Busting the Myths of DLco for Pulmonary Trainees: Isolated Reductions in DLco and the Relationship with VA
Abstract
1. Introduction
Methods of DLco Measurement
- Preparation for the test:
- ○
- No cigarette smoking on the day of the test.
- ○
- No supplemental oxygen for at least 15 min prior to the test. Supplemental oxygen can decrease DLco by approximately 0.35 percent per mmHg change in arterial oxygen tension (PaO2). The DLco test cannot be accurately performed in patients who are unable to discontinue supplemental oxygen for at least 15 min.
- ○
- It can be performed after the bronchodilator test.
- DLco maneuver and techniques:DLco is measured using the following techniques [1].
- Single-breath method;
- Intra-breath method;
- Rebreathing technique.
2. Search Strategy
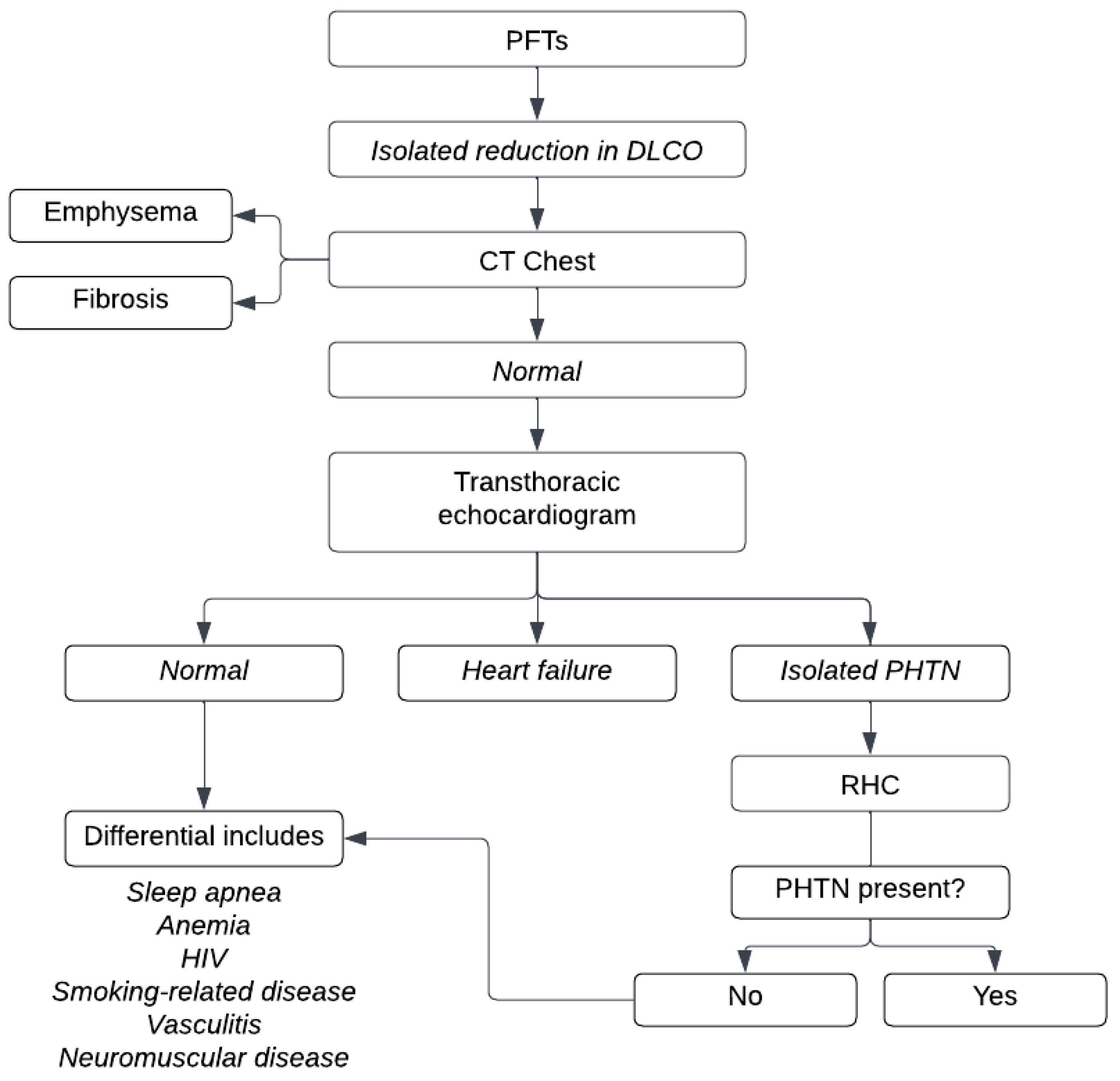
3. Isolated Reduced DLco
3.1. Pulmonary Vascular Disease
3.2. Smoking
3.3. Anemia with Sickle Cell Disease
3.4. Heart Failure
3.5. Interstitial Lung Disease
3.6. Combined Pulmonary Fibrosis and Emphysema
3.7. HIV Infection
3.8. Occupational Exposures
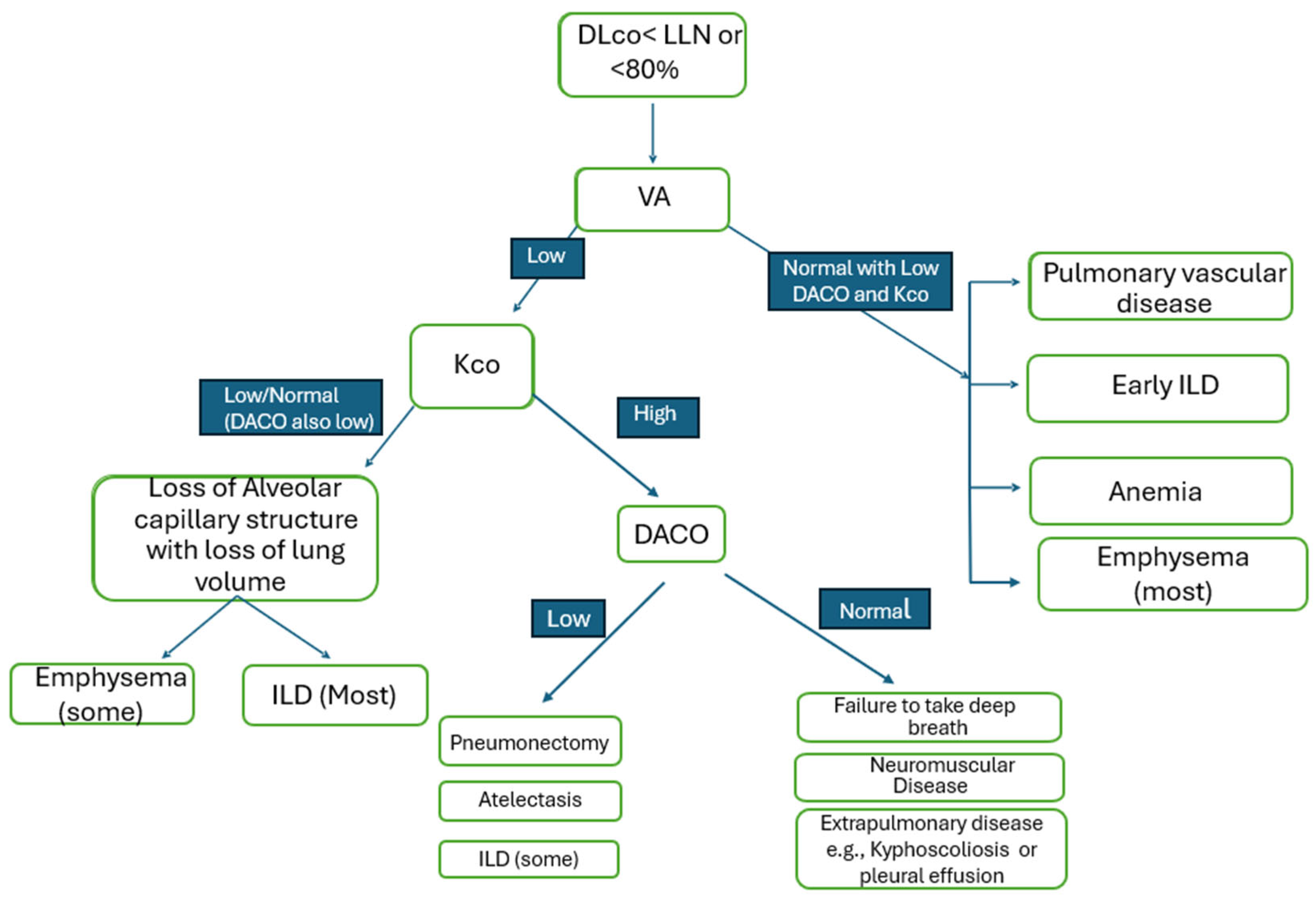
4. Algorithmic Approach
4.1. DLco and Va Relationship
4.2. Components of DLco
4.3. What Is Alveolar Volume (Va)?
4.4. What Is kco?
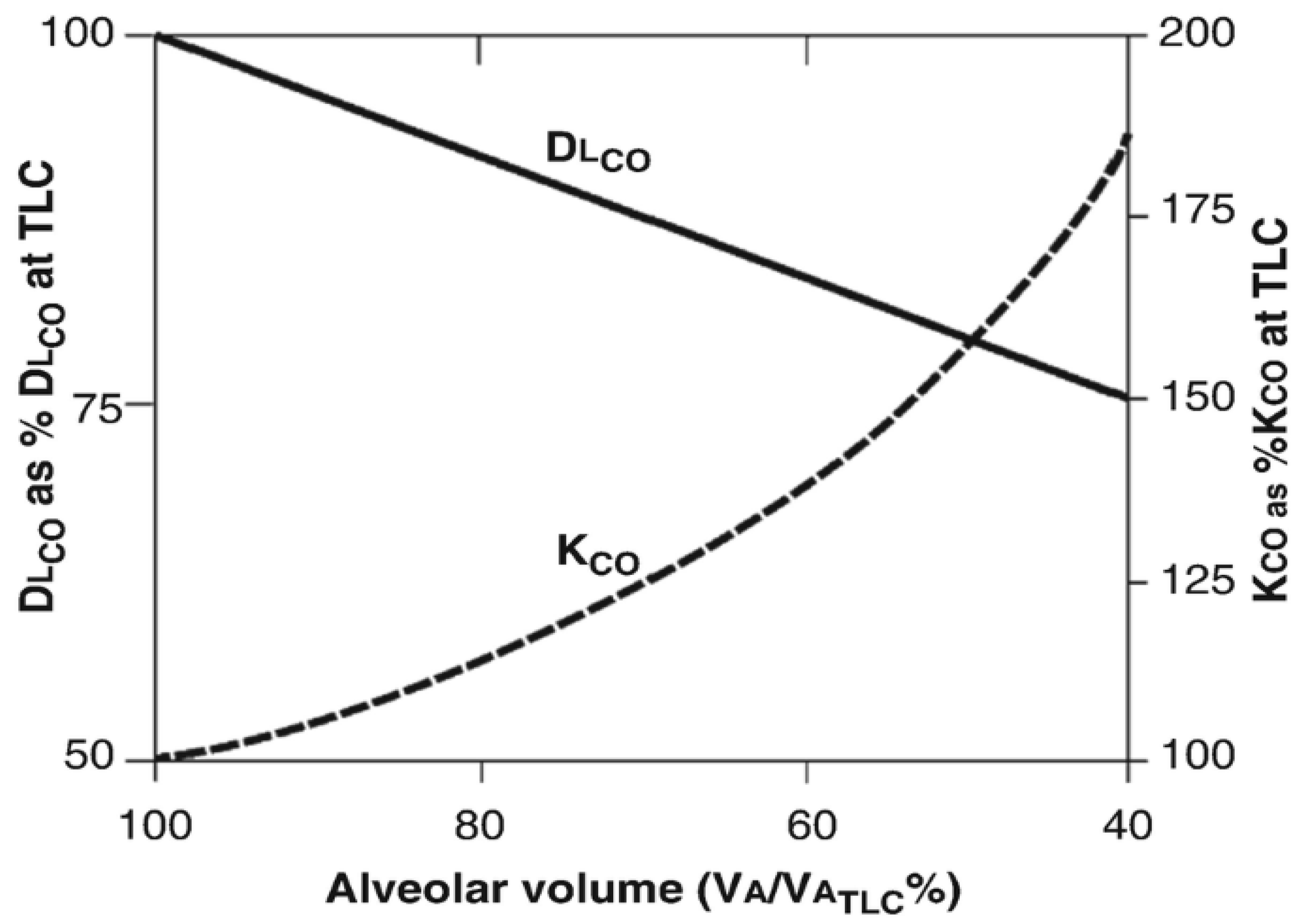
5. Relationship Between Lung Volume (Va) and kco
5.1. Alveolar Po2
5.2. Clearing the Misconception of “Volume-Adjusted DLco (DLco/Va)”
5.3. Integrated Approach Toward Assessment of Lung Diffusion Capacity
6. Conclusions
7. Summary
- ➣
- The diffusion capacity of carbon monoxide (DLco) is a valuable test in evaluating patients with various lung diseases. It assists in diagnosing patients, following them over time, and has prognostic value.
- ➣
- Accurate interpretation of DLco requires understanding its relationship with lung volume (Va) and the diffusion coefficient (kco).
- ➣
- It is a common misunderstanding in everyday clinical practice to interpret DLco/Va as diffusion capacity “corrected” for lung volume. This notion is physiologically incorrect and has clinical implications.
- ➣
- Knowing the physiological basis of this test should help the readers understand the necessity of incorporating all the variables required for its correct interpretation.
- ➣
- Further, a readily available algorithm incorporating the above factors can provide a quick reference guide for trainees and pulmonary physicians to interpret lung diffusion capacity results.
- ➣
- Isolated reduced DLco is a unique abnormality that is often overlooked and not given due importance. It often points to early/developing lung disease and/or pulmonary vascular abnormality.
- ➣
- The presence of isolated DLco on full PFTs should alarm the physicians so that further workups can be initiated to find the cause of this abnormality.
Funding
Acknowledgments
Conflicts of Interest
References
- Enright, P. Office-based DLCO tests help pulmonologists to make important clinical decisions. Respir. Investig. 2016, 54, 305–311. [Google Scholar] [CrossRef]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; Van Der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Crapo, R.O. Diffusing capacity: How to get it right. Respir. Care 2003, 48, 777–782. [Google Scholar] [PubMed]
- Suzuki, T.; Yoshimi, K.; Ueki, J.; Fukuchi, Y. Measurement of diffusing capacity by the intrabreath method. Nihon Kokyuki Gakkai Zasshi J. Jpn. Respir. Soc. 2005, 43, 347–353. [Google Scholar]
- Liu, Q.X.; Zheng, J.P.; Xie, Y.Q.; Guan, W.J.; Jiang, C.Y.; An, J.Y.; Yu, X.X.; Liu, W.T.; Gao, Y. Single-breath and rebreathing methods for measurement of pulmonary diffusing function: A comparative study. Zhonghua Jie He He Hu Xi Za Zhi Chin. J. Tuberc. Respir. Dis. 2013, 36, 510–515. [Google Scholar]
- Abou Daya, I.H.; Anwer, M.U.; Diaz-Fuentes, G.; Blum, S.; Menon, L. Isolated abnormalities of diffusion capacity (DLCO) in pulmonary function tests among inner city patients. Chest 2008, 134, 49S. [Google Scholar] [CrossRef]
- Randhawa, E.; Sherman, M. Causes of Isolated Diffusing Capacity of the Lungs for Carbon Monoxide (Dlco) from Outpatient Pulmonary Function Testing. Chest 2018, 154, 966A. [Google Scholar] [CrossRef]
- Diamanti, E.; Karava, V.; Yerly, P.; Aubert, J.D. Carbon monoxide diffusion capacity as a severity marker in pulmonary hypertension. J. Clin. Med. 2021, 11, 132. [Google Scholar] [CrossRef]
- Farha, S.; Asosingh, K.; Xu, W.; Sharp, J.; George, D.; Comhair, S.; Park, M.; Tang, W.W.; Loyd, J.E.; Theil, K.; et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: A link to the intrinsic myeloid abnormalities. Blood J. Am. Soc. Hematol. 2011, 117, 3485–3493. [Google Scholar] [CrossRef]
- Overbeek, M.J.; Groepenhoff, H.; Voskuyl, A.E.; Smit, E.F.; Peeters, J.W.; Vonk-Noordegraaf, A.; Spreeuwenberg, M.D.; Dijkmans, B.C.; Boonstra, A. Membrane diffusion-and capillary blood volume measurements are not useful as screening tools for pulmonary arterial hypertension in systemic sclerosis: A case control study. Respir. Res. 2008, 9, 68. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Kacprzak, A.; Franczuk, M.; Burakowska, B.; Kurzyna, M.; Fijałkowska, A.; Skoczylas, A.; Wesołowski, S.; Kuś, J.; Torbicki, A. Low DLCO in idiopathic pulmonary arterial hypertension—Clinical correlates and prognostic significance. Adv. Respir. Med. 2016, 84, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Shah, S.J.; Thenappan, T.; Archer, S.L.; Rich, S.; Gomberg-Maitland, M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J. Heart Lung Transplant. 2010, 29, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Meyer, K.; Rademacher, J.; Fuge, J.; Welte, T.; Olsson, K.M. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 441–449. [Google Scholar] [CrossRef]
- Rose, L.; Prins, K.W.; Archer, S.L.; Pritzker, M.; Weir, E.K.; Misialek, J.R.; Thenappan, T. Survival in pulmonary hypertension due to chronic lung disease: Influence of low diffusion capacity of the lungs for carbon monoxide. J. Heart Lung Transplant. 2019, 38, 145–155. [Google Scholar] [CrossRef]
- Garcia-Rio, F.; Miravitlles, M.; Soriano, J.B.; Cosío, B.G.; Soler-Cataluña, J.J.; Casanova, C.; de Lucas, P.; Alfageme, I.; González-Moro, J.M.; Herrero, M.G.; et al. Prevalence of reduced lung diffusing capacity and CT scan findings in smokers without airflow limitation: A population-based study. BMJ Open Respir. Res. 2023, 10, e001468. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Pulmonary function in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2003, 41, 1028–1035. [Google Scholar] [CrossRef]
- Knudson, R.J.; Kaltenborn, W.T.; Burrows, B. The effects of cigarette smoking and smoking cessation on the carbon monoxide diffusing capacity of the lung in asymptomatic subjects. Am. J. Respir. Crit. Care Med. 1989, 140, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Strulovici-Barel, Y.; Kaner, R.J.; Sanders, A.; Vincent, T.L.; Mezey, J.G.; Crystal, R.G. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur. Respir. J. 2015, 46, 1589–1597. [Google Scholar] [CrossRef]
- Neas, L.M.; Schwartz, J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am. J. Epidemiol. 1998, 147, 1011–1018. [Google Scholar] [CrossRef]
- Klings, E.S.; Wyszynski, D.F.; Nolan, V.G.; Steinberg, M.H. Abnormal pulmonary function in adults with sickle cell anemia. Am. J. Respir. Crit. Care Med. 2006, 173, 1264–1269. [Google Scholar] [CrossRef]
- Stinson, J.M.; McPherson, G.L. Lung volumes and diffusion capacity in sickle cell trait. J. Natl. Med. Assoc. 1986, 78, 505. [Google Scholar] [PubMed]
- Siegel, J.L.; Millen, A.; Brown, L.K.; DeLuca, A.; Teirstein, A.S. Pulmonary diffusing capacity in left ventricular dysfunction. Chest 1990, 98, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.D.; Kubo, S.H.; Rector, T.S.; Brunsvold, N.; Bank, A.J. Pulmonary and peripheral vascular factors are important determinants of peak exercise oxygen uptake in patients with heart failure. J. Am. Coll. Cardiol. 1993, 21, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.N.; Khan, R. Retrospective study of isolated reduction of diffusion capacity in pulmonary function test. Chest 2024, 166, A5682. [Google Scholar] [CrossRef]
- Colaci, M.; Giuggioli, D.; Sebastiani, M.; Manfredi, A.; Lumetti, F.; Luppi, F.; Cerri, S.; Ferri, C. Predictive value of isolated DLCO reduction in systemic sclerosis patients without cardio-pulmonary involvement at baseline. Reumatismo 2015, 67, 149–155. [Google Scholar] [CrossRef]
- Morganroth, P.A.; Kreider, M.E.; Okawa, J.; Taylor, L.; Werth, V.P. Interstitial lung disease in classic and skin-predominant dermatomyositis: A retrospective study with screening recommendations. Arch. Dermatol. 2010, 146, 729–738. [Google Scholar] [CrossRef]
- Aduen, J.F.; Zisman, D.A.; Mobin, S.I.; Venegas, C.; Alvarez, F.; Biewend, M.; Jolles, H.I.; Keller, C.A. Retrospective study of pulmonary function tests in patients presenting with isolated reduction in single-breath diffusion capacity: Implications for the diagnosis of combined obstructive and restrictive lung disease. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2007; Volume 82, pp. 48–54. [Google Scholar]
- Crothers, K.; McGinnis, K.; Kleerup, E.; Wongtrakool, C.; Hoo, G.S.; Kim, J.; Sharafkhaneh, A.; Huang, L.; Luo, Z.; Thompson, B.; et al. HIV infection is associated with reduced pulmonary diffusing capacity. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 64, 271–278. [Google Scholar] [CrossRef]
- Jan, A.K.; Moore, J.V.; Wang, R.J.; Mcging, M.; Farr, C.K.; Moisi, D.; Hartman-Filson, M.; Kerruish, R.; Jeon, D.; Lewis, E.; et al. Markers of inflammation and immune activation are associated with lung function in a multi-center cohort of persons with HIV. Aids 2021, 35, 1031–1040. [Google Scholar] [CrossRef]
- Gingo, M.R.; Nouraie, M.; Kessinger, C.J.; Greenblatt, R.M.; Huang, L.; Kleerup, E.C.; Kingsley, L.; McMahon, D.K.; Morris, A. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann. Am. Thorac. Soc. 2018, 15, 192–199. [Google Scholar] [CrossRef]
- Byanova, K.L.; Fitzpatrick, J.; Jan, A.K.; McGing, M.; Hartman-Filson, M.; Farr, C.K.; Zhang, M.; Gardner, K.; Branchini, J.; Kerruish, R.; et al. Isolated abnormal diffusing capacity for carbon monoxide (iso↓ DLco) is associated with increased respiratory symptom burden in people with HIV infection. PLoS ONE 2023, 18, e0288803. [Google Scholar] [CrossRef]
- Gandhi, S.; Cohen, R.A.; Rasmussen, D.L.; Almberg, K.S.; Go, L.H. Impact of Diffusion Capacity Measurement in the Evaluation of Former Coal Miners for Coal Mine Dust Lung Disease. In C15. Occupational Exposures of the 21st Century; American Thoracic Society: New York, NY, USA, 2019; p. A4260. [Google Scholar]
- Johnson, D.C. Importance of adjusting carbon monoxide diffusing capacity (DLCO) and carbon monoxide transfer coefficient (KCO) for alveolar volume. Respir. Med. 2000, 94, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Roughton, F.J.; Forster, R.E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J. Appl. Physiol. 1957, 11, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Cotes, J.E. Carbon monoxide transfer coefficient KCO (TL/VA): A flawed index. Eur. Respir. J. 2001, 18, 893–898. [Google Scholar] [CrossRef][Green Version]
- McGrath, M.W.; Thomson, M.L. The effect of age, body size and lung volume change on alveolar-capillary permeability and diffusing capacity in man. J. Physiol. 1959, 146, 572. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Harper, R.W.; Louie, S. Using and interpreting carbon monoxide diffusing capacity (DLCO) correctly. Consultant 2016, 56, 440–445. [Google Scholar]
- Hughes, J.M.; Pride, N.B. Examination of the carbon monoxide diffusing capacity (DLCO) in relation to its KCO and VA components. Am. J. Respir. Crit. Care Med. 2012, 186, 132–139. [Google Scholar] [CrossRef]
- Stam, H.; Kreuzer, F.J.; Versprille, A. Effect of lung volume and positional changes on pulmonary diffusing capacity and its components. J. Appl. Physiol. 1991, 71, 1477–1488. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Presti, T.P.; Johnson, D.C. Improving pulmonary function test interpretation. Eur. Respir. J. 2023, 61, 2201858. [Google Scholar] [CrossRef]
| Incomplete Alveolar Expansion | Loss of Lung Units (Diffuse or Localized) | Poor Mixing with Normal or Loss of Function (Diffuse or Localized) |
|---|---|---|
| Inadequate inspiration | Pneumonectomy | Asthma |
| Respiratory muscle weakness | Fibrosis | COPD |
| Chest wall or pleural restriction | Bronchiolitis obliterans |
| Factors Affecting kco in Normal Subjects |
|---|
| Lung volume |
| Exercise |
| Anemia |
| Alveolar Po2 (Pao2) |
| Age and height |
| Body position |
| Pathophysiological Mechanism | Clinical Example | |
|---|---|---|
| Causes of increase in kco | ||
| Increased pulmonary blood flow | Asthma, left to right shunt, alveolar hemorrhage | |
| Incomplete alveolar expansion to TLC | Chest wall/pleural abnormalities | |
| Microvascular dilatation | Obesity | |
| Causes of decrease in kco | ||
| Pulmonary microvascular diseases | Pulmonary HTN, pulmonary vasculitis | |
| Alveolar destruction | Emphysema, ILD | |
| Microvascular destruction | Bronchiolitis obliterans, chronic severe heart failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Nadeem, N.; Cardillo, C.; Illipparambil, L.; Ajmeri, A. Busting the Myths of DLco for Pulmonary Trainees: Isolated Reductions in DLco and the Relationship with VA. J. Respir. 2025, 5, 8. https://doi.org/10.3390/jor5030008
Raza A, Nadeem N, Cardillo C, Illipparambil L, Ajmeri A. Busting the Myths of DLco for Pulmonary Trainees: Isolated Reductions in DLco and the Relationship with VA. Journal of Respiration. 2025; 5(3):8. https://doi.org/10.3390/jor5030008
Chicago/Turabian StyleRaza, Ahmad, Nayab Nadeem, Christian Cardillo, Lijo Illipparambil, and Aamir Ajmeri. 2025. "Busting the Myths of DLco for Pulmonary Trainees: Isolated Reductions in DLco and the Relationship with VA" Journal of Respiration 5, no. 3: 8. https://doi.org/10.3390/jor5030008
APA StyleRaza, A., Nadeem, N., Cardillo, C., Illipparambil, L., & Ajmeri, A. (2025). Busting the Myths of DLco for Pulmonary Trainees: Isolated Reductions in DLco and the Relationship with VA. Journal of Respiration, 5(3), 8. https://doi.org/10.3390/jor5030008






