difference score = during lockdown—before lockdown.
References
- Panchal, U.; Salazar de Pablo, G.; Franco, M.; Moreno, C.; Parellada, M.; Arango, C.; Fusar-Poli, P. The impact of COVID-19 lockdown on child and adolescent mental health: Systematic review. Eur. Child. Adolesc. Psychiatry 2023, 32, 1151–1177. [Google Scholar] [CrossRef]
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.G.; Mukunzi, J.N.; McIntee, S.E.; Dalexis, R.D.; Goulet, M.A.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 295, 113599. [Google Scholar] [CrossRef] [PubMed]
- Solé, B.; Verdolini, N.; Amoretti, S.; Montejo, L.; Rosa, A.R.; Hogg, B.; Garcia-Rizo, C.; Mezquida, G.; Bernardo, M.; Martinez-Aran, A.; et al. Effects of the COVID-19 pandemic and lockdown in Spain: Comparison between community controls and patients with a psychiatric disorder. Preliminary results from the BRIS-MHC STUDY. J. Affect. Disord. 2021, 281, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.A.; Hidalgo-Mazzei, D.; Vieta, E.; Radua, J. Coping behaviors associated with decreased anxiety and depressive symptoms during the COVID-19 pandemic and lockdown. J. Affect. Disord. 2020, 275, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Petzold, M.B.; Bendau, A.; Plag, J.; Pyrkosch, L.; Mascarell Maricic, L.; Betzler, F.; Rogoll, J.; Große, J.; Ströhle, A. Risk, resilience, psychological distress, and anxiety at the beginning of the COVID-19 pandemic in Germany. Brain Behav. 2020, 10, e01745. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, P.A.; Kiani, P.; Garssen, J.; Bruce, G.; Verster, J.C. Living alone or together during lockdown: Association with mood, immune fitness and experiencing COVID-19 symptoms. Psychol. Res. Behav. Manag. 2021, 14, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Kaur, W.; Balakrishnan, V.; Chen, Y.Y.; Periasamy, J. Mental health risk factors and coping strategies among students in Asia Pacific during COVID-19 pandemic—A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 8894. [Google Scholar] [CrossRef]
- Merlo, A.; Severeijns, N.R.; Benson, S.; Scholey, A.; Garssen, J.; Bruce, G.; Verster, J.C. Mood and changes in alcohol consumption in young adults during COVID-19 lockdown: A model explaining associations with perceived immune fitness and experiencing COVID-19 symptoms. Int. J. Environ. Res. Public Health 2021, 18, 10028. [Google Scholar] [CrossRef]
- Statistics Netherlands. Overledenen; Geslacht en Leeftijd, per Week. 2023. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/70895ned/table?fromstatweb (accessed on 1 November 2020).
- National Institute for Public Health and the Environment (RIVM). Available online: https://www.rivm.nl/en/node/154271 (accessed on 1 November 2020).
- Lee, K.; Jeong, G.-C.; Yim, J. Consideration of the Psychological and Mental Health of the Elderly during COVID-19: A Theoretical Review. Int. J. Environ. Res. Public Health 2020, 17, 8098. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, 210123. [Google Scholar] [CrossRef]
- Phillips, L.J.; Francey, S.M.; Edwards, J.; McMurray, N. Strategies used by psychotic individuals to cope with life stress and symptoms of illness: A systematic review. Anxiety Stress Coping 2009, 22, 371–410. [Google Scholar] [CrossRef]
- Kozloff, N.; Mulsant, B.H.; Stergiopoulos, V.; Voineskos, A.N. The COVID-19 global pandemic: Implications for people with schizophrenia and related disorders. Schizophr. Bull. 2020, 46, 752–757. [Google Scholar] [CrossRef]
- Nam, S.H.; Nam, J.H.; Kwon, C.Y. Comparison of the mental health impact of COVID-19 on vulnerable and non-vulnerable groups: A systematic review and meta-analysis of observational studies. Int. J. Environ. Res. Public Health 2021, 18, 10830. [Google Scholar] [CrossRef]
- Carstensen, L.L.; Shavit, Y.Z.; Barnes, J.T. Age advantages in emotional experience persist even under threat from the COVID-19 pandemic. Psychol. Sci. 2020, 31, 1374–1385. [Google Scholar] [CrossRef]
- Ceccato, I.; Palumbo, R.; Di Crosta, A.; La Malva, P.; Marchetti, D.; Maiella, R.; Verrocchio, M.C.; Marin, A.; Mammarella, N.; Palumbo, R.; et al. Age-related differences in the perception of COVID-19 emergency during the Italian outbreak. Aging Ment. Health 2021, 25, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.; Ricci, E.; Biondi, S.; Colasanti, M.; Ferracuti, S.; Napoli, C.; Roma, P. A Nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: Immediate psychological responses and associated factors. Int. J. Environ. Res. Public Health 2020, 17, 3165. [Google Scholar] [CrossRef] [PubMed]
- Power, E.; Hughes, S.; Cotter, D.; Cannon, M. Youth mental health in the time of COVID-19. Ir. J. Psychol. Med. 2020, 37, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Palumbo, R.; Sella, E.; Lenti, G.; Di Domenico, A.; Borella, E. Emotional, psychological, and cognitive changes throughout the COVID-19 pandemic in Italy: Is there an advantage of being an older adult? Front. Aging Neurosci. 2021, 13, 712369. [Google Scholar] [CrossRef] [PubMed]
- Bruine de Bruin, W. Age differences in COVID-19 risk perceptions and mental health: Evidence from a National, U.S. survey conducted in March 2020. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Nguyen, M.H.; Do, B.N.; Tran, C.Q.; Nguyen, T.T.P.; Pham, K.M.; Pham, L.V.; Tran, K.V.; Duong, T.T.; Tran, T.V.; et al. People with suspected COVID-19 symptoms were more likely depressed and had lower health-related quality of life: The potential benefit of health literacy. J. Clin. Med. 2020, 31, 965. [Google Scholar] [CrossRef]
- Lei, L.; Huang, X.; Zhang, S.; Yang, J.; Yang, L.; Xu, M. Comparison of prevalence and associated factors of anxiety and depression among people affected by versus people unaffected by quarantine during the COVID-19 epidemic in Southwestern China. Med. Sci. Monit. 2020, 26, e924609. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Rodríguez-Sánchez, I.; Pérez-Rodríguez, P.; Ganz, F.; Torralba, R.; Oliveira, D.V.; Rodríguez-Mañas, L. Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. J. Nutr. Health Aging 2020, 24, 938–947. [Google Scholar] [CrossRef]
- Santini, Z.I.; Jose, P.E.; Cornwell, E.Y.; Koyanagi, A.; Nielsen, L.; Hinrichsen, C.; Meilstrup, C.; Madsen, K.R.; Koushede, V. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): A longitudinal mediation analysis. Lancet Public Health 2020, 5, e62–e70. [Google Scholar] [CrossRef]
- Dalise, S.; Tramonti, F.; Armienti, E.; Niccolini, V.; Caniglia Tenaglia, M.; Morganti, R.; Chisari, C. Psycho-social impact of social distancing and isolation due to the COVID-19 containment measures on patients with physical disabilities. Eur. J. Phys. Rehabil. Med. 2021, 57, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef]
- Negrini, S.; Grabljevec, K.; Boldrini, P.; Kiekens, C.; Moslavac, S.; Zampolini, M.; Christodoulou, N. Up to 2.2 million people experiencing disability suffer collateral damage each day of COVID-19 lockdown in Europe. Eur. J. Phys. Rehabil. Med. 2020, 56, 361–365. [Google Scholar] [CrossRef]
- Physical, E.; Alliance, R.M.B. White book on physical and rehabilitation medicine in Europe. Chapter 2. Why rehabilitation is needed by individual and society. Eur. J. Phys. Rehabil. Med. 2018, 54, 166–176. [Google Scholar]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiat. 2020, 33, e100213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 Pandemic on Mental Health and Quality of Life among Local Residents in Liaoning Province, China: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef]
- Zaninotto, P.; Iob, E.; Demakakos, P.; Steptoe, A. Immediate and longer-term changes in the mental health and well-being of older adults in England during the COVID-19 pandemic. JAMA Psychiatry 2022, 79, 151–159. [Google Scholar] [CrossRef]
- Verster, J.C.; Kraneveld, A.D.; Garssen, J. The assessment of immune fitness. J. Clin. Med. 2023, 12, 22. [Google Scholar] [CrossRef]
- Kiani, P.; Balikji, J.; Kraneveld, A.D.; Garssen, J.; Bruce, G.; Verster, J.C. Pandemic preparedness: The importance of adequate immune fitness. J. Clin. Med. 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Kiani, P.; Merlo, A.; Saeed, H.M.; Benson, S.; Bruce, G.; Hoorn, R.; Kraneveld, A.D.; Severeijns, N.R.; Sips, A.S.M.; Scholey, A.; et al. Immune fitness, and the psychosocial and health consequences of the COVID-19 pandemic lockdown in The Netherlands: Methodology and design of the CLOFIT study. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 199–218. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). Aandoeningen—Welke Aandoeningen Hebben we in de Toekomst? Available online: https://www.vtv2018.nl/aandoeningen (accessed on 5 October 2020).
- Verster, J.C.; Sandalova, E.; Garssen, J.; Bruce, G. The use of single-item ratings versus traditional multiple-item questionnaires to assess mood and health. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 183–198. [Google Scholar] [CrossRef]
- De Boer, A.G.; van Lanschot, J.J.; Stalmeier, P.F.; van Sandick, J.W.; Hulscher, J.B.; de Haes, J.C.; Sprangers, M.A. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual. Life Res. 2004, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Mulder, K.E.W.; Hendriksen, P.A.; Verheul, M.C.E.; van Oostrom, E.C.; Scholey, A.; Garssen, J. Test-retest reliability of single-item assessments of immune fitness, mood and quality of life. Heliyon 2023, 9, e15280. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.C.; Nielsen, E.; Ciliska, D. Lifestyle assessment: Testing the FANTASTIC Instrument. Can. Fam. Physician 1984, 30, 1863–1866. [Google Scholar]
- Sharratt, J.K.; Sharratt, M.T.; Smith, D.M.; Howell, N.J.; Davenport, L. FANTASTIC Lifestyle survey of University of Waterloo employees. Can. Fam. Physician 1984, 30, 1869–1872. [Google Scholar]
- Wilson, D.M.C.; Ciliska, D. Development and use of the FANTASTIC checklist. Can. Fam. Physician 1984, 30, 1527–1532. [Google Scholar]
- Canadian Society for Exercise Physiology. Fantastic Lifestyle Checklist. Available online: https://rowingbc.ca/wp-content/uploads/2016/12/Fantastic-Lifestyle-Checklist.pdf (accessed on 10 October 2020).
- Hendriksen, P.A.; Garssen, J.; Bijlsma, E.Y.; Engels, F.; Bruce, G.; Verster, J.C. COVID-19 lockdown-related changes in mood, health and academic functioning. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 1440–1461. [Google Scholar] [CrossRef] [PubMed]
- Balikji, J.; Koyun, A.H.; Hendriksen, P.A.; Kiani, P.; Stock, A.-K.; Garssen, J.; Hoogbergen, M.M.; Verster, J.C. The impact of COVID-19 lockdowns in Germany on mood, attention control, immune fitness, and quality of life of young adults with self-reported impaired wound healing. J. Clin. Med. 2023, 12, 3205. [Google Scholar] [CrossRef] [PubMed]
- AlSamman, M.; Caggiula, A.; Ganguli, S.; Misak, M.; Pourmand, A. Non-respiratory presentations of COVID-19, a clinical review. Am. J. Emerg. Med. 2020, 38, 2444–2454. [Google Scholar] [CrossRef]
- De Haan, L.; de Haan, H.; Olivier, B.; Verster, J.C. Alcohol mixed with energy drinks: Methodology and design of the Utrecht Student Survey. Int. J. Gen. Med. 2012, 5, 889–898. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).