1. Introduction
The Global Initiative for Asthma Report (GINA 2023) recommends a stepwise strategy for the pharmacological treatment of asthma [
1]. This strategy involves increasing doses of inhaled corticosteroids (ICSs), with or without a long-acting β-2 agonist (LABA) at Steps 1 to 5, as appropriate, as well as a long-acting muscarinic antagonist (LAMA) at Steps 4 and 5. In the preferred therapeutic path (Track 1), in the absence of scientific data confirming the effectiveness of maintenance and reliever therapy (MART) combined with other controller medications, the introduction of LAMAs at Step 4 is not recommended as a first choice; however, such an approach is already indicated at Step 5 [
1,
2]. Similarly, in Track 2, LAMAs are included only as another option for controller medications in Step 4, while the addition of these medications is recommended as a first choice in Step 5. LAMAs should be considered for use as an add-on therapy in separate inhalers for patients aged ≥6 years or for use in combination inhalers (i.e., triple therapy) for patients aged ≥18 years if asthma is uncontrolled despite the use of medium- or high-dose ICS + LABA therapy. Previous studies have shown that adding LAMAs to ICS + LABA therapy modestly reduces the risk of exacerbations compared with medium- or high-dose ICS + LABA comparators alone. Previous meta-analyses revealed that adding LAMAs to medium- or high-dose ICS + LABA therapy resulted in a 17% reduction in the risk of severe exacerbation [
3,
4]. Furthermore, adding LAMAs to medium- or high-dose ICS + LABA therapy modestly has been shown to improve lung function but not asthma symptoms. Patients with uncontrolled asthma experience bothersome symptoms, frequent exacerbations, and side effects of medications; therefore, asthma represents a significant burden to healthcare systems [
5,
6]. The prevalence of uncontrolled asthma varies across studies and ranges from 18% to 49.8%. A global project (NOVELTY cohort) revealed that asthma is still undertreated and not optimally controlled worldwide [
7,
8,
9]. Triple therapy represents a potent and cost-effective therapeutic option for the management of moderate-to-severe asthma at GINA Steps 4 and 5. Although several guidelines recommend triple therapy, little is known about physicians’ opinions on the real-life applicability of this treatment approach [
1,
2].
3. Methods
The EU-LAMA Survey was conducted in Poland, Greece, Sweden, Slovenia, and Austria by Biostat sp. z o.o., a clinical research organization (CRO) using the CATI system (
http://www.cati-system.pl/). The survey was administered via electronic computer-assisted web interviews (CAWIs). The survey was designed by Chiesi Poland in collaboration with clinical experts and the CRO. The required sample size was calculated on the basis of the number of medical professionals with specialties of interest in each country. Subsequently, the survey was translated into local languages and submitted to ethics committees in accordance with local regulations. The questionnaire included 19 items. The first three items assessed the respondent’s specialization, primary workplace, and the size of the city where the workplace is located. The remaining items focused on opinions on the research questions. Physicians from all participating countries, including general practitioners, allergologists, pulmonologists, internal medicine specialists, and fellows in training, were invited to participate. The survey was conducted anonymously, and access to the survey link was restricted with a random unique access code provided by the Chiesi Poland Medical Department. The survey was conducted from April to December 2023. Chiesi Poland Medical Department representatives oversaw the survey and provided support to physicians with respect to survey access. The CRO was responsible for the methodology, survey tool design, and data analysis. The Bioethical Committee at the Medical University of Lodz, Poland, approved this survey (RNN/88/23/KE of 18 April 2023).
5. Results
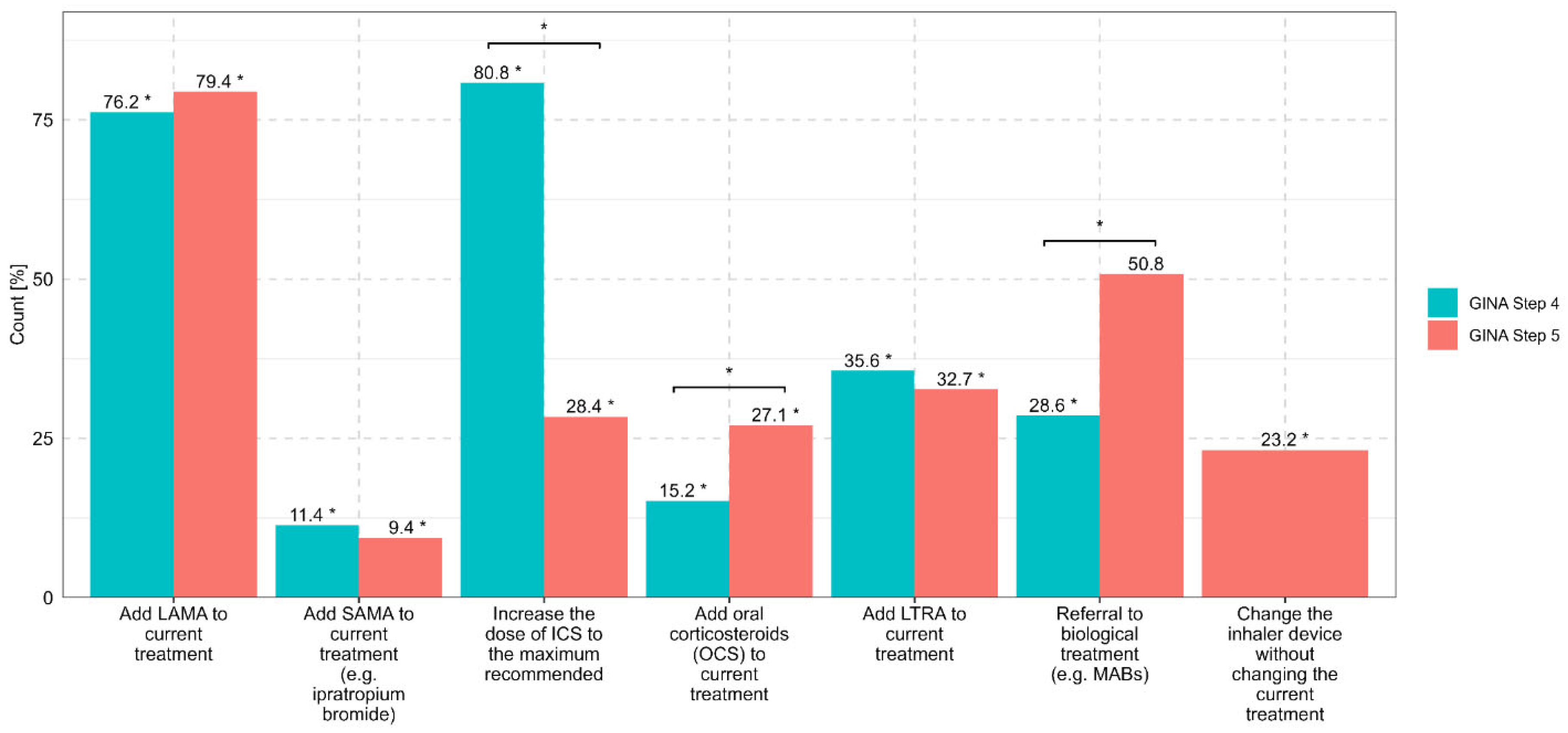
A total of 767 surveys were administered, and 630 were fully completed and included for analysis. The EU-LAMA Survey included responses from physicians from Poland (57.6%), Greece (27.0%), Sweden (6.3%), Slovenia (5.4%) and Austria (3.7%). The most common profession among the respondents was pneumonologist (58.7%), followed by allergologist (15.7%). Detailed descriptive data are provided in
Table 1 and
Table 2 and
Figures S1–S8 (please see the
Supplementary Files). If asthma was uncontrolled at GINA Step 4, which is defined as a medium-dose ICS plus another controller, the most common first choice among the respondents was to increase the ICS dose to the maximum recommended level (80.8%). The most common second choice was to add LAMAs as a controller medication (76.2%). The most common third choice was to add LTRAs (35.6%), followed by a referral for qualification for biological asthma therapy (monoclonal antibodies, MABs) (28.6%). A total of 15.2% of the physicians selected the introduction of OCSs before increasing the dose of ICSs and implementing LAMAs. Increasing the ICS dose to the maximum recommended level was most often selected by internal medicine specialists (87.9%) and least often selected by allergologists (77.8%) and other specialists (76.5%). The strategy of adding LAMAs to a medium dose of ICS was selected by 83.8% of allergologists, 77.6% of pulmonologists, and 68.2% of GPs. OCSs were selected as the optimal option to improve asthma control at GINA Step 4 by 34.8% of internal medicine specialists, 15.9% of GPs, 14.1% of pulmonologists, and 7.1% of allergologists.
If the disease was uncontrolled at GINA Step 5, LAMAs were selected as the preferred option at the first step by 79.4% of the respondents, followed by referral to MABs (50.8%). For 32.7% of the physicians, the optimal choice at this step was to add LTRAs, while for 28.4% of the physicians, the optimal choice was to increase the ICS dose to the maximum recommended level. A total of 27.1% of the respondents proposed adding OCS after achieving asthma control at GINA Step 5. Pulmonologists selected LAMAs most often (84.9% of respondents), followed by internal medicine specialists (63.6%) and other specialists (68.6%). MABs were indicated most often by allergologists (58,6%), followed by pulmonologists (53,5%); only 4.5% of the GPs selected MABs. LTRAs were selected by 43.2% of the GPs and 31.4% of the pulmonologists. Systemic OCSs were selected as the optimal option by 39.4% of the internal medicine specialists, 26.4% of the pulmonologists, 22.2% of the allergologists, and 22.7% of the GPs. At GINA Step 5, 23.2% of the physicians considered changing the inhaler device without changing the current treatment (in
Figure 1, there is no comparison with GINA Step 4; this resulted from the content of the question about the most commonly used treatment regimens in adult patients with asthma who are not achieving adequate control when treated with high-dose ICSs and LABAs).
According to the EU-LAMA Survey, no significant differences were observed in the therapeutic strategies for asthma control at GINA Steps 4 and 5 based on the size and site of the physician’s professional practice. Details of the therapeutic preferences of physicians for patients who are not achieving adequate asthma control at GINA Steps 4 and 5 are presented in
Figure 1.
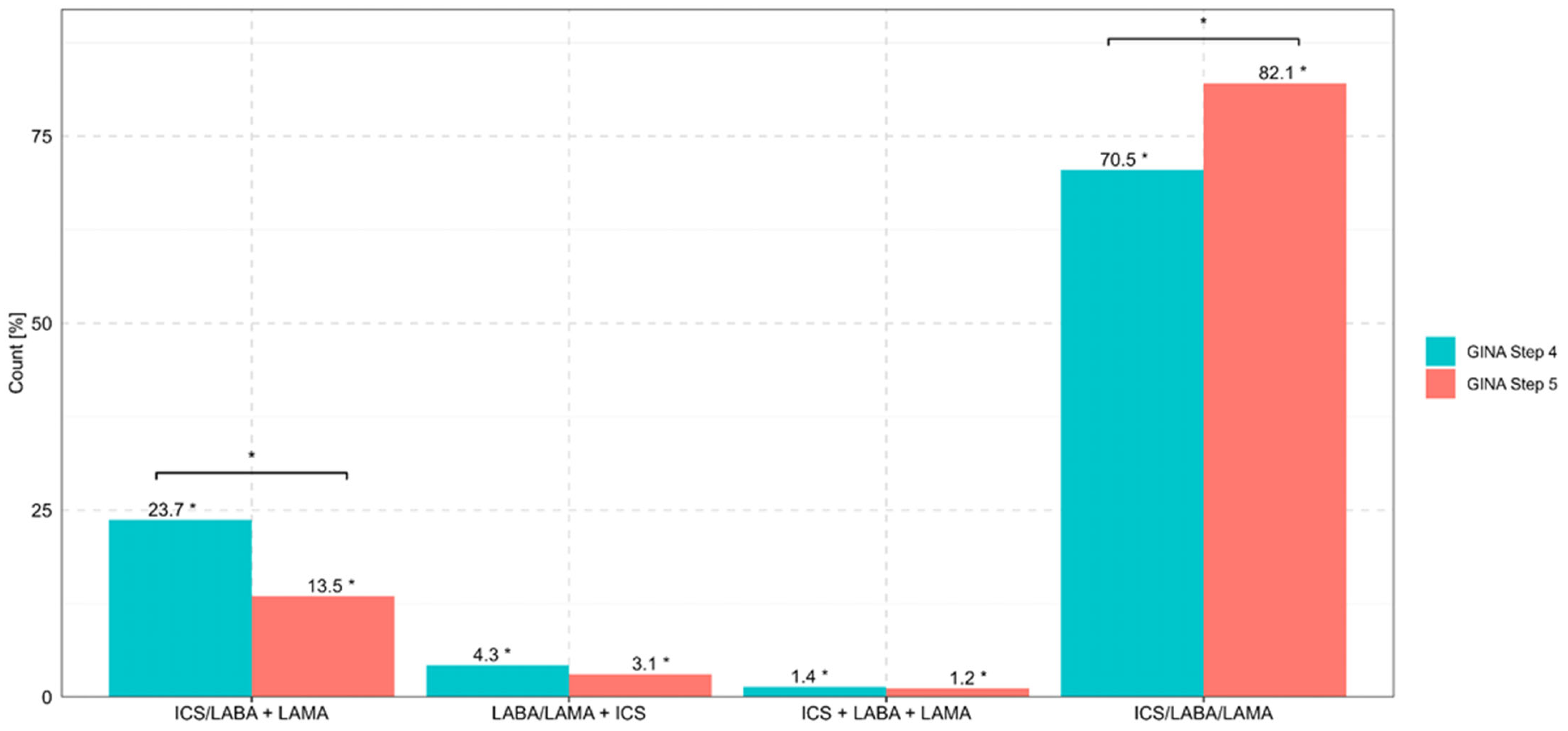
The combination of ICS/LABA/LAMA in one inhaler (i.e., single inhaler triple therapy (SITT) at GINA Step 4 was most often selected in Poland (72.0%) and least often selected in Slovenia (58.8%). Regarding specialties, SITT was most often proposed by GPs (73.1%), followed by allergologists (72.8%) and pulmonologists (71.2%).
SITT at GINA Step 5 was most often selected in Greece (85.5%) and least often selected in Slovenia (58.8%). Regarding specialties, SITT was most often proposed by GPs (84.6%), followed by pulmonologists (84.1%) and allergologists (80.4%). The least frequently used option, namely, ICS + LABA + LAMA from 3 different inhalers, was selected by 1.7% of the pulmonologists and 1.1% of the allergologists. The preferred combinations of ICS, LABA, and LAMA for three-component asthma therapy in patients at GINA Steps 4 and 5 are presented in
Figure 2.
Among all respondents, 91.6% proposed the use of SITT (ICS + LABA + LAMA). In particular, SITT was selected by 100% of respondents in Slovenia, 97.1% in Greece, 91.5% in Poland, 87.0% in Austria, and 65% in Sweden. SITT was most often selected by pulmonologists (96.8%) and least often selected by GPs (59.1%). The frequency of preferred use of frequency of selecting SITT varied across medical sites, ranging from 92.7% in the hospital setting to 94.0% in the specialist hospital policlinic setting and 86.2% in the general policlinic setting. No significant differences were observed based on the size of the city. Details of the potential SITT implementation are presented in
Figure 3.
SITT was the most commonly selected option for patients who received a moderate dose of triple therapy (57.5%) and the least commonly selected option in situations where the physician preferred to start with medium-dose ICS triple therapy (11.7%). A total of 2.2% of the respondents reported that they did not consider the use of triple therapy in asthma patients. High-dose ICS-based SITT was most common in Greece (76.5%) and least common in Austria (43.5%) when there were exacerbations associated with a moderate ICS dose of triple therapy. Adjusting the ICS dose in triple therapy based on the patient’s current condition was most often proposed by allergologists (71.7%) and least often proposed by GPs (40.9%). A detailed description of the high-dose ICS-based SITT with respect to the physician’s country of employment, specialization, and site of work is presented in
Figure S11; please see the
Supplementary Files.
The respondents reported that the main barriers to the applicability in asthma therapy were a lack of reimbursement (30.8%), no/little medical/scientific evidence on the efficacy of LAMAs for treating asthma (12.9%), and no experience in current medical practice (17.9%). No significant differences in the barriers of LAMA implementation were observed based on the site of medical practice or the size of the city. Detailed data on the main barriers to incorporating LAMAs into asthma therapy stratified by the physician’s country of employment and specialization are presented in
Figure S12; please see the
Supplementary Files.
6. Discussion
The 2023 EU-LAMA Survey, which was conducted across five European countries and among medical specialists who care for patients with asthma, provides interesting insights into the perceptions of the roles of and barriers to LAMA implementation at GINA Steps 4 and 5. If asthma is not controlled at GINA Step 4, 80.8% of specialists recommended the implementation of the maximum allowable dose of ICS rather than the addition of LAMAs. Moreover, 28.6% of the respondents considered referral for MAB therapy at GINA Step 4, which is in clear contrast to the recommendation of a high-dose ICS plus other controllers and a lack of optimal asthma control before the initiation of MABs. Surprisingly, 15.2% of the physicians proposed the use of OCSs at this step instead of other therapeutic options, including SITT. Systemic OCS treatment was most likely to be indicated by internal medicine specialists (34.8%) and significantly less often indicated by allergologists (7.1%) or pulmonologists (14.1%), which shows significant therapeutic inertia and a burning need for continuous education in the latest guideline recommendations [
10,
11]
Only 79.4% of the respondents considered the implementation of LAMAs in the absence of asthma control at GINA Step 5; in other words, more than 20% did not prioritize such a recommendation. According to the GINA guidelines, LAMAs are regarded as a potential first choice, and biological treatment may be regarded as an alternative treatment. This approach also seems to be valid from the payer perspective and the optimal healthcare resource utilization perspective with respect to asthma management. Adding a low-dose OCS, accounting for the long-term burden of adverse events, should be considered as the last-line treatment. Surprisingly, 27.1% of the physicians proposed the initiation of OCS treatment at GINA Step 5 before optimizing inhalation therapy with SITT [
12,
13]. Moreover, only 50.8% of the respondents considered qualifications for MAB therapy for suboptimal asthma control at GINA Step 5, although such action is recommended by the guidelines before implementing systemic OCS treatment. In conclusion, the results of the 2023 EU-LAMA Survey revealed that OCS treatment is still considered as the first choice at GINA Steps 4 and 5 by some specialists who care for patients with uncontrolled asthma, and the role of LAMAs or MABs seems to be underestimated [
14,
15]. Allergologists were more willing to propose the use of LTRAs to improve asthma control at GINA Steps 4 (40.4%) and 5 (33.3%) than were pulmonologists (34.6% at GINA Step 4 and 31.4% at GINA Step 5). In contrast, pulmonologists were more willing to implement LAMAs at GINA Steps 4 (77.6%) and 5 (84.9%) for asthma control than were allergologists (83.8% at GINA Step 4 and 77.8% at GINA Step 5). A possible explanation for this finding may be that allergologists more often examine patients with allergic asthma and other allergic conditions, including younger populations of patients. On the other hand, pulmonologists are familiar with LAMAs, which are extensively used in the management of COPD [
2,
16,
17].
The use of SITT with ICSs, LABAs, LAMAs was regarded as an optimal option by 70.5% of respondents at GINA Step 4 and 82.1% at GINA Step 5. The least frequently used combination was ICSs + LABAs + LAMAs from 3 different inhalers (<5%). This finding indicates a clear effort to optimize the treatment of uncontrolled asthma via the use of one inhaler to improve adherence/compliance, thereby limiting issues related to incorrect inhalation technique [
1,
2,
18]. It could be hypothesized that the awareness of asthma exacerbations (past and future) determines the implementation of SITT because physicians prefer high-dose ICS-based SITT if exacerbations occur while on a moderate-dose ICS treatment (57.5%). In contrast, SITT was selected as the optimal option by 11.7% of respondents when the suitable option seems to be a medium ICS-based SITT (11.7%). Thus, the role of medium-dose ICS-based SITT seems to be underestimated in everyday clinical practice [
1,
4].
The discrepancy in the reported use of single-inhaler triple therapy (SITT) 91.6% overall versus lower rates at GINA Steps 4 and 5—arises from differences in the phrasing of the survey questions. The general question referred to the use of SITT as a therapeutic strategy in a broad sense, whereas the questions assigned to specific GINA steps addressed physicians’ preferences in defined clinical scenarios, thereby explaining the observed divergence.
The key findings of the 2023 EU-LAMA Survey include the identification of potential bottlenecks in incorporating LAMAs into asthma management. The respondents noted that reimbursement (30.8%) and a lack of clear positioning of LAMAs in the guidelines/recommendations (24.0%) are among the leading barriers to the use of LAMAs. These findings are surprising, as the position of LAMAs in the GINA guidelines has been clearly described in previous editions of the GINA Report. Moreover, these findings are supported by the results of numerous clinical studies and meta-analyses. Reimbursement is clearly dependent on a national care system, which may significantly limit the implementation of the best therapy in everyday clinical practice. For example, in Austria, LAMAs are only reimbursed for COPD patients. Therefore, there is room for improvement and coordinated actions among experts, policymakers, and patient advocacy groups to promote the implementation of optimal, evidence-based standards of asthma care in particular countries [
1,
2,
19].
This survey had several limitations. The opinions on optimal asthma management were self-reported, which is inherent in the design of survey studies. The collected data were not validated against the prescription database or sale reports. Moreover, the rate of participation in the survey was probably higher for healthcare professionals who are interested in and involved in the care of asthma patients. The countries taking part in the survey differ in their healthcare systems. The largest number of completed questionnaires came from Poland (58%) and Greece (27%), while the smallest number came from Austria (3.7%). Predefined survey questions may lead to a particular selection of answers, which may introduce bias. A good example of some discrepancies is illustrated by the positioning of SITT in asthma care. This point was evaluated in several questions in the survey. As a result of this internal cross-check, we found that as many as 91.6% of the respondents reported using SITT in asthma treatment regimens, whereas in other questions in the same survey, only 70.5% and 82.1% of the respondents reported the use of SITT at GINA Steps 4 and 5, respectively. This finding clearly shows that SITT is recognized as an optimal option; however, its applicability in particular clinical situations remains suboptimal. Heterogeneity in responses is dependent on the country of origin; however, it should be noted that the identification of regional differences was among the goals of this project. All therapeutic options are equally available in each of the surveyed countries; however, not all options are currently eligible for reimbursement, which may have a significant effect on implementation in everyday clinical practice, as extensively discussed above.
Moreover, an error (clerical error) in one of the questions (question 6) was found to be irrelevant to the results collected in the survey; i.e., the answer “Changing the inhaler device without altering the current treatment” was not included in question 6 but was included in question 7. Hence, in
Figure 1, there are no data for GINA Step 4 in the column representing this answer. We underscore the potential risk of systematic bias resulting from the sponsor’s involvement in the preparation and distribution of the survey. We also acknowledge that data derived from physicians’ self-assessments may not fully reflect real-world prescribing patterns, as declarative responses may differ from actual clinical practice.
7. Conclusions
In conclusion, the results of the 2023 EU-LAMA Survey indicate that there is room to improve step-up therapy in patients with severe uncontrolled asthma at GINA Steps 4 and 5. Such improvements can include raising awareness of the evidence underlying the GINA recommendations, specifically regarding the appropriate positioning of SITT with ICS/LABA/LAMA at Steps 4 and 5, the burden of prescribing recurrent OCS courses, and the delay in referral for evaluation for biologic therapy with monoclonal antibodies at GINA Step 5.
We emphasize that improving concordance between clinical practice and GINA recommendations requires both educational interventions targeted at physicians (e.g., structured training programs, workshops) and structural measures (e.g., expanded reimbursement for LAMAs, simplified procedures for qualification for biologic therapies). Furthermore, the findings of this study may serve as a foundation for dialogue with policymakers and patient organizations aimed at developing a more coherent, evidence-based strategy for asthma care across Europe.