1. Introduction
Multiple myeloma (MM) is a B-cell malignancy that is diagnosed based on the presence of clonal bone marrow plasma cells exceeding 10% or confirmed plasmacytoma through biopsy accompanied by one or more myeloma defining events (MDE), including anemia, renal insufficiency, hypercalcemia, and lytic bone lesions by advanced imaging. This along with biomarkers of high potential for progression to symptomatic disease such as bone marrow plasma cells ≥60% and involved to uninvolved serum free light chains ≥100 [
1] confirms the diagnosis. Extramedullary disease (EMD) occurs when myeloma cells survive outside the bone marrow microenvironment, often attributed to hematogenous spread. While rare, EMD typically indicates an aggressive form of MM with an unfavorable prognosis [
2]. This also correlates with features of EMD that are associated with adverse prognostic factors such as high lactate dehydrogenase (LDH) level, 17p deletion, and a high-risk gene expression profile. The incidence of EMD is notably higher in relapsed refractory MM (R/R MM) compared to newly diagnosed MM patients, ranging from 3.4% to 14%, as opposed to 0.5% to 4.8% in the latter. EMD involvement includes skin, muscles, pleura, pericardium, lungs, and the central nervous system [
2]. In addition, EMD involvement at time of relapse has an overall survival of less than 6 months [
3].
EMD with pulmonary and pleural involvement due to malignant infiltration and extranodal growth in MM patients is a very rare phenomenon that can manifest in various ways. This includes pulmonary plasmacytoma presenting as a lung mass, diffuse parenchymal infiltrates mimicking multilobar pneumonia, pleural mass, or malignant/myelomatous pleural effusion (MPE). Evaluation of these conditions can be carried out using various modalities, such as thoracentesis, pleural biopsy, bronchoalveolar lavage, and transbronchial biopsy [
4].
In this case series, we present two patients with extramedullary pulmonary disease in the context of R/R MM, who were treated with several lines of therapies. One patient exhibited myelomatous pleural effusion (MPE) with tension hydrothorax as a relapse feature. The other patient presented with aggressive pulmonary and pleural plasmacytoma. These cases clearly display two rare forms of EMD and also emphasize the aggressiveness of this disease. Additionally, we provide a brief review of the pulmonary complications associated with multiple myeloma and discuss available therapeutic options and future directions for these rare forms of multiple myeloma.
2. Case One
A male in his late 60s with a history of R/R MM, stage III by the Durie–Salmon system, stage II by the revised international staging system (R-ISS), and a light chain disease with Kappa free light chain (FLC). He presented to the emergency department (ED) due to a worsening dyspnea for the past 3 days, accompanied by a dry cough.
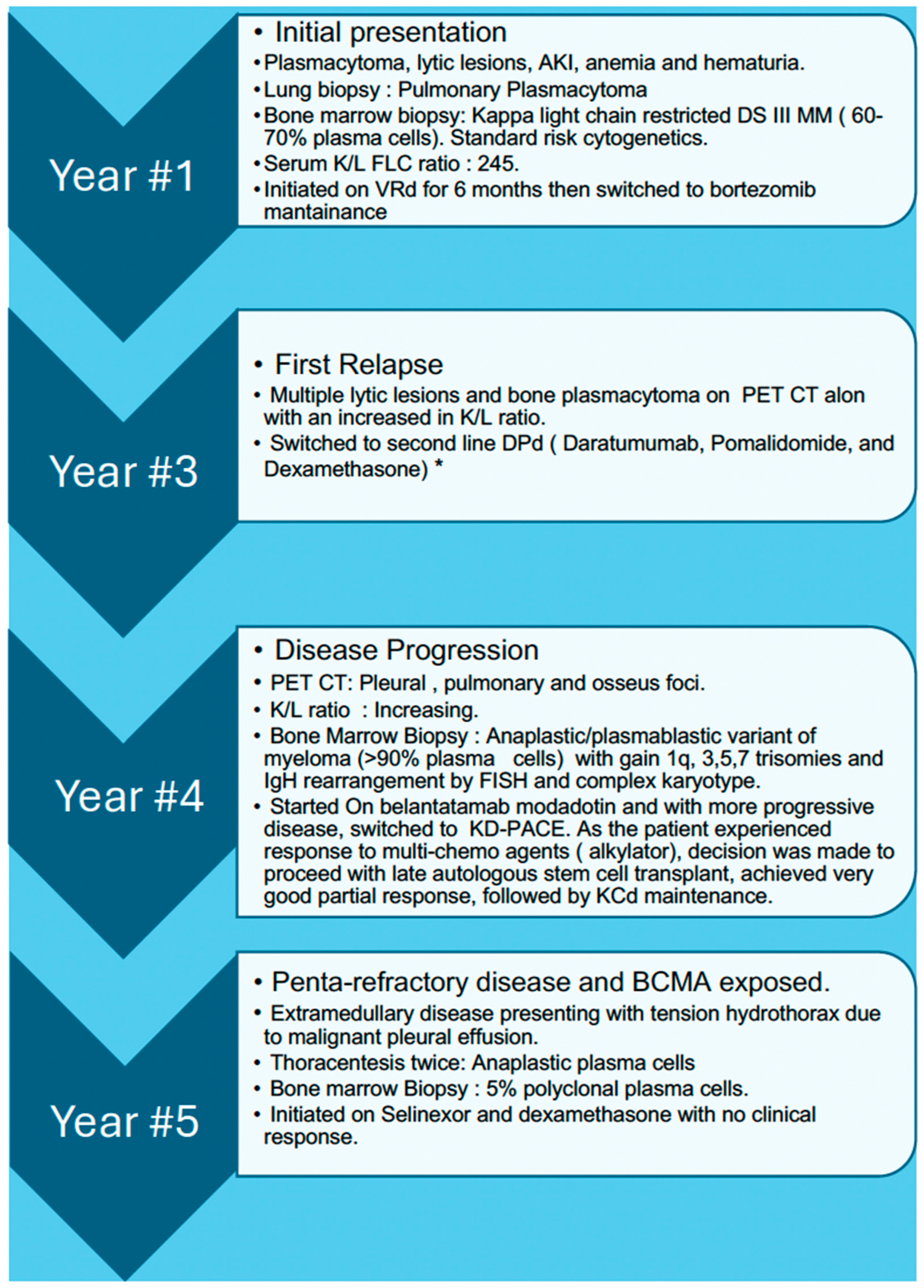
His MM course began with an initial diagnosis five years prior to this admission. At that time, he presented with painless hematuria, multiple lytic lesions, anemia, acute kidney injury, and a right-sided pleural-based lung mass, which was confirmed as pulmonary plasmacytoma through lung biopsy. Subsequent bone marrow biopsy revealed 60–70% kappa-restricted plasma cells confirming the diagnosis of MM. Fluorescence In Situ Hybridization (FISH) showed normal cytogenetics. Despite multiple lines of therapies, including an autologous stem cell transplant (ASCT), the patient had disease progression and transformation to the anaplastic/plasmablastic variant with high-risk features (gain of 1q, trisomy, and IgH rearrangement). A detailed course of the disease is provided in
Figure 1.
Upon this current presentation to the ED with dyspnea, he was afebrile, had a heart rate of 128 beats per minute, a respiratory rate of 40 breaths per minute, blood pressure of 130/79 mmHg, and an oxygen saturation of 95% on 3 L nasal cannula. Pertinent laboratory findings included a serum creatinine of 1.5 mg/dL (near baseline), serum bicarbonate of 19 mmol/L, bilirubin of 2.0 mg/dL with predominantly indirect bilirubinemia, alkaline phosphatase of 290 units/L with a predominant hepatic subtype, and relatively normal liver-associated enzymes. LDH was 810 units (compared to 590 a week prior). A complete blood count showed a hemoglobin of 11.9 g/dL, and the white blood cell count and platelets were within reference ranges, with no circulating plasma cells. Electrocardiogram (EKG) and serum troponins × 2 were normal.
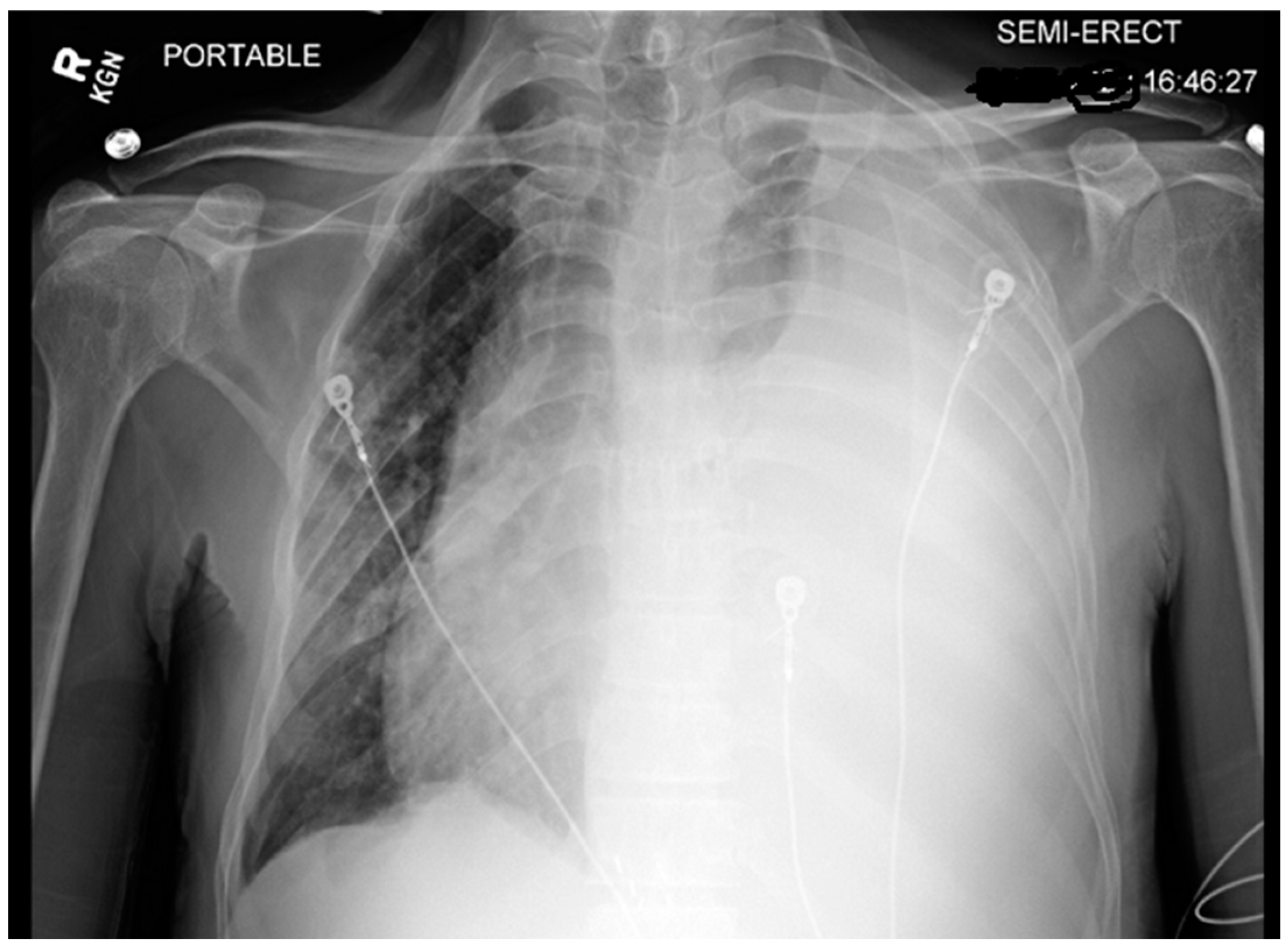
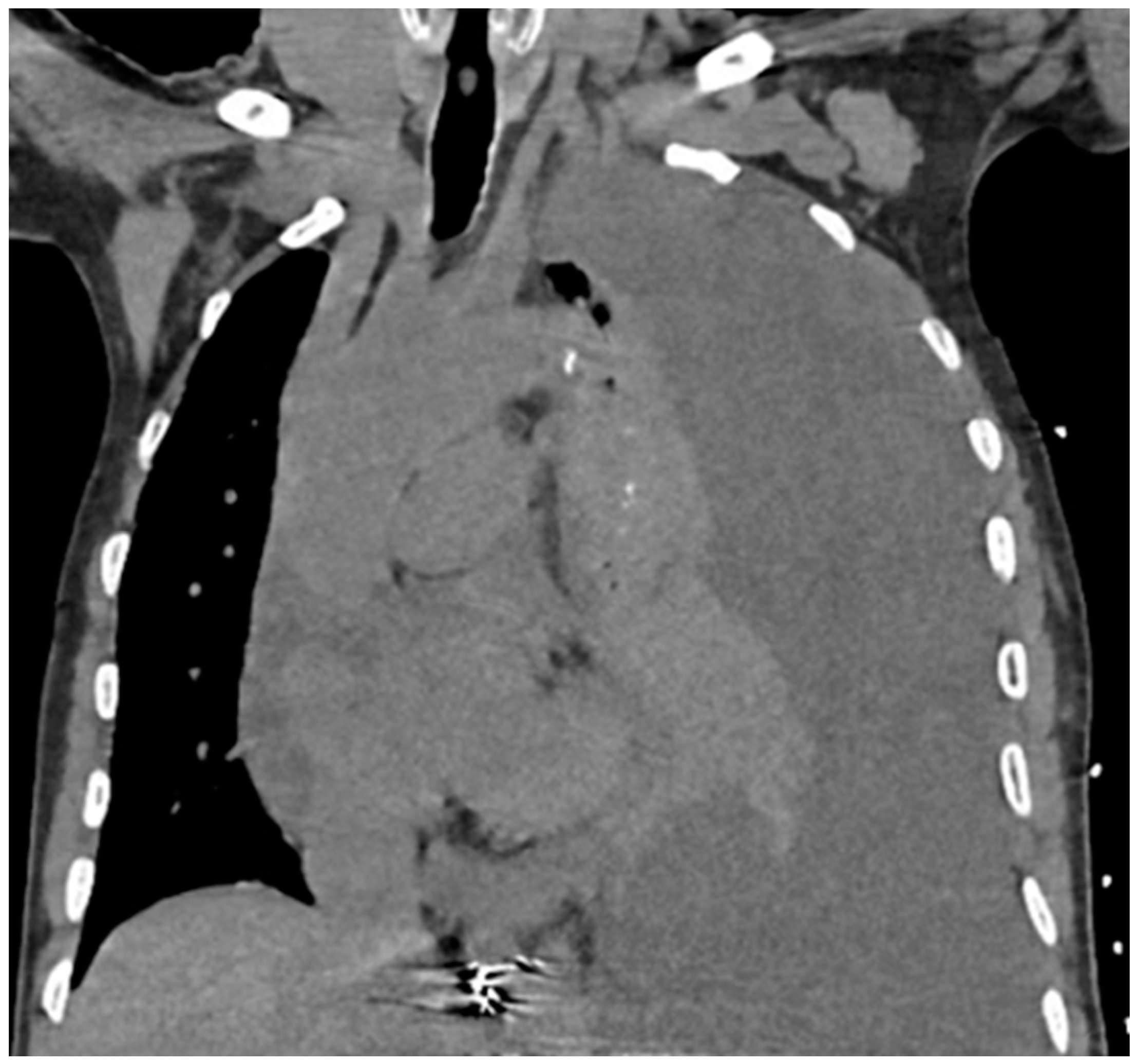
Chest X-ray (CXR) and computed tomography (CT) chest revealed a massive left-sided pleural effusion with mediastinal shift, concerning for tension hydrothorax as depicted in
Figure 2 and
Figure 3. Additionally, the CT scan revealed pleural nodularity with calcification on the left side without surrounding osseous involvement.
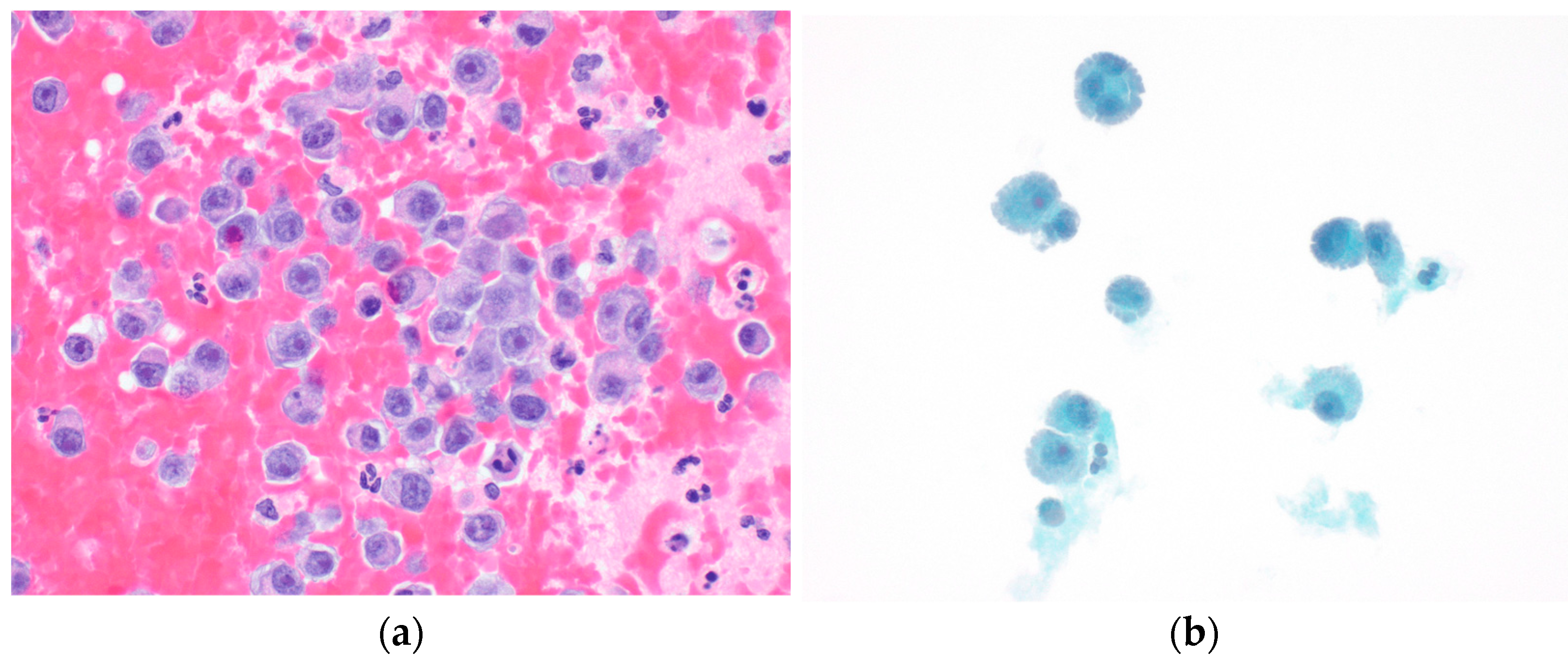
Thoracocentesis revealed a serosanguinous exudative pleural effusion with red blood cell counts of 352,000, a pH of 7.18, and elevated LDH and protein levels. The Gram stain and organism were negative. A total of 3400 cc of pleural fluid was drained, resulting in symptomatic improvement. Pleural fluid cytology showed anaplastic plasma cells consistent with extramedullary myeloma with myelomatous disease (
Figure 4).
A non-tunneled pleural catheter was inserted and alteplase was administered to facilitate removal of the loculations. Interestingly, bone marrow biopsy showed only 5% polyclonal plasma cells without evidence of multiple myeloma suggesting isolated extramedullary disease. The patient had penta-refractory disease that did not exhibit any response to B-cell maturation antigen (BCMA) antibody drug conjugate when it was available in the U.S market. He opted for further therapy, for which he was offered selinexor and dexamethasone as a subsequent line of therapy. Upon discharge, the patient experienced significant symptomatic improvement, and a follow-up CXR revealed a small but persistent pleural effusion.
After three weeks, the patient returned to the ED with worsening fatigue. Oxygen saturation was 96% on room air, and a small, unchanged left-sided effusion was still present. Laboratory results indicated new onset pancytopenia, and the kappa to lambda ratio (K/L ratio) suggested disease progression (increased to 161 from 49 previously). Additionally, the patient’s prolonged hospitalization was complicated by hypoxemic respiratory failure unresponsive to antibiotics. This was thought to be due to multidrug-resistant pneumonia versus possible diffuse parenchymal infiltration by the plasma cells. The patient had an overall worsening status, which led the patient and family to pursue comfort care.
3. Case Two
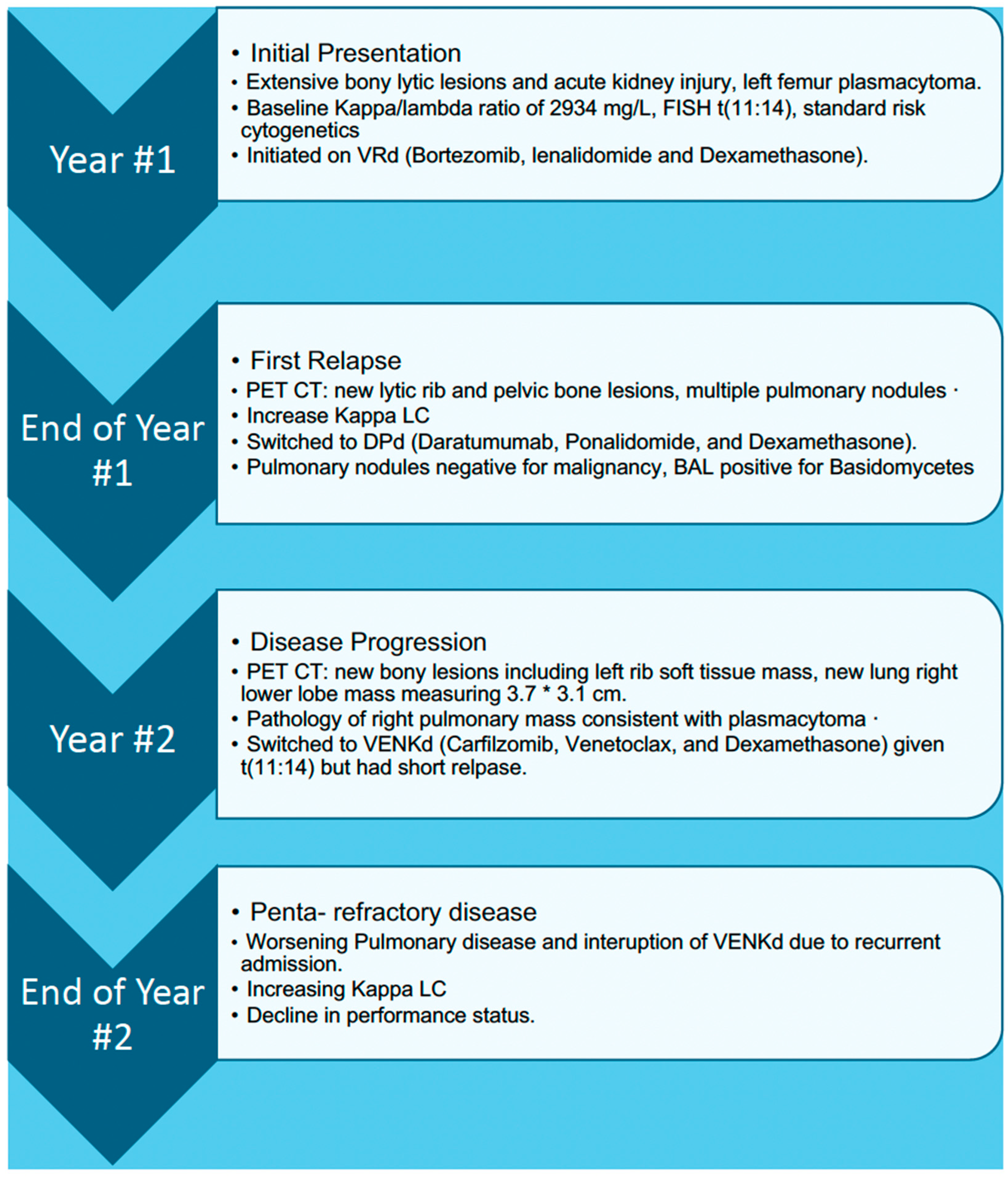
A male in his mid-60s with a past medical history of stage III Durie–Salmon, stage II R-ISS, Kappa FLC MM presented to the ED with worsening dyspnea, lower extremity edema, and generalized weakness for over three weeks.
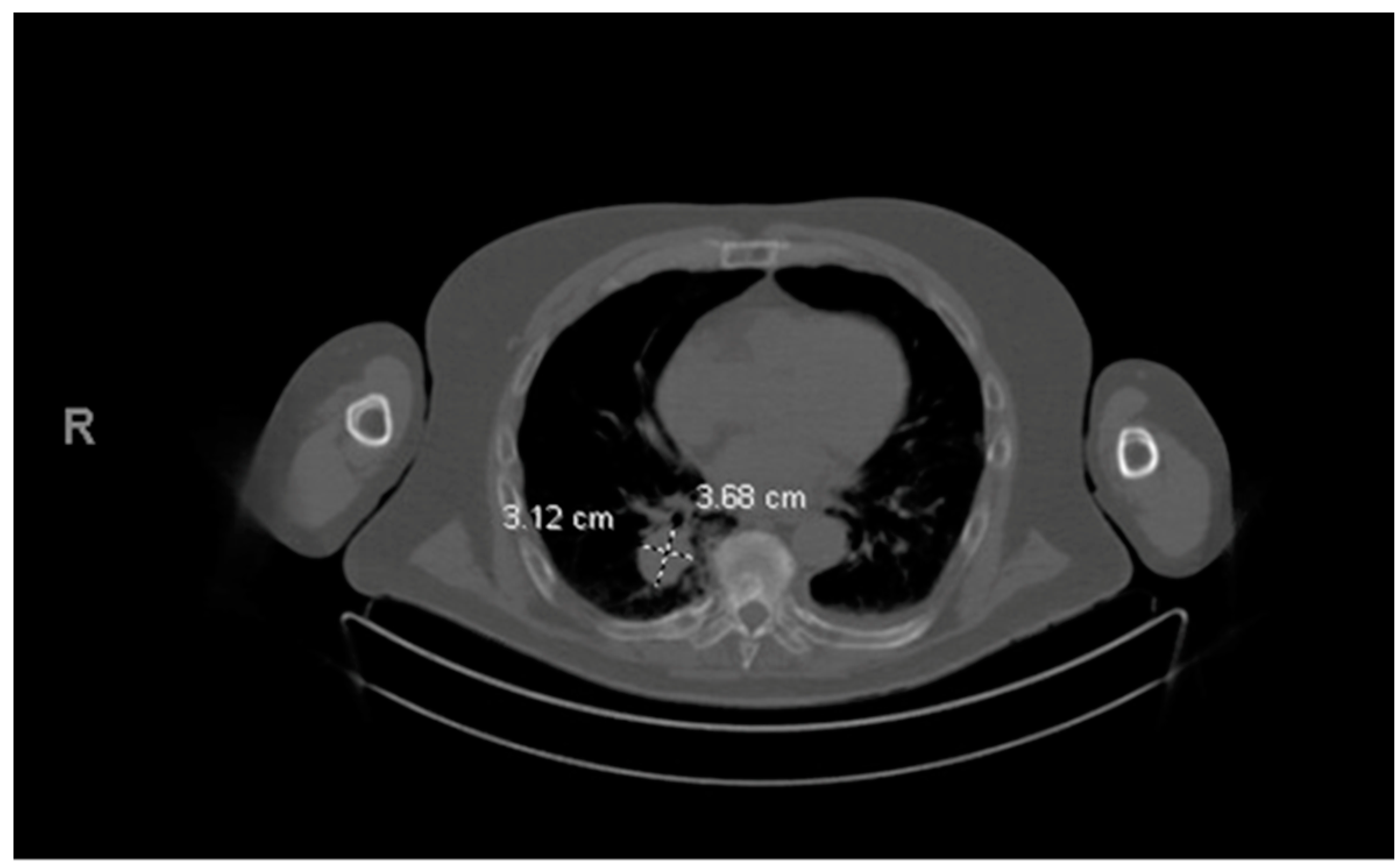
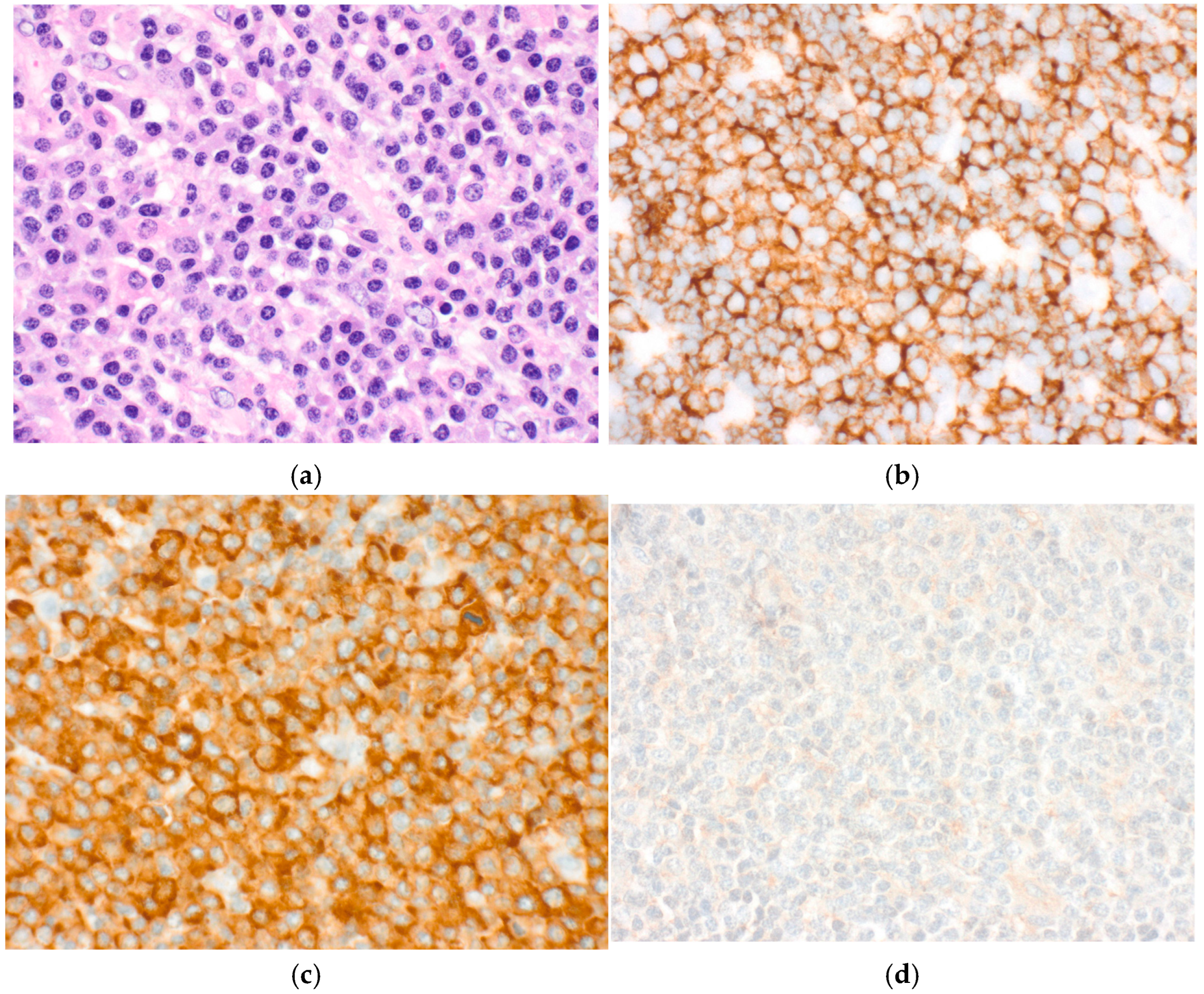
His MM course began with an initial diagnosis of MM one year before admission. At that time, he presented with back pain and was found to have multiple bony lesions. Bone marrow biopsy revealed 52% clonal plasma cells and femur biopsy confirmed bone plasmacytoma finalizing his diagnosis of multiple myeloma. FISH showed t (11:14) rearrangement indicating standard risk cytogenetics. The patient initially responded well to treatment, but unfortunately his disease progressed quickly so he was unable to undergo ASCT. Prior to his hospital stay, a year following the initial diagnosis, a routine positron emission tomography (PET) scan revealed a right lower lobe pulmonary mass, later proven to be a pulmonary (extramedullary) plasmacytoma (
Figure 5 and
Figure 6). A detailed course of the disease is provided in
Figure 7.
Upon this hospitalization for dyspnea and edema, the patient was afebrile with heart rate of 112 beats per minute, blood pressure of 134/80 mmHg, and oxygen saturation of greater than 92% on room air. On physical exam, the patient appeared frail, jaundiced, and had coarse breath sounds bilaterally. Laboratory findings were significant for brain natriuretic peptide (BNP) of 366 pg/mL, serum creatinine of 0.7 mg/dL, hemoglobin of 6.7 g/dL, and serum kappa FLC of 508 mg/L (from 356 mg/L a month prior). Infectious workup, including blood cultures and white blood cell counts, was unremarkable. Cardiac workup, including transthoracic echocardiogram and EKG, was normal. The patient was treated with intravenous diuretics leading to improvement in symptoms and was discharged on oral diuretics.
After hospital discharge, the patient followed up with the oncology clinic and was noted to have a borderline performance status requiring a walker for activities of daily living (ADLs). Repeat kappa FLC had increased to 855.57 mg/L. Cycle three of his current treatment VenKd (Venetoclax, carfilzomib and dexamethasone) was resumed.
Approximately two weeks later, the patient presented back to the hospital and was admitted for shortness of breath and vomiting which had been ongoing for seven days. Vital signs were stable. He endorsed weakness and new onset chest pain, which worsened with exertion and was relieved with aspirin. Cardiac workup, including troponins, echocardiogram, and pharmacologic stress test, was unremarkable. On physical exam, lower extremities were notable for bilateral edema despite compliance with daily oral furosemide. Pertinent labs were BNP 948 pg/mL, Lactate 2.8 mmol/L, and LDH 256 μ/L. CT angiography of the chest showed bilateral pleural effusions and increased size of left chest wall mass and new scapular and splenic lesions not seen on prior scans concerning for progression. The patient was treated with intravenous furosemide. However, his course was complicated by rapid decompensation of symptoms including chest pain and dyspnea due to disease progression. The patient had physical deconditioning and worsening lethargy and ultimately decided to pursue home hospice.
4. Discussion
This case series aims to highlight pleural and pulmonary involvement and the associated poor prognosis, especially in the context of R/R MM. EMD is believed to occur when myeloma cells escape the bone marrow and hematogenously spread to distant organs. The suggested underlying mechanism involves a decrease in the expression and function of molecules responsible for the adhesion and homing of myeloma cells in the bone marrow environment [
5]. EMD is linked to high-risk genetics and is characterized by a shorter progression-free survival time (PFS) and overall survival (OS) time. In a study by Song et al., PFS and OS were reported as 21.8 months and 44 months respectively, for patients with EMD, compared to 38.1 and 61.4 months respectively, for those without EMD [
6]. The prognosis becomes even more unfavorable in the setting of relapsed EMD, with a reported OS of less than 6 months [
7].
Pulmonary EMD is extremely rare, with no consolidated data about its prevalence except one study reporting a prevalence of 2.65% of all EMD, with an OS of approximately 2.8 to 4 months. Most data on clinical presentations and outcomes are seen in case reports given the rarity of pulmonary EMD. It can present in various forms, including pulmonary plasmacytoma presenting as a lung mass or multiple nodules, or pleural plasmacytoma with and without associated myelomatous pleural effusion (MPE) [
8]. Our patients presented with relapsed EMD in the form of pleural and pulmonary plasmacytoma that limited their survival and led to death after failure of several lines of therapies. Despite common patterns on imaging in PEMM being masses or nodules, there are few case reports of PEMM presenting as myeloma cells infiltrating pulmonary parenchyma bilaterally, mimicking antibiotic-resistant multilobar pneumonia or acute respiratory distress syndrome (ARDS) with high mortality (based on a limited number of cases) that may or may not be confirmed with bronchoalveolar lavage or transbronchial biopsy [
9,
10]. Case 1 showed disease progression, reflected by worsening serum K/L ratio, anemia, and clinical status. The patient developed bilateral pulmonary infiltrates on X-ray with negative sputum cultures that were unresponsive to antibiotics. This is hypothesized to have been pulmonary infiltration of myeloma cells. Unfortunately, this could not be confirmed as bronchoscopy was not performed due to patient oxygen requirements and eventual decision to pursue comfort care.
Another interesting finding that can present as PEMM is the presence of MPE as in our patient in case 1. MPE, resulting from malignant plasma cells infiltrating the pleural space, makes up less than 1% of pleural effusions (PEs) in MM patients and is associated with a poor prognosis, with a median survival of approximately four months [
11]. The majority of MPEs are typically characterized by being large, unilateral, serosanguinous, or sanguineous, lymphocytic predominant, and exudative [
12]. Our patient presented with a massive left-sided MPE exhibiting tension physiology, a rare occurrence reported in the literature. MPE may manifest either at the initial presentation of MM or as a late finding. In our patient, although MPE was presented late in the overall course of the disease, it was the presenting feature of the first relapse after autologous stem cell transplant (ASCT). Cytology analysis of pleural fluid often provides diagnostic yield as it did in our patient. Pleural biopsy may aid in diagnosis but may not always be required [
13]. An intriguing aspect of our patient’s myelomatous effusion was the development of MPE within months of undergoing stem cell transplantation, a phenomenon rarely reported before [
14]. However, this may point to the aggressive nature of his relapsed disease. PEMM, particularly in the context of relapsed refractory disease, remains a therapeutic challenge, with no definitive therapies established at these advanced stages. Rezvani et al. [
15] reported a case similar to our patient with an extramedullary relapse as a pleural plasmacytoma without bone marrow involvement after stem cell transplant, treated with DT-PACE, resulting in disease control at a 6-month follow-up. However, this treated case had not reached the advanced stage observed in our patient, who had failed three or four-drug regimens including VD-PACE and DPd and possessed high-risk cytogenetics. Moreover, our patient exhibited plasmablastic/anaplastic transformation, which independently confers an overall poor prognosis.
In addition to malignant pulmonary complications, clinicians should also be watchful about non-malignant pulmonary and pleural findings in patients with MM, such as pulmonary amyloidosis, transudative pleural effusions secondary to congestive heart failure or nephrotic syndrome, pneumonia, and pulmonary hypertension (PH). PH in MM can be multifactorial and may be attributed to drugs, including carfilzomib, which is used in MM, causing pulmonary arterial hypertension (Group 1) [
16], high output heart failure secondary to anemia and lytic lesions (Group 2), and increased thromboembolic risk due to a high prothrombotic state, as well as certain immunomodulatory drugs like lenalidomide leading to chronic thromboembolic pulmonary hypertension (CTEPH) [
17]. Jian et al. [
17] reported shorter PFS and OS in patients with concurrent pulmonary hypertension and multiple myeloma compared to those with multiple myeloma without pulmonary hypertension. PH is an important consideration in such patients, as a sizeable majority of these cases can be reversible or treatable with either correction of underlying causes like anemia, discontinuation of the offending drugs, surgical interventions (for CTEPH), or with ever-advancing therapies for PAH. Another rare but interesting non-malignant pulmonary manifestation of MM includes pulmonary amyloidosis (PA) that presents with diffuse alveolar and interstitial infiltrate that may mimic multilobar pneumomia or diffuse interstitial lung disease and requires a biopsy to confirm the diagnosis. Specifically, AL amyloidosis is a plasma cell proliferative disorder and can be an EMD presentation of multiple myeloma by association. It can be confirmed by Congo red staining and IHC on biopsy to distinguish the subtype of amyloid as well as electrophoresis studies. PA is an important consideration in patients with MM due to poor prognosis and clinical implications including respiratory decompensation and cardiovascular effects like PH [
18].
5. Future Directions
Both patient cases represent difficult to treat MM that have EMD and were penta-refractory, defined by being resistant to at least two immunomodulatory drugs (IMiDs), two different proteasome inhibitors (PIs), and at least one CD38 monoclonal antibody. For penta-refractory patients, outcomes are poor; however, BCMA treatment has shown some benefit in refractory myeloma, including through CAR-T therapy (ide-cel and cilta-cel). One retrospective study evaluated the outcomes in penta-refractory multiple myeloma patients and showed that those who were treated with BCMA therapy had a better median overall survival of 17 vs. 6 months (HR 0.3,
p-value < 0.001) [
19]. In this population of patients, 58% had extramedullary disease. One limitation for our two patients is that BCMA targeting CAR-T treatment was only recently approved when they had progression and so there were long waiting times to receive this therapy at that time. One patient in our case series did receive belantamab mafodotin, a BCMA antibody–drug conjugate; however, they still did have progression. Belantamab mafodotin has since been withdrawn from the market as it did not meet its primary endpoint of progression free survival [
20]. Performance status, high-risk cytogenetics, and white race were associated with poor outcomes in the penta-refractory population, so myeloma treatment must be tailored to the individual patient [
19].
Since then, there have been advancements in myeloma treatment, especially for refractory disease, including new targets such as GPRC5D. BCMA is expressed on mature plasma cells, but GPRC5D is expressed only on the surface of myeloma cells. A recent phase 1 study RedirecTT-1 presented at ASCO 2023 evaluated the combination of teclistamab (a bispecific T-cell engager targeting BCMA) and talquetamab (a bispecific antibody targeting GPRC5D) in patients with relapsed/refractory multiple myeloma [
21]. In this trial, 66% were penta-drug refractory and 38% had extramedullary disease. The overall response rate was 86.6% amongst all dose levels and 96.3% at the recommended phase 2 regimen. The complete response rate was 40.2% in all dose levels. In patients with extramedullary soft tissue plasmacytomas, 85.7% responded to the recommended dose with 28.6% having complete responses [
22]. This study showed an exciting combination of bispecific antibodies targeting pathways for resistant myeloma with no increase in toxicity as well as being effective for those patients with extramedullary disease.
6. Conclusions
This case series adds to the limited literature on PEMM and highlights its poor prognosis even within the era of multiple lines of therapies and improved outcomes in MM. Our cases highlight the various features of PEMM, including masses and pleural effusions, that are important to recognize as a clinician, as these features generally portend a poor prognosis. Relapsed/refractory myeloma remains challenging to treat especially when presenting with EMD; however, there have been many new drugs and treatment combinations that are showing promising results. New targets such as BCMA and GPRC5D have been approved in bispecific formats, as well as CAR-T therapy, and have been shown to be effective even in the penta-refractory population. While this case series underscores the difficulties in treating multiple myeloma and EMD, there is hope that novel drugs may improve outcomes for patients in the future.
Author Contributions
Writing original draft preparation S.K. and A.M.; drafting images, figures, and legends, reviewing the manuscript and editing J.P.; writing review and editing, O.A., M.C., S.K., F.M. and A.M.; supervision O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of South Alabama (1904926-5 approved 5/2/22) for retrospective data collection.
Informed Consent Statement
IRB approval was obtained with a waiver of consent, given that this is a retrospective study and carries no more than minimal risk to the subjects.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors S.K., A.M., M.C., F.M. and J.P. declare no conflicts of interest. O.A.: research funding to the institution related to this manuscript: AbbVie and Bristol-Myers Squibb. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Bladé, J.; Beksac, M.; Caers, J.; Jurczyszyn, A.; von Lilienfeld-Toal, M.; Moreau, P.; Rasche, L.; Rosiñol, L.; Usmani, S.Z.; Zamagni, E.; et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Touzeau, C.; Moreau, P. How I treat extramedullary myeloma. Blood 2016, 127, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Oymak, F.S.; Karaman, A.; Soyuer, I.; Karaman, H.; Gülmez, I.; Demir, R.; Unal, A.; Ozesmi, M. Pulmonary and chest wall involvement in multiple myeloma. Tuberk. Toraks 2003, 51, 27–32. [Google Scholar] [PubMed]
- Bladé, J.; de Larrea, C.F.; Rosiñol, L. Extramedullary involvement in multiple myeloma. Haematologica 2012, 97, 1618. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yue, X.; He, D.; Zhao, Y.; Yang, Y.; Zheng, G.; Han, X.; Wu, W.; Yang, L.; Chen, J. Multiple Extramedullary-Bone Related and/or Extramedullary Extraosseous Are Independent Poor Prognostic Factors in Patients With Newly Diagnosed Multiple Myeloma. Front. Oncol. 2021, 11, 668099. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Rakshit, S.; Kumar, S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; Golovyan, D.; Smith, J. Multiple myeloma causing interstitial pulmonary infiltrates and soft-tissue plasmacytoma. Respir. Med. Case Rep. 2018, 24, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Ravinet, A.; Perbet, S.; Guièze, R.; Lemal, R.; Guérin, R.; Gayraud, G.; Aliane, J.; Tremblay, A.; Pascal, J.; Ledoux, A.; et al. Lung postmortem autopsy revealing extramedullary involvement in multiple myeloma causing acute respiratory distress syndrome. Case Rep. Hematol. 2014, 2014, 635237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Li, Y.Y.; Hu, C.P.; Yang, H.P. Myelomatous pleural effusion as an initial sign of multiple myeloma-a case report and review of literature. J. Thorac. Dis. 2014, 6, E152–E159. [Google Scholar] [PubMed]
- Riveiro, V.; Ferreiro, L.; Toubes, M.E.; Lama, A.; Álvarez-Dobaño, J.M.; Valdés, L. Characteristics of patients with myelomatous pleural effusion. A systematic review. Rev. Clin. Esp. 2018, 218, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, I.B.; Feki, N.B.; Lamloum, M.; Hamzaoui, A.; Khanfir, M.; Salem, T.B.; Said, F.; Ben Romdhane, N.; Houman, M.H. Pleural myelomatous involvement in multiple myeloma: Five cases. Ann. Saudi Med. 2015, 35, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Müzeyyen Aslaner, A.; Barut, F. Myelomatous pleural effusion developing after autologous stem cell transplantation in a patient with multiple myeloma: A rare case. Indian. J. Pathol. Microbiol. 2023, 66, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.; Shahriarirad, R.; Fallahi, M.J.; Zeighami, A. Extramedullary relapse of Immunoglobulin A-kappa myeloma manifesting as plasmacytoma of the pleura without bone marrow involvement and following autologous bone marrow transplant: A case report. J. Med. Case Rep. 2023, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Buckstaff, T.; Narezkina, A.; Fernandes, T.M. Carfilzomib-associated pulmonary arterial hypertension in multiple myeloma. Pulm. Circ. 2021, 11, 20458940211049300. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.N.; Robinson, M.; Jagosky, M.; Slaughter, D.; Arnall, J.; Jandrisevits, E.; Matusz-Fisher, A.; Atrash, S.; Paul, B.; Bhutani, M.; et al. Thromboembolism Incidence and Risk Factors in Multiple Myeloma After First Exposure to Immunomodulatory Drug-Based Regimens. Clin. Lymphoma Myeloma Leuk. 2021, 21, 188–198.e2. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhou, H.; Wang, Y.; Zhang, Z.; Yang, G.; Geng, C.; Said, F.; Ben Romdhane, N.; Habib Houman, M. Echocardiography-defined pulmonary hypertension is an adverse prognostic factor for newly diagnosed multiple myeloma patients. Cancer Med. 2022, 11, 4182–4192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, Z.; Zhang, H.; Zhang, Y.; Shi, M.; Meng, F.; Sun, Q.; Cai, H. Diffuse parenchymal pulmonary amyloidosis associated with multiple myeloma: A case report and systematic review of the literature. BMC Cancer 2018, 18, 802. [Google Scholar] [CrossRef] [PubMed]
- Atrash, S.; Mammadzadeh, A.; Peng, F.; Alkharabsheh, O.; Afrough, A.; Cui, W.; Mahmoudjafari, Z.; Abdallah, A.O.; Hashmi, H. Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy. Cancers 2023, 15, 2891. [Google Scholar] [CrossRef] [PubMed]
- FDA Granted Accelerated Approval to Belantamab Mafodotin-Blmf for Multiple Myeloma. 3 July 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma./ (accessed on 12 June 2024).
- Helwick, C. More on Multiple Myeloma From ASCO 2023: Focus on Bispecific Antibodies. 10 September 2023. Available online: https://ascopost.com/issues/september-10-2023/more-on-multiple-myeloma-from-asco-2023-focus-on-bispecific-antibodies/ (accessed on 12 June 2024).
- Cohen, Y.C.; Morillo, D.; Gatt, M.E.; Sebag, M.; Kim, K.; Min, C.-K.; Oriol, A.; Ocio, E.M.; Yoon, S.-S.; Mateos, M.-V.; et al. First results from the RedirecTT-1 study with teclistamab (tec) + talquetamab (tal) simultaneously targeting BCMA and GPRC5D in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2018, 41, 8. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).