Health Effects and Preventive Strategies for Radon Exposure: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Protocol and Search Strategy
2.2. Inclusion Criteria for the Study
2.3. Data Extraction and Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruano-Ravina, A.; Quindós-Poncela, L.; Sainz Fernández, C.; Barros-Dios, J.M. Radón interior y salud pública en España: Tiempo para la acción. Gac. Sanit. 2014, 28, 439–441. [Google Scholar] [CrossRef]
- Wanty, R.B.; Gundersen, L. Groundwater geochemistry and radon-222 distribution in two sites on the Reading Prong, Eastern Pennsylvania. Mo. Dep. Nat. Resour. Spec. Publ. 1998, 4, 147–156. [Google Scholar]
- Ruano-Ravina, A.; Castro-Bernárdez, M.; Sande-Meijide, M.; Vargas, A.; Barros-Dios, J. Short- versus long-term radon detectors: A comparative study in Galicia, NW Spain. J. Environ. Radioact. 2008, 99, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- EpiCentro. Available online: https://www.epicentro.iss.it/radon/#:~:text=L’Organizzazione%20mondiale%20della%20sanit%C3%A0,cancerogene%20per%20l’essere%20umano (accessed on 9 June 2025).
- EURATOM. DIRETTIVA 2013/59. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32013L0059 (accessed on 9 June 2025).
- International Agency for Research on Cancer. Man-Made Mineral Fibres and Radon; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2005, 330, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EPA US. EPA assessment of risks from radon in homes. In Off Radiat Indoor Air; United States Environ Prot Agency: Washington, DC, USA, 2003. [Google Scholar]
- Rajan, V.; Pandey, B.N. Cytoproliferative effect of low dose alpha radiation in human lung cancer cells is associated with connexin 43, caveolin-1, and survivin pathway. Int. J. Radiat. Biol. 2021, 97, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Howland, M.; Kutzner, B.; McNamee, J.P.; Bellier, P.V.; Wilkins, R.C. Biological effects of alpha particle radiation exposure on human monocytic cells. Int. J. Hyg. Environ. Health 2012, 215, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kouroukla, E.; Gooding, T.D.; Fonseca, H.S. Analysis of radon mitigation methods: 10-year review. J. Radiol. Prot. 2024, 44, 049501, Erratum in J. Radiol Prot. 2024, 44, 031503. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, M.; de Herreros, M.G.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Khan, S.M.; Gomes, J.; Krewski, D.R. Radon interventions around the globe: A systematic review. Heliyon 2019, 5, e01737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Mulrow, C.D.; Tetzlaff, J.M.; Chou, R.; Hróbjartsson, A.; Li, T.; McDonald, S.; Stewart, L.A.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar]
- Palmieri, V.; Colamesta, V.; La Torre, G. Evaluation of methodological quality of studies. Senses Sci. 2016, 3, 235–241. [Google Scholar] [CrossRef]
- Field, R.; Steck, D.J.; Smith, B.J.; Brus, C.P.; Fisher, E.L.; Neuberger, J.S.; Lynch, C.F. The Iowa radon lung cancer study—Phase I: Residential radon gas exposure and lung cancer. Sci. Total Environ. 2001, 272, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.; Dupleac, D. The impact of ventilation rate on radon concentration inside high-rise apartment buildings. Radiat. Prot. Dosim. 2022, 198, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Steck, D.J. The Effectiveness of Mitigation for Reducing Radon Risk in Single-Family Minnesota Homes. Health Phys. 2012, 103, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Finne, I.E.; Kolstad, T.; Larsson, M.; Olsen, B.; Prendergast, J.; Rudjord, A.L. Significant reduction in indoor radon in newly built houses. J. Environ. Radioact. 2019, 196, 259–263. [Google Scholar] [CrossRef]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Pérez-Ríos, M.; Castro-Bernárdez, M.; Abal-Arca, J.; Tojo-Castro, M. Residential Radon Exposure, Histologic Types, and Lung Cancer Risk. A Case–Control Study in Galicia, Spain. Cancer Epidemiol. Biomark. Prev. 2012, 21, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, H.; Kim, H.C.; Sheen, S.S.; Koh, S.B.; Park, K.S.; Cho, N.H.; Lee, C.-M.; Kang, D.R. Residential Radon Exposure and Cigarette Smoking in Association with Lung Cancer: A Matched Case-Control Study in Korea. Int. J. Environ. Res. Public Health 2020, 17, 2946. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, Á.; Ruano-Ravina, A.; Torres-Durán, M.; Provencio, M.; Parente-Lamelas, I.; Vidal-García, I.; Martínez, C.; Hernández-Hernández, J.; Abdulkader-Nallib, I.; Castro-Añón, O.; et al. Residential Radon and Small Cell Lung Cancer. Final Results of the Small Cell Study. Arch. Bronconeumol. 2022, 58, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernández, V.; Abal-Arca, J.; Montero-Martínez, C.; Pena-Álvarez, C.; Castro-Añón, O.; Golpe-Gómez, A.; Martínez, C.; et al. Residential radon and lung cancer characteristics in never smokers. Int. J. Radiat. Biol. 2015, 91, 605–610. [Google Scholar] [CrossRef]
- Xiang-Zhen, X.; Lubin, J.H.; Jun-Yao, L.; Li-Fen, Y.; Sheng, L.Q.; Lan, Y.; Wang, J.-Z.; Blot, W.J. A Cohort Study in Southern China of Tin Miners Exposed to Radon and Radon Decay Products. Health Phys. 1993, 64, 120–131. [Google Scholar] [CrossRef]
- Grzywa-Celinska, A.; Krusinski, A.; Kozak, K.; Mazur, J.; Grzadziel, D.; Szewczyk, K.D.S.; Chmielewska, I.; Milanowski, J. Indoor radon exposure and living conditions in patients with advanced lung cancer in Lublin region, Poland. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7352–7361. [Google Scholar] [CrossRef]
- Kelly-Reif, K.; Sandler, D.P.; Shore, D.; Schubauer-Berigan, M.; Troester, M.; Nylander-French, L.; Richardson, D.B. Lung and extrathoracic cancer incidence among underground uranium miners exposed to radon progeny in the Příbram region of the Czech Republic: A case–cohort study. Occup. Environ. Med. 2022, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, L.; Rogel, A.; Tirmarche, M.; Mitton, N.; Laurier, D. Lung Cancer in French and Czech Uranium Miners: Radon-Associated Risk at Low Exposure Rates and Modifying Effects of Time since Exposure and Age at Exposure. Radiat. Res. 2008, 169, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernández, V.; Abal-Arca, J.; Montero-Martínez, C.; Pena-Álvarez, C.; González-Barcala, F.J.; Castro-Añón, O.; Golpe-Gómez, A.; et al. Lung cancer in never-smokers: A case–control study in a radon-prone area (Galicia, Spain). Eur. Respir. J. 2014, 44, 994–10011. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Krewski, D.; Chen, Y.; Pope, C.A., 3rd; Gapstur, S.; Thun, M.J. Radon and Lung Cancer in the American Cancer Society Cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.S.; Egger, S.; Hughes, S.; Weber, M.; Steinberg, J.; Rahman, B.; Worth, H.; Ruano-Ravina, A.; Rawstorne, P.; Yu, X.Q. Systematic review and meta-analysis of residential radon and lung cancer in never-smokers. Eur. Respir. Rev. 2021, 30, 200230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar] [PubMed]
- Ngoc, L.T.N.; Park, D.; Lee, Y.-C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies. Int. J. Environ. Res. Public Health 2023, 20, 97. [Google Scholar] [CrossRef]
- Henyoh, A.M.S.; Laurent, O.; Mandin, C.; Clero, E. Radon exposure and potential health effects other than lung cancer: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1439355. [Google Scholar] [CrossRef]
- Urrutia-Pereira, M.; Chatkin, J.M.; Chong-Neto, H.J.; Solé, D. Radon exposure: A major cause of lung cancer in nonsmokers. J. Bras. Pneumol. 2023, 49, e20230210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burghele, B.; Botoș, M.; Beldean-Galea, S.; Cucoș, A.; Catalina, T.; Dicu, T.; Dobrei, G.; Florică, Ș.; Istrate, A.; Lupulescu, A.; et al. Comprehensive survey on radon mitigation and indoor air quality in energy efficient buildings from Romania. Sci. Total Environ. 2021, 751, 141858. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, Á.; Torres-Durán, M.; Barros-Dios, J.M.; Ruano-Ravina, A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 2018, 426, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kashkinbayev, Y.; Kazhiyakhmetova, B.; Altaeva, N.; Bakhtin, M.; Tarlykov, P.; Saifulina, E.; Aumalikova, M.; Ibrayeva, D.; Bolatov, A. Radon Exposure and Cancer Risk: Assessing Genetic and Protein Markers in Affected Populations. Biology 2025, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Ravina, A.; Torres-Durán, M.; Kelsey, K.T.; Parente-Lamelas, I.; Leiro-Fernández, V.; Abdulkader, I.; Abal-Arca, J.; Montero-Martínez, C.; Vidal-García, I.; Amenedo, M.; et al. Residential radon, EGFR mutations and ALK alterations in never-smoking lung cancer cases. Eur. Respir. J. 2016, 48, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- López-Abente, G.; Núñez, O.; Fernández-Navarro, P.; Barros-Dios, J.M.; Martín-Méndez, I.; Bel-Lan, A.; Locutura, J.; Quindós, L.; Sainz, C.; Ruano-Ravina, A. Residential radon and cancer mortality in Galicia, Spain. Sci. Total Environ. 2018, 610–611, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Lorenzo, R.; Ruano-Ravina, A.; Cerdeira-Caramés, S.; Raíces-Aldrey, M.; Barros-Dios, J.M. Residential radon and lung cancer: A cohort study in Galicia, Spain. Cad. Saude Publica 2017, 33, e00189415. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, S.; Lyons, S.; Nolan, A. High Radon Areas and lung cancer prevalence: Evidence from Ireland. J. Environ. Radioact. 2018, 182, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Liang, Z.; Hrubec, Z.; Pershagen, G.; Schoenberg, J.B.; Blot, W.J.; Klotz, J.B.; Xu, Z.-Y.; Boice, J.D. Radon exposure in residences and lung cancer among women: Combined analysis of three studies. Cancer Causes Control 1994, 5, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lubin, J.H.; Wang, L.; Zhang, S.; Boice, J.D.; Cui, H.; Zhang, S.; Conrath, S.; Xia, Y.; Shang, B.; et al. Residential Radon and Lung Cancer Risk in a High-exposure Area of Gansu Province, China. Am. J. Epidemiol. 2002, 155, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Hystad, P.; Brauer, M.; Demers, P.A.; Johnson, K.C.; Setton, E.; Cervantes-Larios, A.; Poplawski, K.; McFarlane, A.; Whitehead, A.; Nicol, A.-M. Geographic variation in radon and associated lung cancer risk in Canada. Can. J. Public Health 2014, 105, e4–e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cofone, L.; Sabato, M.; Di Rosa, E.; Colombo, C.; Paglione, L. Evaluating the Environmental Impact of Anthropogenic Activities on Human Health: A Systematic Review. Urban Sci. 2024, 8, 49. [Google Scholar] [CrossRef]

| Author, Year, Country | Field R. W. et al. 2001 USA [16] | Cucu M. et al. 2022 Romania [17] | Steck D. J. 2012 Minnesota [18] |
| n° Patients (sex F%, M%; mean age years, range + SD) | case 413 (100% F; 40–84 years); control 614 (100% F; 40–64 years) | - | - |
| Case | Primary invasive lung carcinoma; residence for 20 consecutive years or more in the current home | - | - |
| Sampling location | Large areas of western Iowa | Urban areas | Minnesota |
| Sampling period | May 1993 and 30 October 1996 | February 2021–June 2021 | Between 1 February and 15 March 2008 |
| Anatomopathological changes | Histologic typing of lung tumours and included the major categories of small cell carcinoma, squamous cell carcinoma, adenocarcinoma, and large cell carcinoma | - | - |
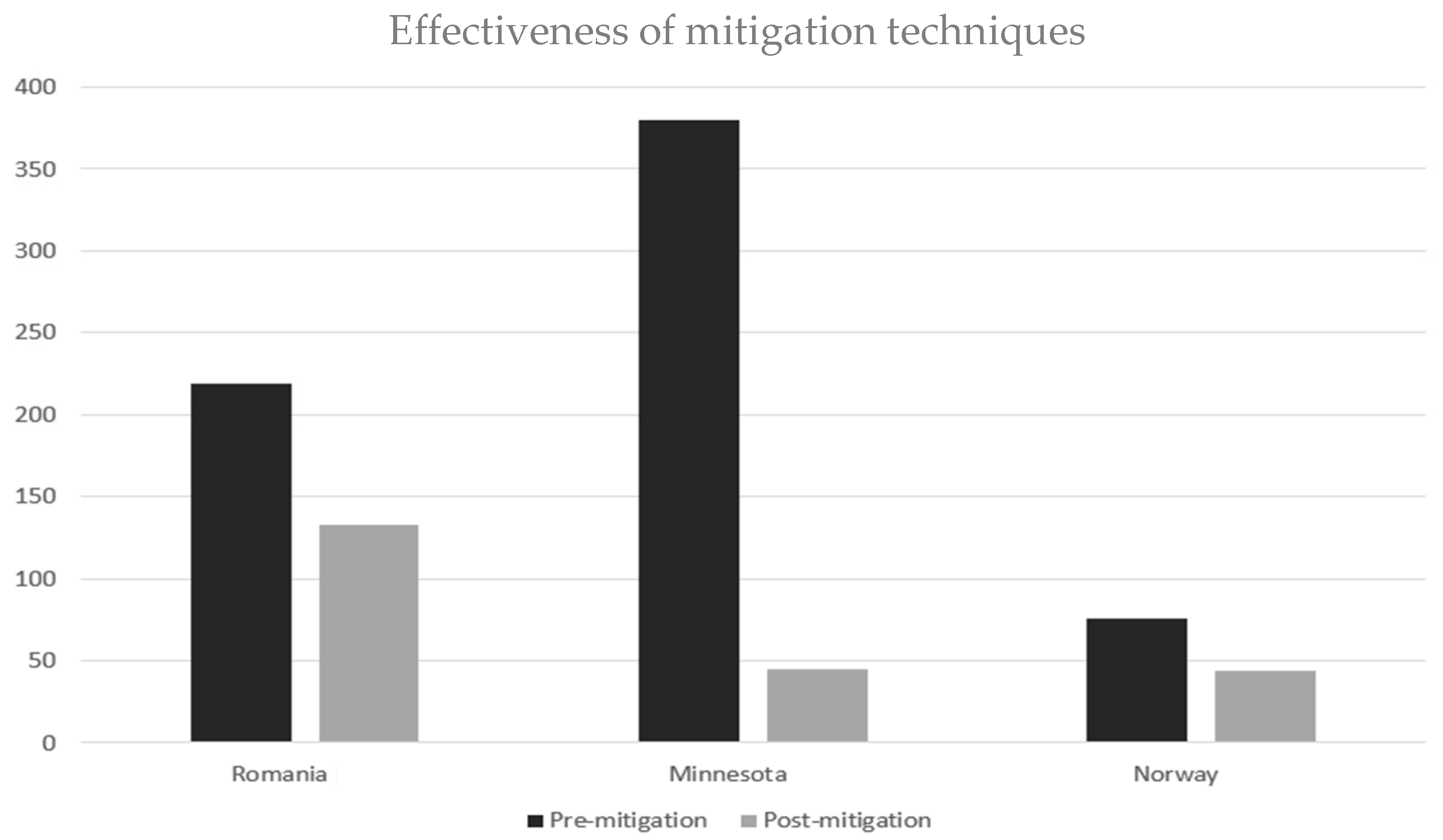
| Environmental concentration measurement | Approximately 60% of the basement radon concentrations and 30% of the first floor radon concentrations of study participants’ homes exceeded the US Environmental Protection Agency Action level of 150 Bq m−3(4 pCi l−1) Large areas of western Iowa had outdoor radon concentrations comparable to the national average indoor value of 55 Bq m−3(1.5 pCi l−1) Excess odds of 0.24 (95 CI = −0.05–0.92) and 0.49 (95% CI = −0.03–1.84) per 11 WLM (Working Level Month) 5–19 were calculated using the continuous radon exposure estimates for all cases and live cases, respectively. Slightly higher excess odds of 0.50 (95% CI = −0.004–1.80) and 0.83 (CI = 0.11–3.34) per 11 WLM5-19 were noted for the categorical radon exposure estimates for all cases and the live cases. | The highest indoor radon concentration was 219 Bq m−3, in the cold season. The lowest measured value for indoor radon concentration (17 Bq m−3) was found in the hot season. The air exchange rate varied from 0.2 to 4.2 h−1. In the cold season (wintertime), the outdoor–indoor exchange of air is made possible by two mechanisms: diffusion and convection. | The pre-mitigation concentration was lognormally distributed with an average of 380 Bq m−3 (10.3 pCi L−1), geometric mean of 295 Bq m−3, and a factor of 1.8 variations (geometric standard deviation). These post-mitigation concentrations averaged 45 Bq m−3 (1.2 pCi L−1). |
| Methodology of environmental measurement | The radon dosimetry assessment consisted of five components: on-site residential assessment survey; on-site radon measurements; regional outdoor radon measurements; assessment of subjects’ exposure when in another building; and linkage of historic subject mobility with residential, outdoor and other building radon concentrations. | 5 residential apartments were subjected to testings of radon concentration and air exchange rates between February 2021 and June 2021. Sample collection for each detector ranged from 4 to 24 h. Two devices were used to carry out indoor radon measurements: a RAD7 active radon detector and an Airthings Wave Plus detector. Temperatures and atmospheric state were also recorded. Other parameters of interest impacting the air exchange rate are: tightness of the building, habitual opening of windows by occupants and outside atmospheric conditions. | A 4 d long and also a winter radon measurement with the activated charcoal detector (AC). Two alpha track detectors (ATD) were sent for long-term measurements in then home during an extensive period of normal house operation. One ATD was to be placed at the primary site. The second ATD was to be placed in a frequently occupied room on another level if possible, preferably a bedroom. The ATDs were returned after 90 or more days of exposure between mid-June and the end of September 2008. |
| Methods of prevention | - | Indoor radon levels are influenced by air exchange rates (ACH), building design, ventilation, materials, and outdoor Indoor radon levels are influenced by air exchange rates (ACH), building design, ventilation, materials, and outdoor conditions. With ACH between 0.2 and 4.2 h−1, radon typically reaches steady state within 24–48 h. Heating and cooling systems mainly recirculate air and do not reduce radon effectively. To lower levels, increased ventilation—such as opening windows—is needed. The critical ACH (ACH_critical) required to reduce radon can range from −1 to 135 h−1, depending on indoor-outdoor differences. Airtight, well-insulated buildings often have higher radon, especially in winter (average 133 Bq/m3) compared to summer (23 Bq/m3). | All the mitigation systems used active soil ventilation. |
| Conclusion | Lung cancer risk is substantially correlated with cumulative radon exposure. | Findings indicate that levels of indoor radon concentration depend on ventilation rates of the unit, but also on building materials, wind speed around building, time of day (higher around noon) and season of the year (higher in the cold season when ventilation rate is lower). | If that reduction was maintained over the lifetime, radon-related lung cancers can be prevented. |
| Author, Year, Country | Finne I. E. et al. 2019 Norway [19] | Barros-Dios J. M. et al. 2012 Spain [20] | Park E. Y. et al. 2020 Korea [21] |
| n° Patients (sex F%, M%; mean age years, range + SD) | - | Case 349 (13.5% F, 86.5% M; 6.6% < 50 y, 50.7% 51–70 y, 42.7% > 70 y) Control 513 (12.3%F, 87.7% M; 10.1% < 50 y, 61.4% 51–70 y, 28.5% >70 y) | Case 519 (51.25% F, 48.75% M; 64, 57–72 y) Control 519 (51.25% F, 48.75% M; 64, 59–72 y) |
| Case | - | Lung cancer | Non-small cell lung cancer |
| Sampling location | Norway | Galicia, Ourense | Seoul, Suwon, Wonju, Changwon, Yangsan |
| Sampling period | 2008 and 2016 | Between 2004 and 2008 | Between October 2015 and March 2018 |
| Anatomopathological changes | - | 46.4% squamous cell carcinoma; 26.4% adenocarcinoma; 15.5% SCC; 5.7% large cell carcinoma; 6.0% other types | - |
| Environmental concentration measurement | For detached houses where the average radon concentration was almost halved from 76 (under construction in 2008) to 40 (under construction in 2016) Bq/m3. For Terraced house/semi-detached houses (vertically divided) were a reduction from 44 (under construction in 2008) to 29 (under construction in 2016) Bq/m3. For Block of flats/terraced apartments, ground and 1st floors were a reduction from 35 (under construction in 2008) to 34 (under construction in 2016) Bq/m3. | About 18.6% and 20.1% of cases were exposed to 101–147 and >147 Bq/m3 compared with 14% and 15% of controls. | Mean residential radon levels were 65.46 Bq/m3 and 73.75 Bq/m3 (p = 0.013) in the case and control groups, respectively. Among the cases and controls, the proportions of individuals exposed to high levels of residential radon (≥100 Bq/m3) were 13.7% and 17.7% (p = 0.007); smokers comprised 42.8% and 34.9% (p = 0.009); and second-hand smokers accounted for 46.1% and 21.2% (p < 0.001), respectively. |
| Methodology of environmental measurement | Integrated radon measurements were performed with alpha track detectors for two months or longer, in two occupied rooms, preferably in the living room and a bedroom. | Radon measurements were conducted with alpha-track detectors. Detectors were placed away from doors, windows, and electric devices, between 60 and 180 cm from the floor. Radon was measured for a period of 3 to 6 months. | Residential radon levels were measured in the living room and the bedroom with Alpha-track detectors, as a passive radon measuring device. The measuring devices were positioned away from household electrical appliances, windows, or sealed drawers. The measurements were made over 3 months, and the average of measurements at both locations in the house was taken. Given that indoor radon levels are highest in the winter and lowest in the summer, seasonal corrections were made with average temperature and wind speed. |
| Methods of prevention | Balanced ventilation (mechanical supply and exhaust ventilation) with heat recovery in new buildings. | - | - |
| Conclusion | It appears that radon concentrations are lower in terraced and semi-detached homes, which were previously thought to be about the same as detached homes in terms of radon hazards. | Compared to people exposed to lesser quantities, those exposed to concentrations greater than 50 Bq/m3 are about twice as likely to get lung cancer. Additionally, radon exposure modifies the effects of tobacco use. The increased radon exposure raises the risk of lung cancer for people who smoke regularly. | Residential radon exposure and cigarette smoking were synergistically associated with a greater risk of lung cancer. Although addictive interaction (p = 0.344) and multiplicative interaction (p = 0.367) did not reach statistical significance, the difference in ORs for lung cancer according to radon exposure was much greater in current smokers than in non-smokers. |
| Author, Year, Country | Rodríguez-Martínez A. et al. 2021 Spain [22] | Torres-Duràn M. et al. 2015 Spain [23] | Xiang-Zhen X. et al. 1993 China [24] |
| n° Patients (sex F%, M%; mean age years, range + SD) | Case 375 (24.5% F, 75.5% M; 6.4% < 50 y, 62.4% 51–70 y, 29.9% > 70 y) Control 902 (33.0%F, 77.0% M; 20.2% < 50 y, 61.5% 51–70 y, 18.1% > 70 y) | Case 199 (79.9% F, 13.5% M; 70, 61–77 y) Control 275 (78.8%F, 21.2% M; 70, 63–79 y) | 17,000 male and 2800 female employees |
| Case | Small cell lung cancer | Never-smoking cases with primary lung cancer | Lung cancer |
| Sampling location | Santiago de Compostela, Ourense, A Coruna, Asturias, Ávila, Puerta de Hierro, Porto | Galicia and Asturias | Workers of the Yunnan Tin Corporation (YTC) in Yunnan Province, Southern China |
| Sampling period | - | Between January 2011 and October 2013 | The mean durations of radon exposure and of arsenic exposure are about 13 years |
| Anatomopathological changes | - | Squamous cell carcinoma; Adenocarcinoma; Small cell carcinoma; Large cell carcinoma; other histological types | - |
| Environmental concentration measurement | Median radon concentration (25–75th percentiles) 152,5 Bq/m3 (91–260) in case’s houses; 142 Bq/m3 (89–267) in control’s houses | Median radon concentration (25–75th percentiles): 223 Bq/m3 (123–587) in squamous cell carcinoma; 189 Bq/m3 (106–375) in adenocarcinoma; 173 Bq/m3 (57–218) in small cell carcinoma; 109 Bq/m3 (77–130) in large cell carcinoma; 309 Bq/m3 (238–516) in other histological types. | The mean lifetime cumulative radon exposure was 275.4 WLM with a maximum exposure of 1653 WLM. |
| Methodology of environmental measurement | Alpha-track device which was placed for a period of at least three months in each dwelling. | Alpha-track types were placed for a minimum of 3 months in the participant’ s bedroom, away from doors, windows, heating and electrical devices and at a height between 60 and 180 cm off the floor. | - |
| Methods of prevention | - | - | - |
| Conclusion | It can be observed that for people exposed to more than 147 Bq/m3 the risk of lung cancer increased with tobacco consumption. For heavy smokers, the risk of lung cancer also increases with radon exposure. Those exposed to more than 147 Bq/m3 and heavy smokers showed an OR of 72.6 (95%CI 18.0–499.4) compared to never-smokers exposed to less than 50 Bq/m3. The synergy index, calculated for 2 categories of radon exposure (<100 and >147 Bq/m3) and tobacco consumption (never smokers and heavy smokers) was not significant: 2.18 (95%CI: 0.88–5.43). | We can observe that the risks are very similar for the different histologies, though there is statistical significance only for adenocarcinoma (OR 2.19; 95% CI 1.44–3.33). The remaining histological types did not reach statistical significance, though for all histological types, the lowest confidence interval was 0.85. | The risk of lung cancer declines significantly with attained age, radon exposure rate, and years since last radon exposure, and does not vary in a consistent way with age at first radon exposure. |
| Author, Year, Country | Grzywa-Celińska A. et al. 2023 Poland [25] | Kelly-Reif K. et al. 2022 USA [26] | Tomasek L. et al. 2012 Czech Republic [27] |
| n° Patients (sex F%, M%; mean age years, range + SD) | 102 patients 39 women (38.2%) and 63 men (61.8%). | 16,434 underground uranium miners | 11842 people (5858 men and 5984 women) |
| Case | lung cancer | - | Lung cancer |
| Sampling location | Lublin | Uranium miners in the Czech Republic. | 80 villages of Mid-Bohemia Pluton |
| Sampling period | - | The cohort included male workers who were listed in the employment registry between 1 January 1949 and 31 December 1975, worked underground for at least 1 year | The study was established in 1990 as a retro-prospective follow-up covering period since 1961 |
| Anatomopathological changes | 38.2% of the study group. Patients with the diagnosis of non-small cell carcinoma accounted for 78.4% (n = 80), 41.2% (n = 42) had adenocarcinoma subtype, squamous subtype occurred in 26.5% (n = 27), and not otherwise specified (NOS) due to an uncertain histological subtype was found in 6.9% (n = 7). Four patients were diagnosed with rare types of lung cancer. In this subgroup of patients, there were neuroendocrine, mixed histology of adenosquamous and two patients with large cell tumours. Small cell carcinoma was treated in 21.6% (n = 22) of patients. | - | - |
| Environmental concentration measurement | The average concentration of radon during the exposure of the detector in the residential premises of the respondents was at the level of 69.0 Bq/m3 [37.0–117.0]. Significantly higher values of the average radon concentration during the exposure of the detector in residential premises were observed if the measurement was performed in the countryside compared to a large city (82.0 vs. 3 9.5 B q/m3; p = 0.0232; It was found that there were significantly lower values); in the cases where the basement floors were wooden compared to other possible materials (wood vs. ceramic tiles or concrete or soil, respectively: 30.5 vs. 119.0 or 69.0 or 76.5 Bq/m3; p = 0.0024); Significantly higher values of the average radon concentration during detector exposure were observed in patients with air conditioning (95.0 vs. 67.0 Bq/m3; p = 0.0456). Moreover, in patients whose window frames were made of plastic instead of wood, the mean values of radon concentration during detector exposure were significantly higher (75.0 vs. 40.0 Bq/m3; p = 0.0350) | Radon (WLM) 115 (0–1022) | The measurement as were conducted in 2154 houses (mean 499 Bq/m3). |
| Methodology of environmental measurement | The measurements of indoor radon concentration were made with the use of a passive method with solid-state nuclear track detectors (SSNTD) of CR-39 type. The detectors of RSKS type (Radosys Ltd., Hungary) have been used. One month of radon exposure measurement was performed with alpha-track detectors. | - | Passive track detectors and radon gas by electrets and closed CR-39 detectors |
| Methods of prevention | There are some practical solutions to reduce indoor radon, using wooden window frames rather than plastic frames and limiting the use of air conditioning. | - | - |
| Conclusion | That higher concentrations of radon occurs in buildings located in the countryside. The level of radon was higher in the presence of air conditioning, which intensifies the transport of radon from the soil to the interior of the building. The higher radon concentrations were also associated with the presence of plastic window frames compared to wooden frames. | There is a positive exposure-response relationship between cumulative exposure to radon and lung cancer. | The study confirmed that the risk from radon exposure in houses is indubitable. |
| Author, Year, Country | Torres-Duran M. et al. 2014 Spain [28] | Turner M. et al. 2011 Canada [29] | |
| n° Patients (sex F%, M%; mean age years, range + SD) | 521 (192 cases and 329 controls); Female 153 (79.7) 259 (78.7) Male 39 (20.3) 70 (21.3) | 811,961 (55.3%F, 44.7%M; 4.6% < 40 y, 21.4% 40–49 y; 36.6% 50–59 y; 26.3% 60–69 y; 9.4% 70–79 y; 1.7% > 80) | |
| Case | Lung cancer | - | |
| Sampling location | Hospitals of Northwest of Spain (Galicia and Asturias) | Florida, New Jersey, South Carolina, New Hampshire, New York, Iowa, Idaho, Ohio, Utah | |
| Sampling period | Between January 2011 and June 2013 | 1982–1986 | |
| Anatomopathological changes | Adenocarcinoma, Squamous cell carcinoma, Small cell carcinoma, Large cell carcinoma, Other histological types | - | |
| Environmental concentration measurement | 48% of cases had residential radon exposure >200 Bq·m−3; ≤100 to ≥200 | Mean Bq/m3 (SD): Northeast 58.3 (42.3); South 35.6 (21.7); Midwest 73.7 (36.6); West 46.9 (40.3) | |
| Methodology of environmental measurement | Alpha-track type | Mean county level residential radon concentrations were linked to study participants according to ZIP code information | |
| Methods of prevention | - | - | |
| Conclusion | The results of the present study show that residential radon increases the risk of lung cancer in never-smokers when they are exposed to indoor levels >200 Bq·m−3. | A significant positive linear trend was observed between categories of radon concentrations and lung cancer mortality (p ¼ 0.02). A 15% (95% CI, 1–31) increase in the risk of lung cancer mortality was observed per 100 Bq/m3 increase in radon. Participants with mean radon concentrations above the EPA guideline value (148 Bq/m3) experienced a 34% (95% CI, 7–68) increase in risk for lung cancer mortality relative to those below the guideline value. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofone, L.; Sabato, M.; Colombo, C.; Scalingi, S.; Montesi, A.; Paglione, L.; Patania, F. Health Effects and Preventive Strategies for Radon Exposure: A Systematic Review of the Literature. J. Respir. 2025, 5, 16. https://doi.org/10.3390/jor5040016
Cofone L, Sabato M, Colombo C, Scalingi S, Montesi A, Paglione L, Patania F. Health Effects and Preventive Strategies for Radon Exposure: A Systematic Review of the Literature. Journal of Respiration. 2025; 5(4):16. https://doi.org/10.3390/jor5040016
Chicago/Turabian StyleCofone, Luigi, Marise Sabato, Chiara Colombo, Stefania Scalingi, Antonio Montesi, Lorenzo Paglione, and Federica Patania. 2025. "Health Effects and Preventive Strategies for Radon Exposure: A Systematic Review of the Literature" Journal of Respiration 5, no. 4: 16. https://doi.org/10.3390/jor5040016
APA StyleCofone, L., Sabato, M., Colombo, C., Scalingi, S., Montesi, A., Paglione, L., & Patania, F. (2025). Health Effects and Preventive Strategies for Radon Exposure: A Systematic Review of the Literature. Journal of Respiration, 5(4), 16. https://doi.org/10.3390/jor5040016