Effects of Structured Exercise Programs on Self-Reported Health-Related Quality of Life in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Study Characteristics
2.2.2. Population
2.2.3. Intervention
2.2.4. Comparator/Control
2.2.5. Outcome
2.3. Study Design
Search Strategy
2.4. Data Collection and Analysis
2.4.1. Selection of Studies
2.4.2. Data Extraction and Management
2.4.3. Quality and Risk of Bias Assessment
2.4.4. Measurement of Treatment Effect
2.4.5. Data Synthesis
3. Results
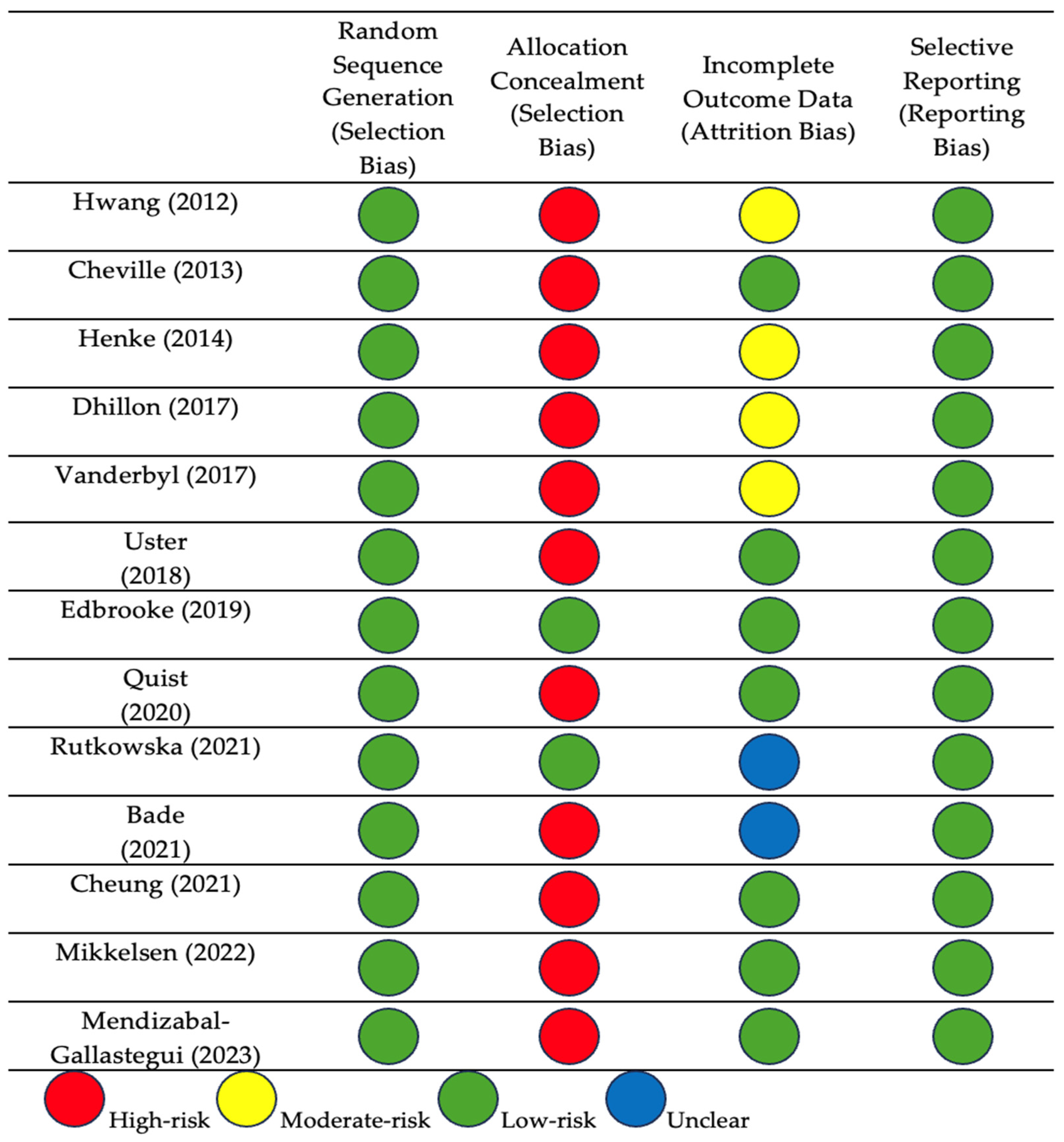
3.1. Risk of Bias
3.2. Synthesis Without Meta-Analysis (SWiM)
3.3. GRADE Analysis of Safety Results
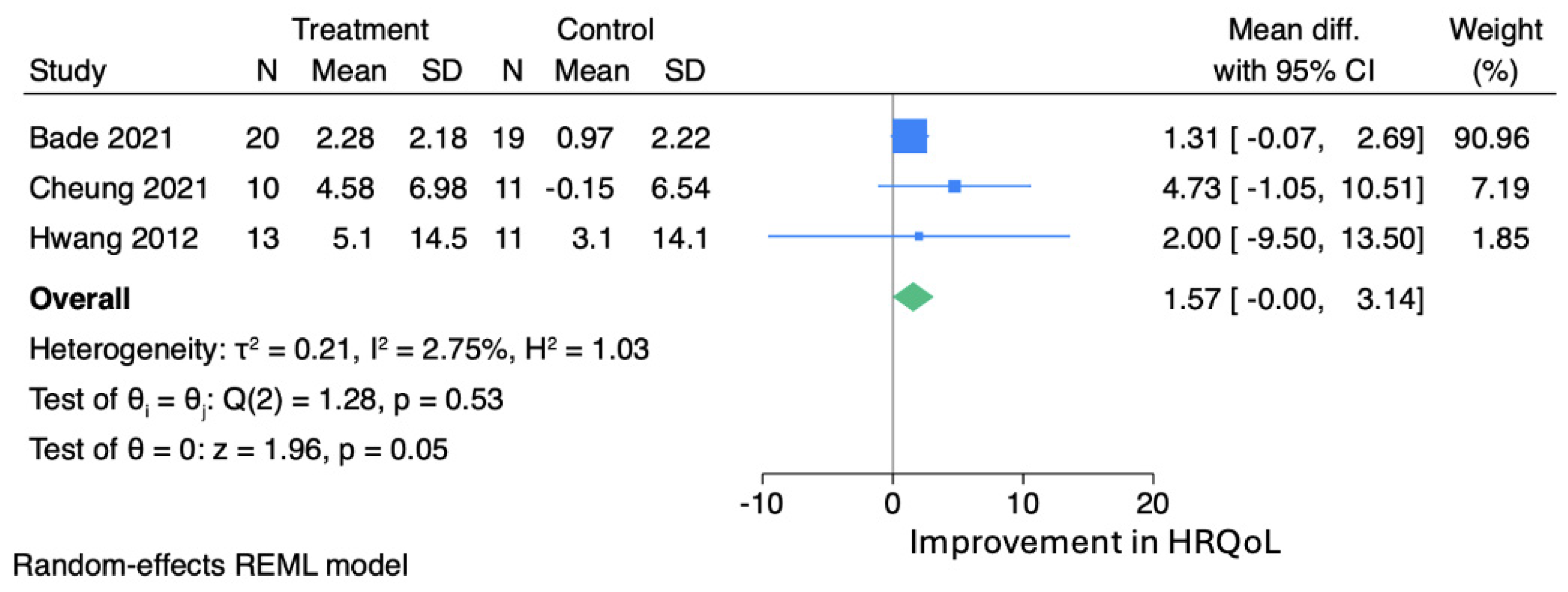
3.4. Meta-Analysis
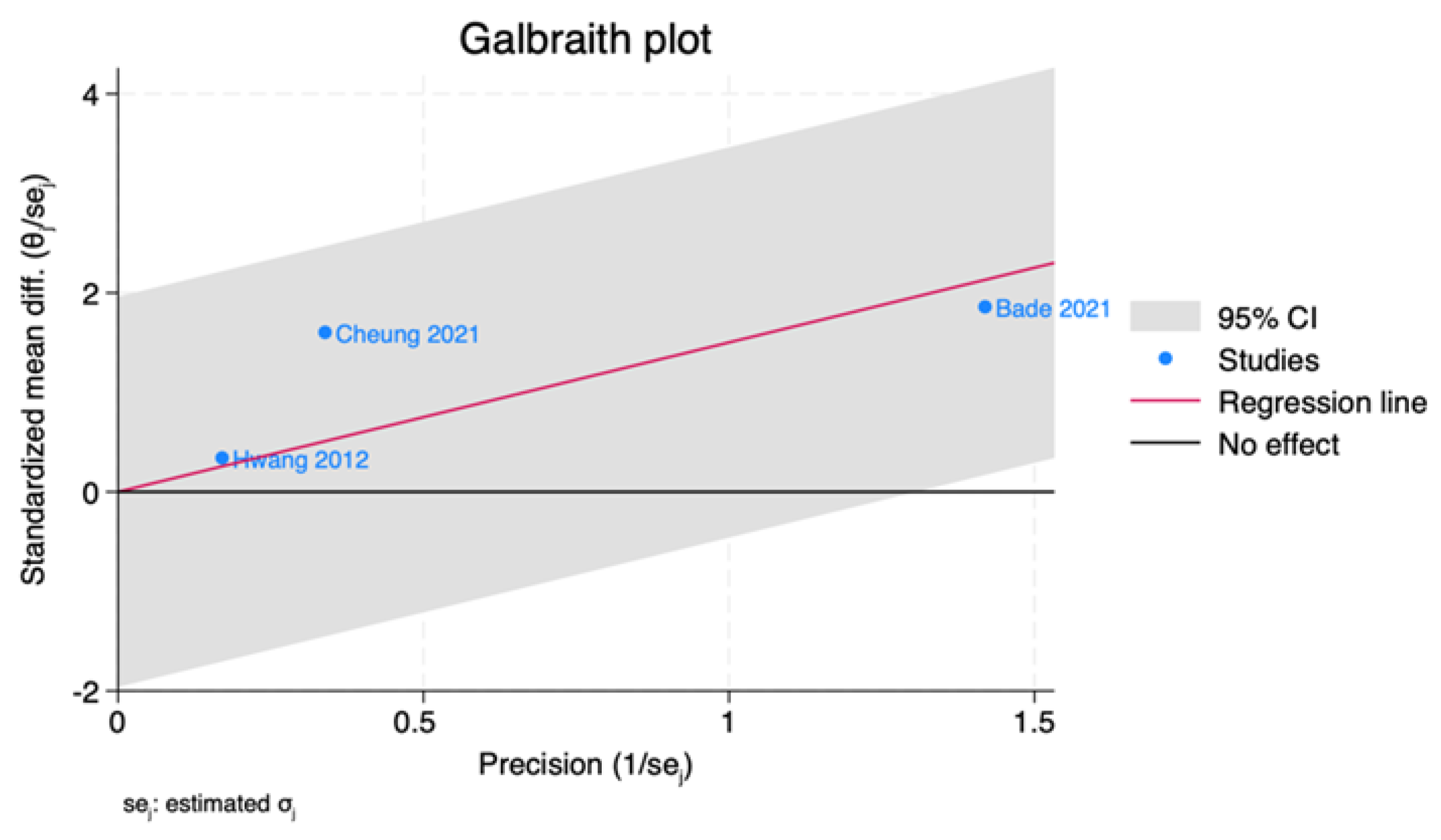
3.5. Publication Bias
3.6. Assessment of Heterogeneity
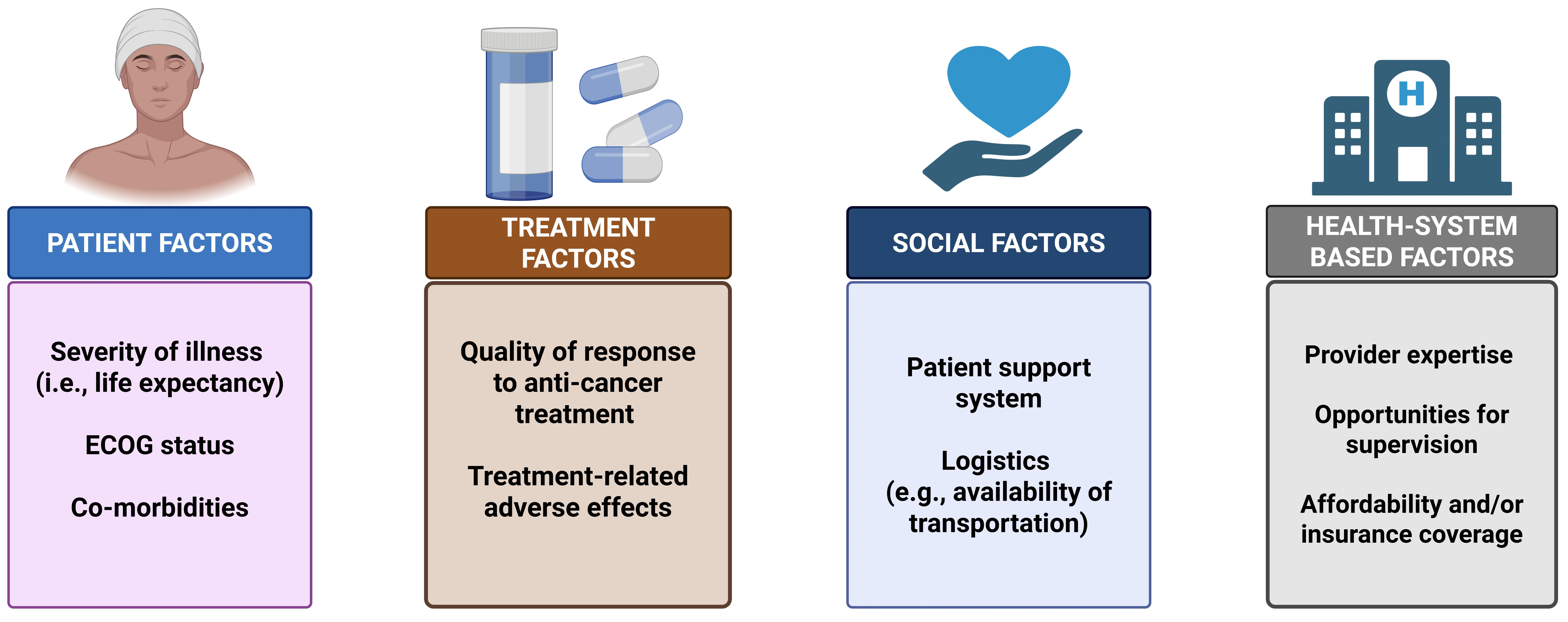
4. Discussion
- Prescribing aerobic and resistance exercises in advanced/metastatic NSCLC patients is generally safe for patients with good functional capacity (PS 0–2), controlled comorbidities, a life expectancy of at least 4 months, and an absence of bone metastases that could precipitate skeletal-related events.
- Any exercise regimen must be closely supervised by professionals, with regular follow-up periods.
- An individualized approach to the regimen, duration, intensity, and goals of exercise should be implemented based on the patient’s capacity and functional status.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 5 March 2025).
- Walsh, E.; Boutros, G.H.; Bastarache, G.; Faust, A.; Comtois, A.S. Feasibility of a Six Week High Intensity Interval Training Program in Patients with Non-Small Cell Lung Cancer: A Randomized Pilot Study. Int. J. Sports Exerc. Med. 2021, 7, 200. [Google Scholar]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023. [Google Scholar]
- Michaels, C. The importance of exercise in lung cancer treatment. Transl. Lung Cancer Res. 2016, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Gravier, F.E.; Smondack, P.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.F.; Baste, J.M.; Cuvelier, A.; Boujibar, F.; Bonnevie, T. Effects of exercise training in people with non-small cell lung cancer before lung resection: A systematic review and meta-analysis. Thorax 2021, 77, 486–496. [Google Scholar] [CrossRef]
- Eisenstein, M. New lung-cancer drugs extend survival times. Nature 2020, 587, S10. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020, 25, 80–87. [Google Scholar] [CrossRef]
- Peddle-McIntyre, C.J.; Singh, F.; Thomas, R.; Newton, R.U.; Galvão, D.A.; Cavalheri, V. Exercise training for advanced lung cancer. Cochrane Database Syst. Rev. 2019, 2, CD012685. [Google Scholar] [CrossRef]
- Health-Related Quality of Life (HRQOL). US Centers for Disease Control and Prevention. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/hrqol/index.htm (accessed on 2 April 2022).
- Fayers, P.; Bottomley, A.E.; EORTC Quality of Life Group. Quality of life research within the EORTC—The EORTC QLQ-C30. Eur. J. Cancer 2002, 38, 125–133. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 2 April 2022).
- Higgins, J.P.T.; Lasserson, T.; Chandler, J.; Tovey, D.; Thomas, J.; Flemyng, E.; Churchill, R. Methodological Expectations of Cochrane Intervention Reviews; Cochrane: London, UK, 2022. [Google Scholar]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Schalock, R.L.; Keith, K.D. Quality of Life Questionnaire (QOL.Q) [Database Record]. PsycTESTS: Worthington, OH, USA, 1993. [Google Scholar]
- Patrick, D.L. Functional Limitations Profile. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Cham, Switzerland, 2014; pp. 2376–2383. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36). Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Moriarty, D.G.; Zack, M.M.; Kobau, R. The Centers for Disease Control and Prevention’s Healthy Days Measures–Population tracking of perceived physical and mental health over time. Health Qual. Life Outcomes 2003, 1, 37. [Google Scholar] [CrossRef]
- Andrade, C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: As simple as it gets. J. Clin. Psychiatry 2020, 81, 11349. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Cao, L.; Li, R.; Wang, X.; Yao, L.; Yan, P.; Li, Y.; Chu, X.; Li, H.; et al. PROTOCOL: Examining the best time of day for exercise: A systematic review and network meta-analysis. Campbell Syst. Rev. 2021, 17, e1144. [Google Scholar] [CrossRef]
- Schunemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October, 2013; The GRADE Working Group, Ed.; The Cochrane Colaboration: Oxford, UK, 2013. [Google Scholar]
- Lin, L.; Shi, L.; Chu, H.; Murad, M.H. The magnitude of small-study effects in the Cochrane Database of Systematic Reviews: An empirical study of nearly 30 000 meta-analyses. BMJ Evid.-Based Med. 2020, 25, 27–32. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Hwang, C.L.; Yu, C.J.; Shih, J.Y.; Yang, P.C.; Wu, Y.T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Kollasch, J.; Vandenberg, J.; Shen, T.; Grothey, A.; Gamble, G.; Basford, J.R. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: A randomized controlled trial. J. Pain Symptom Manag. 2013, 45, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; de Wit, M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef]
- Dhillon, H.M.; Bell, M.L.; van der Ploeg, H.P.; Turner, J.D.; Kabourakis, M.; Spencer, L.; Lewis, C.; Hui, R.; Blinman, P.; Clarke, S.J.; et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann. Oncol. 2017, 28, 1889–1897. [Google Scholar] [CrossRef]
- Vanderbyl, B.L.; Mayer, M.J.; Nash, C.; Tran, A.T.; Windholz, T.; Swanson, T.; Kasymjanova, G.; Jagoe, R.T. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support. Care Cancer 2017, 25, 1749–1758. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Edbrooke, L.; Aranda, S.; Granger, C.L.; McDonald, C.F.; Krishnasamy, M.; Mileshkin, L.; Clark, R.A.; Gordon, I.; Irving, L.; Denehy, L. Multidisciplinary home-based rehabilitation in inoperable lung cancer: A randomised controlled trial. Thorax 2019, 74, 787–796. [Google Scholar] [CrossRef]
- Quist, M.; Langer, S.W.; Lillelund, C.; Winther, L.; Laursen, J.H.; Christensen, K.B.; Rørth, M.; Adamsen, L. Effects of an exercise intervention for patients with advanced inoperable lung cancer undergoing chemotherapy: A randomized clinical trial. Lung Cancer 2020, 145, 76–82. [Google Scholar] [CrossRef]
- Rutkowska, A.; Rutkowski, S.; Wrzeciono, A.; Czech, O.; Szczegielniak, J.; Jastrzębski, D. Short-Term Changes in Quality of Life in Patients with Advanced Lung Cancer during In-Hospital Exercise Training and Chemotherapy Treatment: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 1761. [Google Scholar] [CrossRef]
- Bade, B.C.; Gan, G.; Li, F.; Lu, L.; Tanoue, L.; Silvestri, G.A.; Irwin, M.L. Randomized trial of physical activity on quality of life and lung cancer biomarkers in patients with advanced stage lung cancer: A pilot study. BMC Cancer 2021, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.S.T.; Takemura, N.; Lam, T.C.; Ho, J.C.M.; Deng, W.; Smith, R.; Yan, Y.; Lee, A.W.M.; Lin, C.C. Feasibility of Aerobic Exercise and Tai-Chi Interventions in Advanced Lung Cancer Patients: A Randomized Controlled Trial. Integr. Cancer Ther. 2021, 20, 15347354211033352. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.K.; Lund, C.M.; Vinther, A.; Tolver, A.; Johansen, J.S.; Chen, I.; Ragle, A.M.; Zerahn, B.; Engell-Noerregaard, L.; Larsen, F.O.; et al. Effects of a 12-Week Multimodal Exercise Intervention Among Older Patients with Advanced Cancer: Results from a Randomized Controlled Trial. Oncologist 2022, 27, 67–78. [Google Scholar] [CrossRef]
- Mendizabal-Gallastegui, N.; Arietaleanizbeaskoa, M.S.; Latorre, P.M.; García-Álvarez, A.; Sancho, A.; Iruarrizaga, E.; López-Vivanco, G.; Grandes, G. Nurse-Supervised Exercise for People with Stage IV Cancer: The EFICANCER Randomized Clinical Trial. Semin. Oncol. Nurs. 2023, 39, 151448. [Google Scholar] [CrossRef] [PubMed]
- Sweegers, M.G.; Altenburg, T.M.; Chinapaw, M.J.; Kalter, J.; Verdonck-de Leeuw, I.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Brug, J.; et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of Randomised Controlled Trials. Br. J. Sports Med. 2017, 52, 505–513. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; de Castro, G.; Martyn St-James, M.; Fayers, P.M.; Brown, J.M. Evidence-based guidelines for interpreting change scores for the European Organisation for the research and treatment of cancer quality of Life Questionnaire Core 30. Eur. J. Cancer 2012, 48, 1713–1721. [Google Scholar] [CrossRef]
- Nadler, M.B.; Desnoyers, A.; Langelier, D.M.; Amir, E. The effect of exercise on quality of life, fatigue, physical function, and safety in advanced solid tumor cancers: A meta-analysis of Randomized Control Trials. J. Pain Symptom Manag. 2019, 58, 899–908.e7. [Google Scholar] [CrossRef]
- Trotti, A.; Colevas, A.D.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N.; et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003, 13, 176–181. [Google Scholar] [CrossRef]
- Republic of the Philippines. National Ethical Guidelines for Health and Health-Related Research 2017. Available online: https://www.pchrd.dost.gov.ph/wp-content/uploads/2022/03/Annex-5.-National-Ethical-Guidelines-for-Health-and-Health-Related-Research-2017-1.pdf (accessed on 10 May 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenos, C.L.; Huan-Jacinto, F.Y.D.C.; Lucero, J.A.; Ting, F.I.; Mendoza, M.J.; Amante, M.; Sacdalan, D.B.; Vergara, J.P. Effects of Structured Exercise Programs on Self-Reported Health-Related Quality of Life in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Respir. 2025, 5, 7. https://doi.org/10.3390/jor5020007
Arenos CL, Huan-Jacinto FYDC, Lucero JA, Ting FI, Mendoza MJ, Amante M, Sacdalan DB, Vergara JP. Effects of Structured Exercise Programs on Self-Reported Health-Related Quality of Life in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Respiration. 2025; 5(2):7. https://doi.org/10.3390/jor5020007
Chicago/Turabian StyleArenos, Carl Lawrence, Franessa Ysabel Dianne Chan Huan-Jacinto, Josephine Anne Lucero, Frederic Ivan Ting, Marvin Jonne Mendoza, Madelaine Amante, Danielle Benedict Sacdalan, and John Paulo Vergara. 2025. "Effects of Structured Exercise Programs on Self-Reported Health-Related Quality of Life in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Respiration 5, no. 2: 7. https://doi.org/10.3390/jor5020007
APA StyleArenos, C. L., Huan-Jacinto, F. Y. D. C., Lucero, J. A., Ting, F. I., Mendoza, M. J., Amante, M., Sacdalan, D. B., & Vergara, J. P. (2025). Effects of Structured Exercise Programs on Self-Reported Health-Related Quality of Life in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Respiration, 5(2), 7. https://doi.org/10.3390/jor5020007