The Association Between Asthma and Endometriosis: A Systematic Review and Metanalysis
Abstract
1. Introduction
- (1)
- Host Factors:
- Genetics: A complex inheritance pattern influences asthma expression, IgE production, and response to therapies [5].
- Sex: Epidemiological studies have also shown gender differences in asthma risk [6,7,8,9]. While asthma prevalence is higher in male children compared to females, this trend reverses around the ages of 15 to 19, after which asthma becomes more common among women [2]. Additionally, after puberty, women are observed to have a higher incidence and greater severity of asthma compared to men [10,11,12]. Furthermore, premenopausal women often experience a decline in pulmonary function along with increased asthma exacerbations and hospitalizations during the premenstrual and menstrual phases. Studies suggest that the use of oral contraceptives and hormone replacement therapy can improve pulmonary function and reduce the frequency of asthma exacerbations, providing potential therapeutic benefits during these phases [13].
- (2)
- Environmental Factors [4]
- Allergens: Exposure to specific allergens (e.g., house dust mites) contributes significantly to asthma’s onset and persistence.
- Respiratory Infections: Viral infections in early life, such as RSV, are associated with asthma development, although certain infections may also offer protective effects.
- (3)
2. Materials and Methods
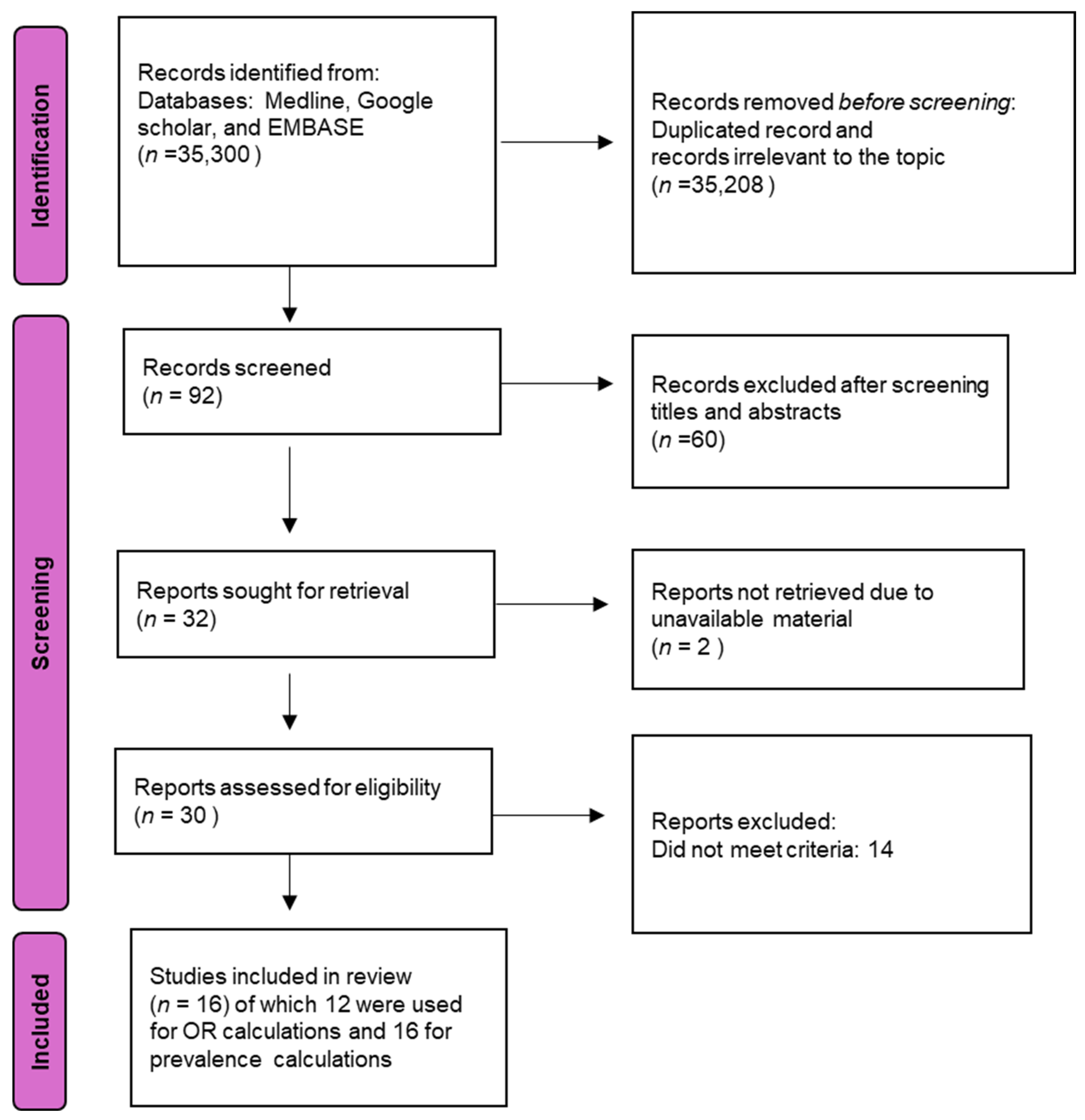
2.1. Data Sources and Search Strategy
2.2. Study Selection and Eligibility
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
Quality Assessment and Publication Bias
3.2. Asthma in Endometriosis Patients
- (a)
- The region of the study (Figure 7):
- America (OR: 2.24 [1.59, 3.16], I2 = 100%, p < 0.00001);
- Europe (OR: 1.27 [0.88, 1.84], I2 = 6%, p = 0.34);
- Asia (OR: 1.46 [1.10, 1.95], I2 = 90%, p = 0.009).
- (b)
- Study design (Figure 8):
- Cross-sectional studies (OR: 1.39 [0.96, 2.02], I2 = 90%, p = 0.09);
- Cohort studies (OR: 2.12 [1.24, 3.64], I2 = 98%, p = 0.006);
- Case–control studies (OR: 1.22 [1.15, 1.30], I2 = 0%, p < 0.00001).
4. Discussion
- (1)
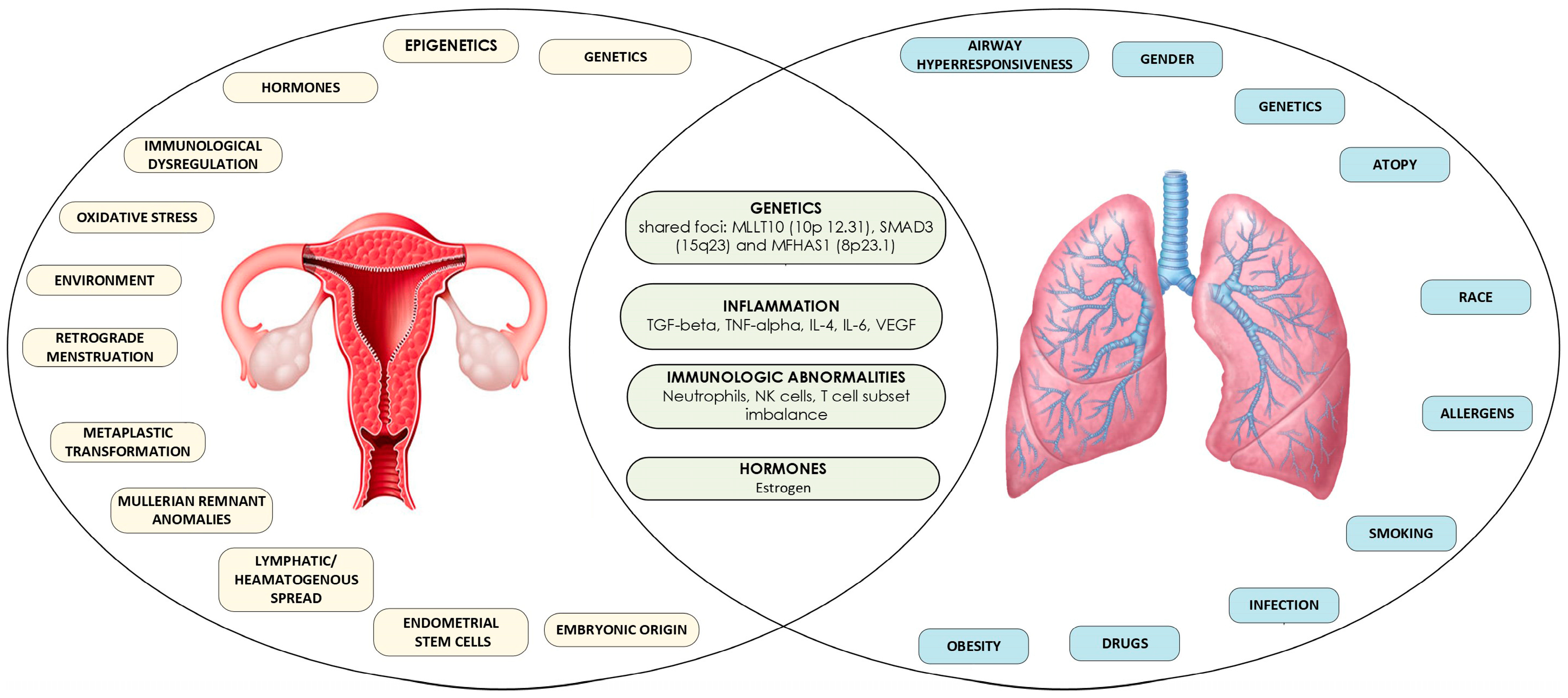
- Genetics: A genome-wide association (GWA) study conducted by Adewuyi et al. on individuals of European descent has identified a significant genetic overlap between endometriosis and asthma. Utilizing SNP-level assessments through SECA and LDSC methods, the study suggests shared genetic predispositions among patients. This finding is further supported by independent gene-based analyses, which highlight genetic connections at the gene level between the two disorders. These results align with some earlier studies indicating co-occurrence, despite conflicting evidence from previous observational research. A meta-analysis of GWAS data from the IEC endometriosis and UK Biobank asthma studies identified 14 genomic loci that reached genome-wide significance for both conditions, with five being potentially novel. Notably, three loci—MLLT10, SMAD3, and MFHAS1—were replicated in an independent asthma GWAS and confirmed through additional analyses. The MFHAS1 gene is expressed in several tissues, including the lungs and endometrium, and is associated with systemic lupus erythematosus [55], while MLLT10, a transcription factor, is expressed in various tissues, including reproductive organs and the lungs [56,57]. These genes are among the most plausible candidates for the association between endometriosis and asthma. However, Mendelian Randomization (MR) analyses did not provide evidence of a causal relationship between the two disorders, suggesting that the observed association may not be causal [15].
- (2)
- Inflammation: Inflammation plays a critical role in both asthma and endometriosis. Key cytokines, such as transforming growth factor-beta (TGF-beta), tumor necrosis factor-alpha (TNF-alpha), interleukin-4 (IL-4), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF), contribute to airway remodeling in asthma [58,59,60,61,62] as well as the proliferation of endometriotic cells [63,64,65,66]. Additionally, elevated TGF-beta activity in the peritoneal fluid of women with endometriosis correlates with disease severity, while TGF-beta produced by Th2 cells is increased in the airways of asthma patients [67]. Chronic inflammation, a hallmark of asthma, is believed to exert systemic effects that may extend beyond the respiratory system, potentially impacting reproductive organs [68,69,70,71].
- (3)
- Hormones (Estrogen): A study by Peng et al. [35] found that estrogen use was higher among asthmatic patients and was associated with a lower risk of endometriosis. This suggests that estrogen may play a dual role in influencing both conditions. Supportive of these findings GWAS studies identified informative biological pathways, including the sex hormone-related pathology shared by these disorders [15]. Furthermore, previous observational studies support the role of sex hormones in the development of both endometriosis and asthma [72,73]. High estrogen levels and frequent exposure to the hormone increase the risks for both disorders [50,74,75,76,77]. Early menarche and frequent menstrual cycles, driven by estrogen, are established risk factors for endometriosis [75], while female sex hormones are believed to influence pulmonary inflammation and smooth muscle function, which can lead to asthma [13,78].
- (4)
- Immunological Factors: The immune system’s failure to eliminate ectopic endometrial cells is a key factor in endometriosis. Endometriotic cells may resist immune surveillance, or there may be deficits in the immune response [18,79]. Immune cells, particularly neutrophils and peritoneal macrophages, are involved in inducing inflammation in endometriosis [26,80,81], while NK cells and macrophages exhibit reduced ability to clear endometrial cells from the peritoneal cavity [26]. Both IL-6 and TGF-b are known to suppress NK cell activity [82,83].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Kiley, J.P.; Gibbons, G.H. Generating Evidence to Inform an Update of Asthma Clinical Practice Guidelines: Perspectives from the National Heart, Lung, and Blood Institute. J. Allergy Clin. Immunol. 2018, 142, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Carson-Chahhoud, K.; Karamzad, N.; Sullman, M.J.M.; Nejadghaderi, S.A.; Taghizadieh, A.; Bell, A.W.; Kolahi, A.-A.; Ansarin, K.; Mansournia, M.A.; et al. Prevalence, Deaths, and Disability-Adjusted Life-Years Due to Asthma and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019. Chest 2022, 161, 318–329. [Google Scholar] [CrossRef] [PubMed]
- CDC Asthma. Most Recent National Asthma Data. 2021. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 31 March 2025).
- Sinyor, B.; Perez, L.C. Pathophysiology of Asthma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ekpruke, C.D.; Silveyra, P. Sex Differences in Airway Remodeling and Inflammation: Clinical and Biological Factors. Front. Allergy 2022, 3, 875295. [Google Scholar] [CrossRef]
- Postma, D.S. Gender Differences in Asthma Development and Progression. Gend. Med. 2007, 4, S133–S146. [Google Scholar] [CrossRef]
- Kynyk, J.A.; Mastronarde, J.G.; McCallister, J.W. Asthma, the Sex Difference. Curr. Opin. Pulm. Med. 2011, 17, 6–11. [Google Scholar] [CrossRef]
- Mccallister, J.W.; Mastronarde, J.G. Sex Differences in Asthma. J. Asthma 2008, 45, 853–861. [Google Scholar] [CrossRef]
- Zein, J.G.; Erzurum, S.C. Asthma Is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef]
- Greenblatt, R.; Mansour, O.; Zhao, E.; Ross, M.; Himes, B.E. Gender-Specific Determinants of Asthma among U.S. Adults. Asthma Res. Pract. 2017, 3, 2. [Google Scholar] [CrossRef]
- Rei, C.; Williams, T.; Feloney, M. Endometriosis in a Man as a Rare Source of Abdominal Pain: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2018, 2018, 2083121. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Ness, R.B.; Kelsey, S.; Waterer, G.W. The Impact of Estrogen and Progesterone on Asthma. Ann. Allergy. Asthma Immunol. 2003, 90, 284–291. [Google Scholar] [CrossRef]
- Louisias, M.; Ramadan, A.; Naja, A.S.; Phipatanakul, W. The Effects of the Environment on Asthma Disease Activity. Immunol. Allergy Clin. N. Am. 2019, 39, 163–175. [Google Scholar] [CrossRef]
- Adewuyi, E.O.; Mehta, D.; International Endogene Consortium (IEC); Sapkota, Y.; Yoshihara, K.; Nyegaard, M.; Steinthorsdottir, V.; Morris, A.P.; Fassbender, A.; Rahmioglu, N.; et al. Genetic Overlap Analysis of Endometriosis and Asthma Identifies Shared Loci Implicating Sex Hormones and Thyroid Signalling Pathways. Hum. Reprod. 2022, 37, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.W.; Cho, H.J.; Kim, J.Y.; Jeong, K.A.; Kim, S.K.; Cho, D.J.; Song, C.H.; Park, K.H. Endometriosis in an Adolescent Population: The Severance Hospital in Korean Experience. Yonsei Med. J. 2002, 43, 48. [Google Scholar] [CrossRef]
- World Health Organization Sixty-Fourth World Health Assembly. Resolution WHA 64.28: Youth and Health Risks. Resolut. WHA 6428 2011, 2, 119–120. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.T.A.A.; Bucu, M.E.M. Clinical Profile and Symptoms of Young Women Aged 10–24 Years Diagnosed with Pelvic Endometriosis: A 5-Year Experience of a Tertiary Hospital in the Philippines. J. Endometr. Uterine Disord. 2024, 8, 100085. [Google Scholar] [CrossRef]
- Prescott, J.; Farland, L.V.; Tobias, D.K.; Gaskins, A.J.; Spiegelman, D.; Chavarro, J.E.; Rich-Edwards, J.W.; Barbieri, R.L.; Missmer, S.A. A Prospective Cohort Study of Endometriosis and Subsequent Risk of Infertility. Hum. Reprod. 2016, 31, 1475–1482. [Google Scholar] [CrossRef]
- Halis, G.; Arici, A. Endometriosis and Inflammation in Infertility. Ann. N. Y. Acad. Sci. 2004, 1034, 300–315. [Google Scholar] [CrossRef]
- Ballard, K.; Seaman, H.; De Vries, C.; Wright, J. Can Symptomatology Help in the Diagnosis of Endometriosis? Findings from a National Case–Control Study—Part 1. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Farquhar, C. Endometriosis. BMJ 2007, 334, 249–253. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde Menstruation in Healthy Women and in Patients with Endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar] [PubMed]
- Lebovic, D.I.; Mueller, M.D.; Taylor, R.N. Immunobiology of Endometriosis. Fertil. Steril. 2001, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of Immune Cells in the Pathogenesis of Endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef]
- De Barros, I.B.L.; Malvezzi, H.; Gueuvoghlanian-Silva, B.Y.; Piccinato, C.A.; Rizzo, L.V.; Podgaec, S. What Do We Know about Regulatory T Cells and Endometriosis? A Systematic Review. J. Reprod. Immunol. 2017, 120, 48–55. [Google Scholar] [CrossRef]
- Barrier, B.F. Immunology of Endometriosis. Clin. Obstet. Gynecol. 2010, 53, 397–402. [Google Scholar] [CrossRef]
- Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Yoshino, O.; Taketani, Y. Lymphocytes in Endometriosis. Am. J. Reprod. Immunol. 2011, 65, 1–10. [Google Scholar] [CrossRef]
- Badawy, S.Z.; Cuenca, V.; Stitzel, A.; Tice, D. Immune Rosettes of T and B Lymphocytes in Infertile Women with Endometriosis. J. Reprod. Med. 1987, 32, 194–197. [Google Scholar]
- Nothnick, W.B. Treating Endometriosis as an Autoimmune Disease. Fertil. Steril. 2001, 76, 223–231. [Google Scholar] [CrossRef]
- Oosterlynck, D.J.; Cornillie, F.J.; Waer, M.; Vandeputte, M.; Koninckx, P.R. Women with Endometriosis Show a Defect in Natural Killer Activity Resulting in a Decreased Cytotoxicity to Autologous Endometrium. Fertil. Steril. 1991, 56, 45–51. [Google Scholar] [CrossRef]
- Vassilopoulou, L.; Matalliotakis, M.; Zervou, M.; Matalliotaki, C.; Krithinakis, K.; Matalliotakis, I.; Spandidos, D.; Goulielmos, G. Defining the Genetic Profile of Endometriosis (Review). Exp. Ther. Med. 2019, 17, 3267–3281. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Su, S.-Y.; Liao, W.-C.; Huang, C.-W.; Hsu, C.Y.; Chen, H.-J.; Wu, T.-N.; Ho, W.-C.; Wu, C.-C. Asthma Is Associated with Endometriosis: A Retrospective Population-Based Cohort Study. Respir. Med. 2017, 132, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Coiplet, E.; Courbiere, B.; Agostini, A.; Boubli, L.; Bretelle, F.; Netter, A. Endometriosis and Environmental Factors: A Critical Review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102418. [Google Scholar] [CrossRef] [PubMed]
- Sinaii, N. High Rates of Autoimmune and Endocrine Disorders, Fibromyalgia, Chronic Fatigue Syndrome and Atopic Diseases among Women with Endometriosis: A Survey Analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Smorgick, N.; Marsh, C.A.; As-Sanie, S.; Smith, Y.R.; Quint, E.H. Prevalence of Pain Syndromes, Mood Conditions, and Asthma in Adolescents and Young Women with Endometriosis. J. Pediatr. Adolesc. Gynecol. 2013, 26, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Petrera, P.; Colombo, B.M.; Navaratnarajah, R.; Parisi, M.; Anserini, P.; Remorgida, V.; Ragni, N. Asthma in Women with Endometriosis. Hum. Reprod. 2005, 20, 3514–3517. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Zubrzycki, M.; Perdas, E.; Zubrzycka, M. Genetic, Epigenetic, and Steroidogenic Modulation Mechanisms in Endometriosis. J. Clin. Med. 2020, 9, 1309. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberatî, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Caserta, D.; Mallozzi, M.; Pulcinelli, F.M.; Mossa, B.; Moscarini, M. Endometriosis Allergic or Autoimmune Disease: Pathogenetic Aspects—A Case Control Study. Clin. Exp. Obstet. Gynecol. 2016, 43, 354–357. [Google Scholar] [CrossRef]
- Al-Jefout, M.; Nesheiwat, A.; Odainat, B.; Sami, R.; Alnawaiseh, N. Questionnaire-Based Prevalence of Endometriosis and Its Symptoms in Jordanian Women. Biomed. Pharmacol. J. 2017, 10, 699–706. [Google Scholar] [CrossRef]
- Alqaisi, S.; Yaqoob, Z.; Fadhil, S.; Al-Kindi, S.; Zein, J.G. Endometriosis Is Associated with Increased Risk of Asthma in Reproductive Age Woman. In C37. Optimizing Asthma Care Across Diverse Patients; American Journal of Respiratory and Critical Care Medicine: San Diego, CA, USA, 2018; Volume 197, p. A4840. [Google Scholar]
- Shafrir, A.L.; Palmor, M.C.; Fourquet, J.; DiVasta, A.D.; Farland, L.V.; Vitonis, A.F.; Harris, H.R.; Laufer, M.R.; Cramer, D.W.; Terry, K.L.; et al. Co-Occurrence of Immune-Mediated Conditions and Endometriosis among Adolescents and Adult Women. Am. J. Reprod. Immunol. 2021, 86, e13404. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, E.; Yamana, H.; Ono, S.; Matsui, H.; Yasunaga, H. Association between Allergic or Autoimmune Diseases and Incidence of Endometriosis: A Nested Case-control Study Using a Health Insurance Claims Database. Am. J. Reprod. Immunol. 2021, 86, e13486. [Google Scholar] [CrossRef]
- Jöud, A.; Nilsson-Condori, E.; Schmidt, L.; Ziebe, S.; Vassard, D.; Mattsson, K. Infertility, Pregnancy Loss and Assisted Reproduction in Women with Asthma: A Population-Based Cohort Study. Hum. Reprod. 2022, 37, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, P.; Li, S.; Cao, L.; Yang, C. Association of Endometriosis with Asthma: A Study of the NHANES Database in 1999–2006. J. Health Popul. Nutr. 2024, 43, 50. [Google Scholar] [CrossRef]
- Imbroane, M.R.; Kim, H.; Falcone, T.; Richards, E.G. The Association Between Asthma and Endometriosis in the United States: A Retrospective Cohort Study. Int. J. Clin. Stud. Med. Case Rep. 2024, 42, 1–3. [Google Scholar] [CrossRef]
- Matalliotakis, I.; Cakmak, H.; Matalliotakis, M.; Kappou, D.; Arici, A. High Rate of Allergies among Women with Endometriosis. J. Obstet. Gynaecol. 2012, 32, 291–293. [Google Scholar] [CrossRef]
- Nowakowska, A.; Kwas, K.; Fornalczyk, A.; Wilczyński, J.; Szubert, M. Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits. Medicina 2022, 58, 1038. [Google Scholar] [CrossRef]
- Matalliotakis, M.; Goulielmos, G.N.; Matalliotaki, C.; Trivli, A.; Matalliotakis, I.; Arici, A. Endometriosis in Adolescent and Young Girls: Report on a Series of 55 Cases. J. Pediatr. Adolesc. Gynecol. 2017, 30, 568–570. [Google Scholar] [CrossRef]
- Lötvall, J.; Ekerljung, L.; Rönmark, E.P.; Wennergren, G.; Lindén, A.; Rönmark, E.; Torén, K.; Lundbäck, B. West Sweden Asthma Study: Prevalence Trends over the Last 18 Years Argues No Recent Increase in Asthma. Respir. Res. 2009, 10, 94. [Google Scholar] [CrossRef]
- Loftus, P.A.; Wise, S.K. Epidemiology and Economic Burden of Asthma. Int. Forum Allergy Rhinol. 2015, 5, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Zhang, Y.; Zhu, Z.; Wang, T.-Y.; Morris, D.L.; Shen, J.J.; Zhang, H.; Pan, H.-F.; Yang, J.; Yang, S.; et al. Identification of ST3AGL4, MFHAS1, CSNK2A2 and CD226 as Loci Associated with Systemic Lupus Erythematosus (SLE) and Evaluation of SLE Genetics in Drug Repositioning. Ann. Rheum. Dis. 2018, 77, 1078–1084. [Google Scholar] [CrossRef]
- Linder, B.; Newman, R.; Jones, L.K.; Debernardi, S.; Young, B.D.; Freemont, P.; Verrijzer, C.P.; Saha, V. Biochemical Analyses of the AF10 Protein: The Extended LAP/PHD-Finger Mediates Oligomerisation 1 1Edited by T. Richmond. J. Mol. Biol. 2000, 299, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, H.; Yamagata, K.; Nakao, T.; Sandell, L.L.; Yamamoto, A.; Yamashita, A.; Tanga, N.; Suzuki, M.; Abe, T.; Kitabayashi, I.; et al. Mllt10 Knockout Mouse Model Reveals Critical Role of Af10-Dependent H3K79 Methylation in Midfacial Development. Sci. Rep. 2017, 7, 11922. [Google Scholar] [CrossRef]
- Halwani, R.; Al-Muhsen, S.; Al-Jahdali, H.; Hamid, Q. Role of Transforming Growth Factor–β in Airway Remodeling in Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 127–133. [Google Scholar] [CrossRef]
- Berry, M.; Brightling, C.; Pavord, I.; Wardlaw, A. TNF-α in Asthma. Curr. Opin. Pharmacol. 2007, 7, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Joos, G.F.; Brusselle, G.G. Targeting Interleukin-4 in Asthma: Lost in Translation? Am. J. Respir. Cell Mol. Biol. 2012, 47, 261–270. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Meyer, N.; Akdis, C.A. Vascular Endothelial Growth Factor as a Key Inducer of Angiogenesis in the Asthmatic Airways. Curr. Allergy Asthma Rep. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Young, V.J.; Ahmad, S.F.; Brown, J.K.; Duncan, W.C.; Horne, A.W. Peritoneal VEGF-A Expression Is Regulated by TGF-Β1 through an ID1 Pathway in Women with Endometriosis. Sci. Rep. 2015, 5, 16859. [Google Scholar] [CrossRef]
- OuYang, Z.; Hirota, Y.; Osuga, Y.; Hamasaki, K.; Hasegawa, A.; Tajima, T.; Hirata, T.; Koga, K.; Yoshino, O.; Harada, M.; et al. Interleukin-4 Stimulates Proliferation of Endometriotic Stromal Cells. Am. J. Pathol. 2008, 173, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.; Garrido, N.; Coperias, J.L.; Pardo, F.; Desco, J.; García-Velasco, J.A.; Simón, C.; Pellicer, A. Serum Interleukin-6 Levels Are Elevated in Women with Minimal–Mild Endometriosis. Hum. Reprod. 2007, 22, 836–842. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J. Vascular Endothelial Growth Factor and Endometriotic Angiogenesis. Hum. Reprod. Update 2000, 6, 45–55. [Google Scholar] [CrossRef]
- Schmidt-Weber, C.B.; Blaser, K. The Role of TGF-β in Allergic Inflammation. Immunol. Allergy Clin. N. Am. 2006, 26, 233–244. [Google Scholar] [CrossRef]
- Juul Gade, E.; Thomsen, S.F.; Lindenberg, S.; Backer, V. Female Asthma Has a Negative Effect on Fertility: What Is the Connection? ISRN Allergy 2014, 2014, 131092. [Google Scholar] [CrossRef]
- Denburg, J.A.; Sehmi, R.; Saito, H.; Pil-Seob, J.; Inman, M.D.; O’Byrne, P.M. Systemic Aspects of Allergic Disease: Bone Marrow Responses. J. Allergy Clin. Immunol. 2000, 106, S242–S246. [Google Scholar] [CrossRef]
- Bláfoss, J.; Hansen, A.V.; Malchau Lauesgaard, S.S.; Ali, Z.; Ulrik, C.S. Female Asthma and Atopy—Impact on Fertility: A Systematic Review. J. Asthma Allergy 2019, 12, 205–211. [Google Scholar] [CrossRef]
- Wasilewska, E.; Małgorzewicz, S. Impact of Allergic Diseases on Fertility. Adv. Dermatol. Allergol. 2019, 36, 507–512. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of Endometriosis and Its Comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- De Boer, W.I. Perspectives for Cytokine Antagonist Therapy in COPD. Drug Discov. Today 2005, 10, 93–106. [Google Scholar] [CrossRef]
- Missmer, S.A. Incidence of Laparoscopically Confirmed Endometriosis by Demographic, Anthropometric, and Lifestyle Factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Keselman, A.; Heller, N. Estrogen Signaling Modulates Allergic Inflammation and Contributes to Sex Differences in Asthma. Front. Immunol. 2015, 6, 568. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- McCleary, N.; Nwaru, B.I.; Nurmatov, U.B.; Critchley, H.; Sheikh, A. Endogenous and Exogenous Sex Steroid Hormones in Asthma and Allergy in Females: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2018, 141, 1510–1513.e8. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Women Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Milewski, Ł.; Dziunycz, P.; Barcz, E.; Radomski, D.; Roszkowski, P.I.; Korczak-Kowalska, G.; Kamiński, P.; Malejczyk, J. Increased Levels of Human Neutrophil Peptides 1, 2, and 3 in Peritoneal Fluid of Patients with Endometriosis: Association with Neutrophils, T Cells and IL-8. J. Reprod. Immunol. 2011, 91, 64–70. [Google Scholar] [CrossRef]
- Guo, S.-W.; Du, Y.; Liu, X. Platelet-Derived TGF-Β1 Mediates the down-Modulation of NKG2D Expression and May Be Responsible for Impaired Natural Killer (NK) Cytotoxicity in Women with Endometriosis. Hum. Reprod. 2016, 31, 1462–1474. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jeung, I.C.; Park, A.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. An Increased Level of IL-6 Suppresses NK Cell Activity in Peritoneal Fluid of Patients with Endometriosis via Regulation of SHP-2 Expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef]
- Podgaec, S.; Abrao, M.S.; Dias, J.A.; Rizzo, L.V.; De Oliveira, R.M.; Baracat, E.C. Endometriosis: An Inflammatory Disease with a Th2 Immune Response Component. Hum. Reprod. 2007, 22, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Olkowska-Truchanowicz, J.; Bocian, K.; Maksym, R.B.; Bialoszewska, A.; Wlodarczyk, D.; Baranowski, W.; Zabek, J.; Korczak-Kowalska, G.; Malejczyk, J. CD4+ CD25+ FOXP3+ Regulatory T Cells in Peripheral Blood and Peritoneal Fluid of Patients with Endometriosis. Hum. Reprod. 2013, 28, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D. Epigenetic Regulation and T-Cell Responses in Endometriosis—Something Other than Autoimmunity. Front. Immunol. 2022, 13, 943839. [Google Scholar] [CrossRef]
- Pasoto, S.G.; Abrao, M.S.; Viana, V.S.T.; Bueno, C.; Leon, E.P.; Bonfa, E. Endometriosis and Systemic Lupus Erythematosus: A Comparative Evaluation of Clinical Manifestations and Serological Autoimmune Phenomena. Am. J. Reprod. Immunol. 2005, 53, 85–93. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hessel, E.M. Functions of T Cells in Asthma: More than Just TH2 Cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]






| Year | Study Source | Age | Study | Study Design | Region | Asthma/ Endo Cases | Total | Diagnosis Criteria Asthma/Endo | Quality Score * |
|---|---|---|---|---|---|---|---|---|---|
| 2002 | Endometriosis Association (1998) | All | Sinaii N [37] | Cross-sectional | America (USA, Canada) | 442/ 3680 | 3680 | Self-reported/Laparoscopy | NA |
| 2005 | San Martino Hospital (2001–2004) | ~28–40 | Ferrero S [39] | Cross-sectional | Europe (Italy) | 45/ 467 | 879 | ATS criteria/Histology | 8 |
| 2012 | Yale University School of Medicine (1996–2002) | ~28–41 | Matalliotakis, H [50] | Retrospective Cohort | America (USA) | 53/ 501 | 689 | Medical records and interview/American Society for Reproductive Medicine | 7 |
| 2013 | University of Michigan Medical Center (2001–2011) | ≤21 | Smorgick N [38] | Retrospective cohort | America (USA) | 31/ 138 | 138 | Records/IC-9 617.1 to 617.5 and 617.8–617.9 | NA |
| 2016 | Andrea Hospital (2009–2013) | 19–53 | Caserta D [42] | Case–Control | Europe (Italy) | 8/ 304 | 622 | Medical Records/Laparoscopy–Histology | 5 |
| 2017 | LHID 2000 database (1996–2013) | 12–50 | Peng Y-H [35] | Retrospective Cohort | Asia (Taiwan) | 7337/ 1297 | 36,685 | ICD-9-CM 493.xx and treatment/CD-9-CM 617.xx | 9 |
| 2017 | Self-administered questionnaire (2015) | 15 to 55 | Al-Jefout M [43] | Cross-sectional | Asia (Jordan) | 42/ 45 | 1772 | Questionnaire/Laparoscopy or Laparotomy with histology report | 7 |
| 2017 | Yale University Hospital, University of Crete and Venizeleio General Hospital of Heraklion (1996–2016) | 13–21 | Matalliotakis M [52] | Retrospective cohort | America (USA) and Greece | 267/ 900 | 900 | Medical Records/Medical records | NA |
| 2018 | Explorys® database (2012–2017) | 20–40 | Alqaisi S [44] | Cross-sectional | America (USA) | 437,470/ 64,150 | 327,202 | Medical records/Medical records | 6 |
| 2021 | Woman Health Study (2012–2018) | 7–55 | Shafir AL [45] | Cross-sectional | America (USA) | 267/ 551 | 1203 | Questionnaire/Surgical diagnosis | 8 |
| 2021 | JMDC Claims Database (2011–2018) | 16–40 | Yoshii E [46] | Case–Control | Asia (Japan) | 5801/ 30,516 | 151,492 | ICD-10 codes: J45.0,J45.8,J46/N80 | 8 |
| 2022 | Skåne Healthcare Register (1998–2019) | 15–45 | Joud A [47] | Retrospective Cohort | Europe (Sweden) | 6445/ 413 | 206,693 | ICD-10 codes: J45- j46 (all subcategories) plus health visits/N80 | 9 |
| 2022 | Internet-based | ≥18 | Nowakoska A [51] | Cross-sectional | Open | 43/ 364 | 501 | Questionnaire/Mixed (included 49.6% laparoscopy or laparotomy) | 2 |
| 2024 | NHANES (1999–2006) | ≥20 | Pan G [48] | Cross-sectional | America (USA) | 782/ 380 | 5556 | Questionnaire: RHQ_D/MCQ | 6 |
| 2024 | TriNetX: US collaborative network (2023) | 12 to 50 | Imbroane MR [49] | Retrospective Cohort | America (USA) | 1,581,644/ 196,857 | 24,647,797 | ICD-10 J45/ICD-10 N80 | 7 |
| 2024 | Pediatric Surgery | 10–24 | Cialuj TA [19] | Retrospective Cohort | Asia (Philippines) | 5/ 50 | 50 | Medical records/Medical records | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Nino, M.E.; Obadiah, A.A.; Ozugha, I.O.; Ramdass, P.V.A.K. The Association Between Asthma and Endometriosis: A Systematic Review and Metanalysis. J. Respir. 2025, 5, 6. https://doi.org/10.3390/jor5020006
Ramos-Nino ME, Obadiah AA, Ozugha IO, Ramdass PVAK. The Association Between Asthma and Endometriosis: A Systematic Review and Metanalysis. Journal of Respiration. 2025; 5(2):6. https://doi.org/10.3390/jor5020006
Chicago/Turabian StyleRamos-Nino, Maria E., Abraham Agaya Obadiah, Ifesinachi Ogochukwu Ozugha, and Prakash V. A. K. Ramdass. 2025. "The Association Between Asthma and Endometriosis: A Systematic Review and Metanalysis" Journal of Respiration 5, no. 2: 6. https://doi.org/10.3390/jor5020006
APA StyleRamos-Nino, M. E., Obadiah, A. A., Ozugha, I. O., & Ramdass, P. V. A. K. (2025). The Association Between Asthma and Endometriosis: A Systematic Review and Metanalysis. Journal of Respiration, 5(2), 6. https://doi.org/10.3390/jor5020006







