TUS-EPIC: Thoracic Ultrasonography for Exclusion of Iatrogenic Pneumothorax in Post Transbronchial Lung Cryobiopsy—A Safe Alternative to Chest X-Ray
Abstract
1. Introduction
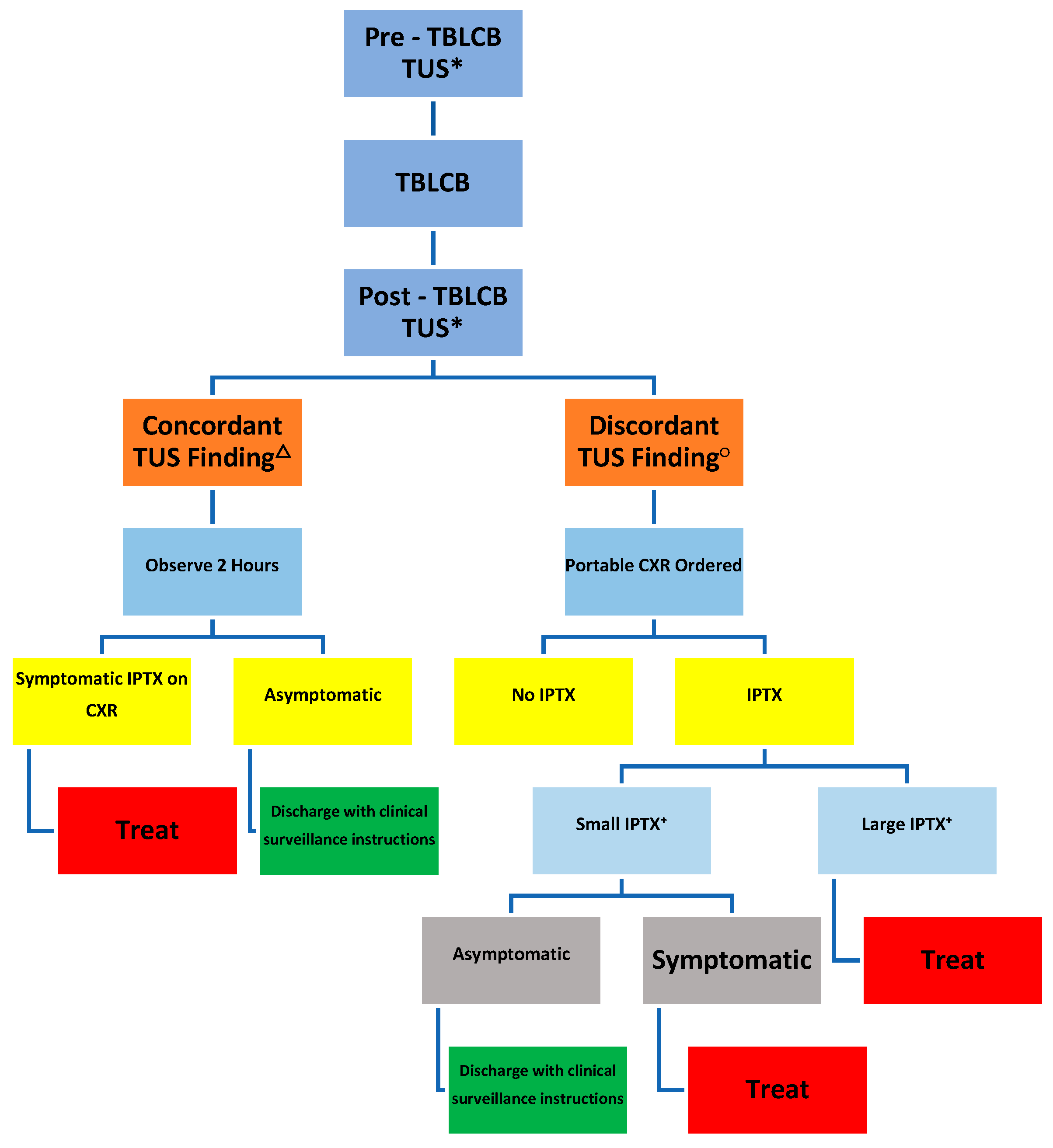
2. Materials and Methods
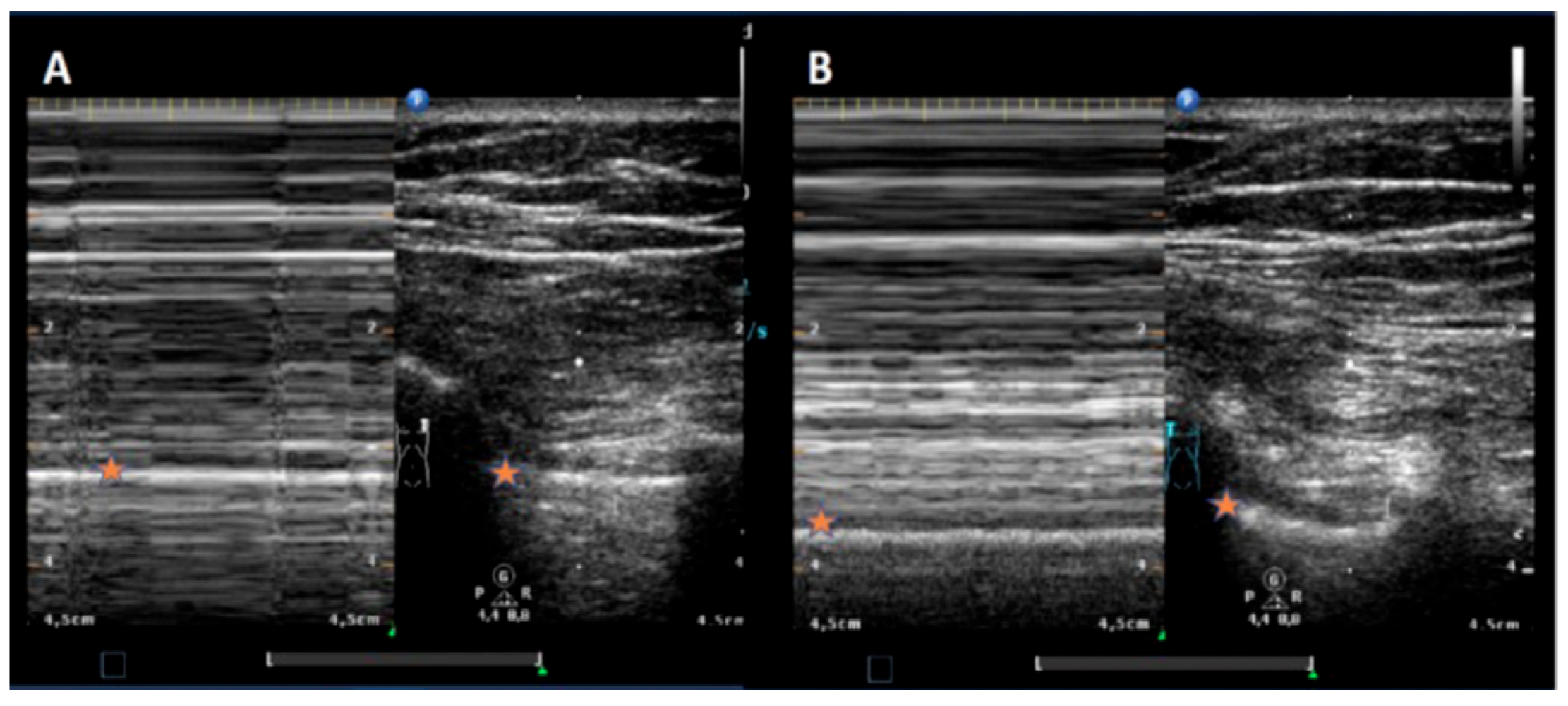
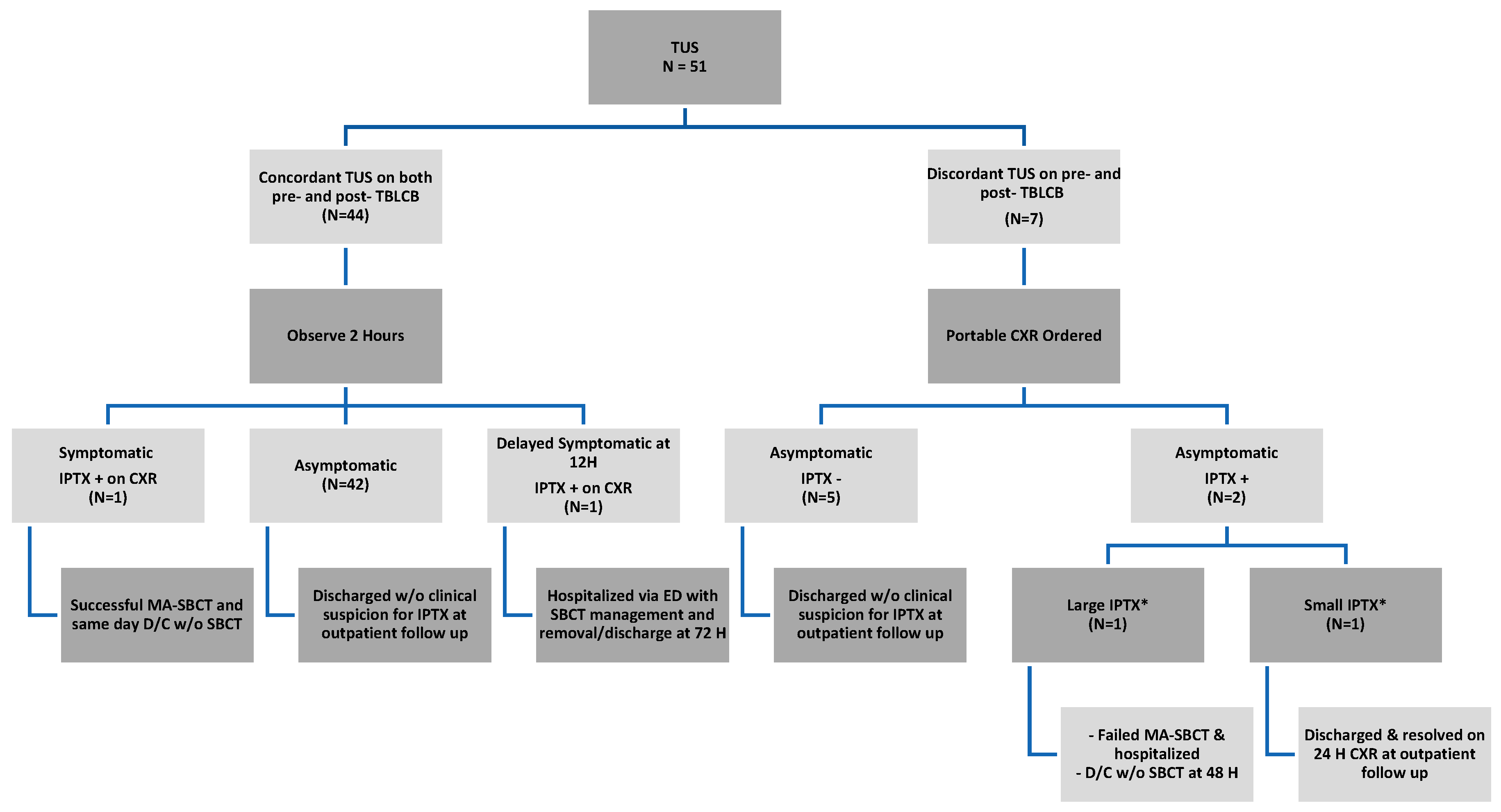
3. Results
Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society bronchoscopy guideline group. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68 (Suppl. S1), i1–i44. [Google Scholar] [CrossRef]
- Facciolongo, N.; Patelli, M.; Gasparini, S.; Agli, L.L.; Salio, M.; Simonassi, C.; Del Prato, B.; Zanoni, P. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch. Chest Dis. 2009, 71, 8–14. [Google Scholar] [CrossRef]
- Pue, C.A.; Pacht, E.R. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995, 107, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Colt, H.G.; Matsuo, T. Hospital charges attributable to bronchoscopy-related complications in outpatients. Respiration 2001, 68, 67–72. [Google Scholar] [CrossRef]
- Milman, N.; Faurschou, P.; Munch, E.P.; Grode, G. Transbronchial lung biopsy through the fibre optic bronchoscope. Results and complications in 452 examinations. Respir. Med. 1994, 88, 749–753. [Google Scholar] [CrossRef]
- Matus, I.; Raja, H. Protocolized thoracic ultrasonography in transbronchial lung cryobiopsies: A potential role as an exclusion study for pneumothorax. J. Bronchol. Interv. Pulmonol. 2018, 26, 172–178. [Google Scholar] [CrossRef]
- Ultrasound Lung Zones. Volpicelli Method for Thoracic Exam. Available online: https://www.teachingmedicine.com/Lesson.aspx?l_id=95 (accessed on 2 June 2024).
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Ravaglia, C.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Gurioli, C.; Dubini, A.; Cavazza, A.; Colby, T.V.; Piciucchi, S.; Puglisi, S.; et al. Diagnostic yield and risk/benefit analysis of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: A large cohort of 699 patients. BMC Pulm. Med. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Danoff, S.K.; Wells, A.U.; Colby, T.V.; Ryu, J.H.; Liberman, M.; Wahidi, M.M.; Frazer, L.; Hetzel, J.; Rickman, O.B.; et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases. Chest 2020, 157, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, D.A.; Colella, S.; Fally, M.; Camuset, J.; Colby, T.V.; Hagmeyer, L.; Hetzel, J.; Maldonado, F.; Morais, A.; Ravaglia, C.; et al. European Respiratory Society guidelines on transbronchial biopsy in the diagnosis of interstital lung diseases. Eur. Respir. J. 2022, 60, 2200425. [Google Scholar] [CrossRef]
- Dibardino, D.; Haas, A.; Lanfranco, A.; Litzky, L.A.; Sterman, D.; Bessich, J.L. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann. Am. Thorac. Soc. 2017, 14, 851–885. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Alghothani, L.; Sardi, A.; Berkowitz, D.; Musani, A.I. Transbronchial Lung Cryobiopsy and Video-assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease. A Meta-analysis of Diagnostic Test Accuracy. Ann. Am. Thorac. Soc. 2017, 14, 1197–1211. [Google Scholar] [CrossRef]
- Sharp, C.; McCabe, M.; Adamali, H.; Medford, A. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—A systematic review and cost analysis. QJM Int. J. Med. 2017, 110, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zayed, Y.; Alzghoul, B.N.; Hyde, R.; Wadood, Z.; Banifadel, M.; Khasawneh, M.; Maharrey, P.B.; Saker, H.; Harden, C.; Barnes, G.; et al. Role of Transbronchial Lung Cryobiopsy in the Diagnosis of Interstitial Lung Disease: A Meta-analysis of 68 Studies and 6300 Patients. J. Bronchol. Interv. Pulmonol. 2023, 30, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Raices, M.; Bequis, M.A.; Heras, M.L.; Wainstein, E.; Castro, R.; Montagne, J.; Dietrich, A. Post-transbronchial biopsy complications: The role of cryobiopsy in the incidence of postoperative pneumothorax. J. Fac. Med. Sci. Córdoba 2021, 78, 29–32. [Google Scholar] [CrossRef]
- Cardoso, A.C.G.; Serino, M.; Coelho, D.B.; Guimarães, S.; Moura, C.S.; Mota, P.C.; Melo, N.; Pereira, J.M.; Cunha, R.; Carvalho, A.; et al. Predictors of pneumothorax after transbronchial lung cryobiopsy for diagnosis of interstitial lung diseases. Eur. Respir. J. 2021, 58 (Suppl. S65), PA852. [Google Scholar] [CrossRef]
- Davidsen, J.R.; Skov, I.R.; Louw, I.G.; Laursen, C.B. Implementation of transbronchial lung cryobiopsy in a tertiary referral center for interstitial lung diseases: A cohort study on diagnostic yield, complications, and learning curves. BMC Pulm. Med. 2021, 21, 67. [Google Scholar] [CrossRef]
- MacDuff, A.; Arnold, A.; Harvey, J. Group BTSPDG: Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guidelines 2010. Thorax 2010, 65 (Suppl. S2), ii18–ii31. [Google Scholar] [CrossRef]
- Baumann, M.H.; Strange, C.; Heffner, J.E.; Light, R.; Kirby, T.J.; Klein, J.; Luketich, J.D.; Panacek, E.A.; Sahn, S.A.; Forthe ACCP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2001, 119, 590–602. [Google Scholar] [CrossRef]
- Manhire, A.; Charig, M.; Clelland, C.; Gleeson, F.; Miller, R.; Moss, H.; Pointon, K.; Richardson, C.; Sawicka, E. Guidelines for radiologically guided lung biopsy. Thorax 2003, 58, 920–936. [Google Scholar] [CrossRef]
- Perlmutt, L.; Braun, S.; Newman, G.; Oke, E.; Dunnick, N. Timing of chest film follow-up after transthoracic needle aspiration. Am. J. Roentgenol. 1986, 146, 1049–1050. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Krueter, M.; Eberhardt, R.; Wenz, H.; Schmitteckert, H.; Heussel, C.P.; Herth, F. Diagnostic value of transthoracic ultrasound compared to chest radiography in the detection of a post-interventional pneumothorax. Ultraschall Med. 2011, 32 (Suppl. S2), E2–E23. [Google Scholar]
- Kumar, S.; Agarwal, R.; Aggarwal, A.N.; Gupta, D.M.; Jindal, S.K.M. Role of ultrasonography in the diagnosis and management of pneumothorax following transbronchial lung biopsy. J. Bronchol. Interv. Pulmonol. 2015, 22, 14–19. [Google Scholar] [CrossRef]
- Bensted, K.; McKenzie, J.; Havryk, A.; Plit, M.; Ben-Menachem, E. Lung ultrasound after transbronchial biopsy for pneumothorax screening in post-lung transplant patients. J. Bronchol. Interv. Pulmonol. 2018, 25, 42–47. [Google Scholar] [CrossRef]
- Viglietta, L.; Inchingolo, R.; Pavano, C.; Tomassetti, S.; Piciucchi, S.; Smargiassi, A.; Ravaglia, C.; Dubini, A.; Gurioli, C.; Gurioli, C.; et al. Ultrasonography for the diagnosis of pneumothorax after trans bronchial lung cryobiopsy in diffuse parenchymal lung diseases. Respiration 2017, 94, 232–236. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point of care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, M.; Melniker, L.A.; Volpicelli, G.; Neri, L.; Petrovic, T.; Storti, E.; Blaivas, M. Development of evidence-based clinical recommendations and consensus statements in critical ultrasound field: Why and how? Crit. Ultrasound J. 2010, 2, 93–95. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Accuracy of transthoracic sonography in excluding post-interventional pneumothorax and hydropneumothorax: Comparison to chest radiography. Eur. J. Radiol. 2005, 53, 463–470. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Sirois, M.; Laupland, K.B.; Liu, D.; Rowan, K.; Ball, C.G.; Hameed, S.M.; Brown, R.; Simons, R.; Dulchavsky, S.A.; et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: The extended focused assessment with sonography for trauma (EFAST). J. Trauma 2004, 57, 288–295. [Google Scholar] [CrossRef]
- Volpicelli, G. monographic diagnosis of pneumothorax. Intensive Care Med. 2011, 37, 224–232. [Google Scholar] [CrossRef]
- Blaivas, M.; Lyon, M.; Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad. Emerg. Med. 2005, 12, 844–849. [Google Scholar] [CrossRef]
- Rowan, K.R.; Kirkpatrick, A.W.; Liu, D.; Forkheim, K.E.; Mayo, J.R.; Nicolaou, S. Traumatic pneumothorax detection with thoracic US: Correlation with chest radiography and CT—Initial experience. Radiology 2002, 225, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Pignataro, G.; Portale, G.; Biasucci, D.G.; Leone, A.; Silveri, N.G. The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med. Biol. 2006, 32, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult traumatic pneumothorax: Diagnostic accuracy or lung ultrasonography in the emergency department. Chest 2008, 133, 204–2011. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.H.; Yang, J.X.; Gan, J.-X.; Xu, S.-W.; You, X.-D.; Jiang, G.-Y. Rapid detection of pneumothorax by ultrasonography in patients with multiple trauma. Crit. Care 2006, 10, R112. [Google Scholar] [CrossRef]
- Izbicki, G.; Romem, A.; Arish, N.; Cahan, C.; Azulai, H.; Chen-Shuali, C.; Tennenhaus, E.; Bar-Yosef, Z.; Zlotkevich, E.; Rokach, A. Avoiding Routine Chest Radiography after Transbronchial Biopsy Is Safe. Respiration 2016, 92, 176–181. [Google Scholar] [CrossRef]
- Matus, I.; Mertens, A.; Wilton, S.; Raja, H.; Roedder, T. Safety and Efficacy of Manual Aspiration Via Small Bore Chest Tube in Facilitating the Outpatient Management of Transbronchial Biopsy-related Iatrogenic Pneumothorax. J. Bronchol. Interv. Pulmonol. 2021, 28, 272–280. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | |
|---|---|
| Median Age (Y) | 70 (22–88) |
| Gender | n (%) |
| Male | 24 (47.6%) |
| Female | 27 (52.94%) |
| Indication for TBLCB | n (%) |
| Suspected ILD +/− nondiagnostic | 43 (84.31%) |
| bronchoscopy | |
| Other computed tomographic | 8 (15.69%) |
| Abnormality (ie, diffuse infiltrates) +/− | |
| nondiagnostic bronchoscopy | |
| Complications | n (%) |
| Pneumothorax | 4 (7.8%) |
| Bleeding * | 5 (9.8%) |
| Grade 1 | 1 (1.96%) |
| Grade 2 | 4 (7.8%) |
| Study | PTX (+) | PTX (−) |
|---|---|---|
| Discordant TUS | 2 | 5 |
| Concordant TUS | 2 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matus, I.; Akhtar, S.; Matta, V. TUS-EPIC: Thoracic Ultrasonography for Exclusion of Iatrogenic Pneumothorax in Post Transbronchial Lung Cryobiopsy—A Safe Alternative to Chest X-Ray. J. Respir. 2025, 5, 18. https://doi.org/10.3390/jor5040018
Matus I, Akhtar S, Matta V. TUS-EPIC: Thoracic Ultrasonography for Exclusion of Iatrogenic Pneumothorax in Post Transbronchial Lung Cryobiopsy—A Safe Alternative to Chest X-Ray. Journal of Respiration. 2025; 5(4):18. https://doi.org/10.3390/jor5040018
Chicago/Turabian StyleMatus, Ismael, Sameer Akhtar, and Vamsi Matta. 2025. "TUS-EPIC: Thoracic Ultrasonography for Exclusion of Iatrogenic Pneumothorax in Post Transbronchial Lung Cryobiopsy—A Safe Alternative to Chest X-Ray" Journal of Respiration 5, no. 4: 18. https://doi.org/10.3390/jor5040018
APA StyleMatus, I., Akhtar, S., & Matta, V. (2025). TUS-EPIC: Thoracic Ultrasonography for Exclusion of Iatrogenic Pneumothorax in Post Transbronchial Lung Cryobiopsy—A Safe Alternative to Chest X-Ray. Journal of Respiration, 5(4), 18. https://doi.org/10.3390/jor5040018






